2019 年 67 巻 11 号 p. 1250-1254
2019 年 67 巻 11 号 p. 1250-1254
Methotrexate is a folate antagonist cytotoxic drug employed in the therapy of cancers and rheumatoid arthritis. Hypobromous acid (HOBr) and hypochlorous acid (HOCl) are generated by eosinophils and neutrophils at inflammation sites. The administered methotrexate may encounter HOBr and HOCl, and react with them to generate products. When methotrexate was incubated with HOBr or HOCl at pH 7.4 and 37°C for 30 min, a single product was generated almost exclusively in each case, identified as 3′-bromomethotrexate for HOBr and 3′-chloromethotrexate for HOCl. When methotrexate was incubated with HOCl in the presence of NaBr, the concentration of 3′-bromomethotrexate increased with decreasing concentration of 3′-chloromethotrexate in a dose-dependent manner with NaBr, probably due to the formation of HOBr. Free amino acids suppressed the reactions of methotrexate with HOBr and HOCl. Taurine suppressed the HOCl reaction but not the HOBr reaction. These results suggest that 3′-bromomethotrexate and 3′-chloromethotrexate may be generated from methotrexate at inflammation sites in humans, although their formation will be suppressed by coexistent amino acids.
Methotrexate (MTX), also known as amethopterin, is one of the oldest drugs but is still frequently used.1–3) MTX is structurally similar to folic acid and acts as an antimetabolite, interfering with the metabolism of folic acid. MTX competitively inhibits dihydrofolate reductase synthesizing tetrahydrofolate that provides single carbon groups for the synthesis of precursors of DNA and RNA.4) Thus, MTX can inhibit cell proliferation. High doses of MTX are prescribed for the treatment of cancer,5) while low doses MTX are widely prescribed for the treatment of rheumatoid arthritis.6,7) These diseases treated with MTX involve inflammation. At sites of inflammation, eosinophils and neutrophils are recruited. Eosinophils, one of the white blood cell types, are abundant in blood and tissues in various inflammatory disorders.8) Eosinophil peroxidase, secreted by eosinophils, generates hypobromous acid (HOBr) using H2O2 and Br−.9,10) Although the plasma concentrations are approx. 60 µM for Br− and 100 mM for Cl−, eosinophil peroxidase uses Br− preferentially to generate HOBr.11,12) Meanwhile, neutrophils are a major component of white blood cells. Myeloperoxidase, secreted by neutrophils, generates hypochlorous acid (HOCl) from H2O2 and Cl−,13–15) which plays a central role in host defense mechanisms against infection. HOCl can oxidize Br− to generate HOBr.16,17) Thus, a portion of the HOCl formed by the myeloperoxidase system in humans should react with Br−, converting to HOBr. Under inflammation conditions, MTX may encounter HOBr and HOCl, and react with them. In the present study, we examined the reactions of MTX with HOBr and HOCl, and report that both HOBr and HOCl react with MTX to generate monohalogenated products. To the best of our knowledge, this is the first study to examine the reactions of MTX with HOBr and HOCl under neutral conditions, although the reactions of MTX with Br2 and Cl2 in hydrochloric acid were reported.18)
A solution of 100 µM MTX was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 30 min. When the reaction mixture was analyzed by reversed-phase (RP) HPLC, a product peak appeared in the chromatogram detected at 260 nm (Fig. 1). The product peak showed a UV spectrum (λmax = 260 and 372 nm) as shown in the inset of Fig. 1. The product was isolated by RP-HPLC and identified using MS (electrospray ionization time of flight mass spectrometry, ESI-TOF/MS) and NMR (1H-1d, 1H–1H correlation spectroscopy (COSY), 1H–13C heteronuclear multiple quantum correlation (HMQC), 1H–13C heteronuclear multiple bond correlation (HMBC), 13C-1d). The product showed an ESI-TOF/MS spectrum including peaks of m/z = 531 and 533 (1 : 1) in negative mode. High-resolution (HR) ESI-TOF/MS values of the molecular ion (m/z 531) for the product agreed with the theoretical molecular mass for C20H2179BrN8O5− attributable to [MTX + Br–2H]− within 3 ppm. A singlet aromatic 1H signal attributable to the 7-position of the pteridine moiety was observed. For the benzoyl group, three aromatic protons were observed. Two of them, attributable to 2′ and 6′ protons, showed correlations with the carbonyl carbon on the 1H–13C HMBC spectrum. From these data, the product was identified as 3′-bromomethotrexate (3′-Br-MTX) (Fig. 2).
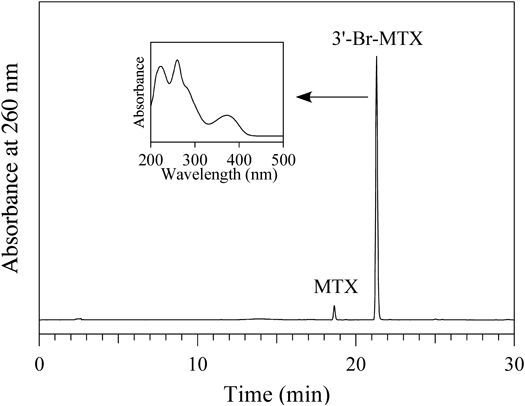
A solution of 100 µM MTX was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. The HPLC system consisted of LC-10ADvp pumps and an SPD-M10Avp UV-vis photodiode-array detector (Shimadzu). For the RP-HPLC, an Inertsil ODS-3 octadecylsilane column of 4.6 × 250 mm and particle size 5 µm (GL Sciences) was used. The eluent was 20 mM ammonium acetate (pH 7.0) containing methanol. The methanol concentration was increased from 0 to 50% during 15 min in linear gradient mode, and maintained at 50% from 15 to 30 min. The column temperature was 40°C and the flow rate was 1 mL/min.
Figure 3A shows the time-dependence of the reaction of MTX with HOBr when 100 µM MTX and 100 µM HOBr were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 0–4 h. The reaction was fast and completed within 10 min. Figure 3B shows the HOBr dose-dependence of the reaction of MTX with HOBr when 100 µM MTX and 0–100 µM HOBr were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. With increasing HOBr concentration, the consumption of MTX and the formation of 3′-Br-MTX increased. MTX was converted to 3′-Br-MTX almost exclusively.

(B) HOBr dose-dependence of the concentration changes in MTX and 3′-Br-MTX when a solution of 100 µM MTX was incubated with 0–100 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. MTX (closed circle) and 3′-Br-MTX (open triangle). All the reaction mixtures were analyzed by RP-HPLC. Means ± standard deviation (S.D.) (n = 3) are presented.
A solution of 100 µM MTX was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 30 min. A product peak appeared in the RP-HPLC chromatogram detected at 260 nm (Fig. 4). The peak showed a UV spectrum (λmax = 261 and 372 nm) similar to 3′-Br-MTX. The product was isolated by RP-HPLC and identified using MS and NMR. The product showed a ESI-TOF/MS spectrum including peaks of m/z = 487 and 489 (3 : 1) in negative mode. HR-ESI-TOF/MS values of the molecular ion (m/z 487) for the product agreed with the theoretical molecular mass for C20H2135ClN8O5− attributable to [MTX + Cl–2H]− within 3 ppm. For NMR, results similar to those of 3′-Br-MTX were obtained. A singlet aromatic 1H signal attributable to the 7-position of the pteridine moiety was observed. For the benzoyl group, three aromatic protons were observed. Two of them, attributable to 2′ and 6′ protons, showed correlations with the carbonyl carbon on the 1H–13C HMBC spectrum. From these data, the product was identified as 3′-chloromethotrexate (3′-Cl-MTX) (Fig. 5).
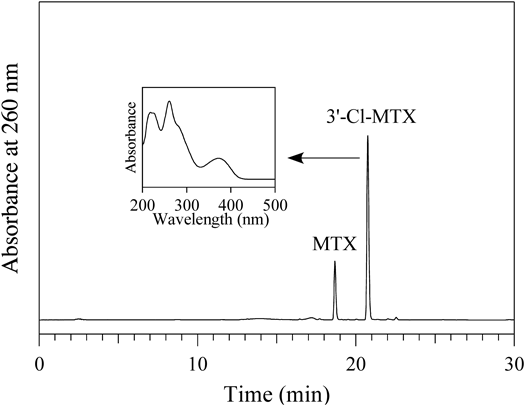
A solution of 100 µM MTX was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. The HPLC conditions are the same as shown in Fig. 1.

Figure 6A shows the time-dependence of the reaction of MTX with HOCl when 100 µM MTX and 100 µM HOCl were incubated in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 0–4 h. The reaction was fast and completed within 10 min. Figure 3B shows the HOCl dose-dependence of the reaction of MTX with HOCl when 100 µM MTX and 0–100 µM HOCl were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. With increasing HOCl concentration, the consumptions of MTX and the formation of 3′-Cl-MTX increased. MTX was converted to 3′-Cl-MTX almost exclusively.
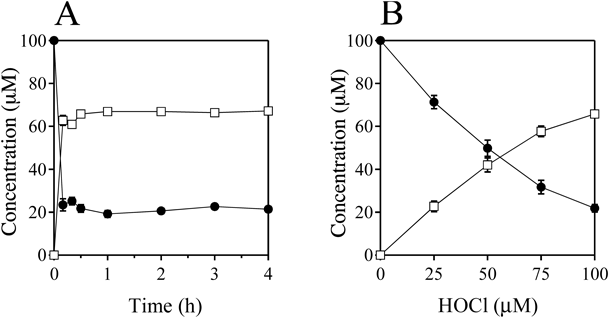
(B) HOCl dose-dependence of the concentration changes in MTX and 3′-Cl-MTX when a solution of 100 µM MTX was incubated with 0–100 µM HOCl in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. MTX (closed circle) and 3′-Cl-MTX (open square). All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
A solution of 100 µM MTX was incubated with 100 µM HOCl in the presence of 0–100 µM NaBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 30 min (Fig. 7). As the NaBr dose was increased up to 100 µM, the concentration of 3′-Cl-MTX decreased, while the concentration of 3′-Br-MTX increased.
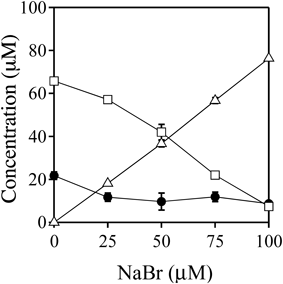
MTX (closed circle), 3′-Br-MTX (open triangle), and 3′-Cl-MTX (open square). All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
To obtain information about the effect of coexistent compounds on the reactions of MTX with HOBr, experiments were carried out in the presence of various additives. Table 1 shows the concentrations of MTX and 3′-Br-MTX when a solution of 100 µM MTX and 1 mM additives was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h. Ammonium chloride suppressed the reaction. Methylamine and dimethylamine showed little effect, while trimethylamine and tetramethylammounium showed no effect. Amino acids suppressed the reaction. Taurine and urea showed no effect. Ascorbic acid suppressed the reaction. Glucose and sodium acetate showed no effect.
| Additives | MTX (µM) | 3′-Br-MTX (µM) |
|---|---|---|
| None | 2.6 ± 0.7 | 95.9 ± 1.1 |
| NH4Cl | 50.4 ± 4.9 | 42.5 ± 4.6 |
| CH3NH2/HCl | 10.3 ± 2.9 | 87.1 ± 2.6 |
| (CH3)2NH/HCl | 19.1 ± 3.7 | 77.7 ± 3.8 |
| (CH3)3N/HCl | 0.2 ± 0.3 | 95.8 ± 0.4 |
| (CH3)4NCl | 0.0 ± 0.0 | 95.3 ± 1.9 |
| Gly | 59.6 ± 1.0 | 38.5 ± 0.3 |
| Lys | 89.2 ± 0.1 | 9.0 ± 0.1 |
| Met | 95.8 ± 0.1 | 4.1 ± 0.1 |
| Cys | 98.2 ± 0.1 | 1.6 ± 0.0 |
| Taurine | 0.9 ± 1.2 | 95.6 ± 0.7 |
| Urea | 0.7 ± 0.6 | 94.3 ± 1.1 |
| Ascorbic acid | 78.4 ± 0.9 | 4.9 ± 1.0 |
| Glucose | 0.7 ± 0.5 | 93.8 ± 4.8 |
| CH3COONa | 4.0 ± 2.5 | 92.7 ± 2.4 |
a) Concentrations of MTX and 3′-Br-MTX when a solution of 100 µM MTX was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h in the presence of 1 mM of additives. All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
To obtain information about the effect of coexistent compounds on the reactions of MTX with HOCl, experiments were carried out in the presence of various additives. Table 2 shows the concentrations of MTX and 3′-Cl-MTX when a solution of 100 µM MTX and 1 mM additives was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h. Ammonium chloride, methylamine, and dimethylamine strongly suppressed the reaction, while trimethylamine and tetramethylammounium showed little effect. Amino acids and taurine suppressed the reaction almost completely. Urea, glucose, and sodium acetate showed little suppression. Ascorbic acid suppressed the formation of 3′-Cl-MTX almost completely, although a certain amount of MTX was consumed.
| Additives | MTX (µM) | 3′-Cl-MTX (µM) |
|---|---|---|
| None | 19.1 ± 3.4 | 64.6 ± 2.5 |
| NH4Cl | 84.0 ± 2.3 | 3.0 ± 0.2 |
| CH3NH2/HCl | 98.5 ± 0.7 | 1.0 ± 0.1 |
| (CH3)2NH/HCl | 97.3 ± 0.7 | 2.1 ± 0.2 |
| (CH3)3N/HCl | 29.6 ± 3.0 | 64.5 ± 3.4 |
| (CH3)4NCl | 23.1 ± 2.2 | 63.5 ± 2.2 |
| Gly | 99.1 ± 0.5 | 0.9 ± 0.1 |
| Lys | 98.4 ± 0.3 | 0.7 ± 0.0 |
| Met | 100.1 ± 0.4 | 0.7 ± 0.0 |
| Cys | 99.8 ± 0.3 | 0.7 ± 0.1 |
| Taurine | 98.8 ± 0.3 | 0.8 ± 0.0 |
| Urea | 21.5 ± 8.2 | 62.4 ± 6.6 |
| Ascorbic acid | 81.4 ± 0.2 | 0.6 ± 0.1 |
| Glucose | 27.9 ± 3.9 | 58.9 ± 3.4 |
| CH3COONa | 26.3 ± 9.8 | 59.7 ± 8.3 |
a) Concentrations of MTX and 3′-Cl-MTX when a solution of 100 µM MTX was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h in the presence of 1 mM of additives. All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
3′-Br-MTX and 3′-Cl-MTX were reportedly synthesized from MTX in hydrochloric acid by Br2 and Cl2, respectively.18) In the present study, we showed that MTX reacted with HOBr and HOCl to generate 3′-Br-MTX and 3′-Cl-MTX in a neutral solution, respectively. The reactions were fast and generated the corresponding halogenated MTX almost exclusively. Recently, we showed that rebamipide, a therapeutic agent for gastric ulcers and chronic gastritis, reacted with HOBr to yield several products including 3-bromorebamipide as a major product.19) Rebamipide did not react with HOCl. Under similar conditions, MTX reacted with not only HOBr but also HOCl to generate the corresponding monohalogenated products. Generally, the reactivity of HOBr to organic compounds is higher than that of HOCl due to the higher electrophilicity of HOBr compared to HOCl.20) MTX reacted with HOCl comparably to HOBr, especially at a low dose (0.25 mM) of the hypohalous acids as shown in Figs. 3B and 6B. This suggests that the reactivity of HOCl to MTX is relatively high. In the presence of NaBr, HOCl generated 3′-Br-MTX from MTX in addition to 3′-Cl-MTX as shown in Fig. 7. At around serum concentration of Br− (approx. 60 µM),11,12) the formation of 3′-Br-MTX was comparable to that of 3′-Cl-MTX, suggesting that 3′-Br-MTX, in addition to 3′-Cl-MTX, may also generated in vivo by HOCl formed by myeloperoxidase.
CH3NH2 and (CH3)2NH had little effect on the reactions of MTX with HOBr, whereas they suppressed the reactions with HOCl almost completely (Tables 1, 2). CH3NH2 and (CH3)2NH react with HOBr and HOCl to generate corresponding bromamines and chloramines, respectively. It has been reported that second-order rate constants for the reactions of phenol with bromamines of CH3NH2 and (CH3)2NH are several orders higher than those with chloramines.21) Similarly, MTX would react with bromamines of CH3NH2 and (CH3)2NH to generate 3′-Br-MTX, whereas MTX would not react with chloramines. The reactions of both HOBr and HOCl were suppressed by amino acids, even Gly. It has been reported that α-amino acids react with both HOBr and HOCl to yield corresponding aldehydes and nitriles via monobromamines and dibromamines of the α-amino acids, respectively.22) The reaction was suppressed more strongly by Lys, Met, and Cys than by Gly. This implies that, in addition to the reaction on the α-amino group, a γ-amino group for Lys, a methyl thiol group for Met, or a thiol group for Cys in the side chain reacts with HOBr and HOCl. The reaction of MTX with HOBr and HOCl in plasma should be suppressed by free amino acids, since the total concentration of free amino acids in human plasma is several mM.23) Taurine suppressed the HOCl reaction almost completely, whereas taurine did not suppress the HOBr reaction. Taurine reacts with HOBr and HOCl resulting in taurine bromamine (Tau-NHBr) and taurine cholamine (Tau-NHCl), respectively. It has been reported that the reactivity of Tau-NHBr to amino acids is higher than that of Tau-NHCl.24) Tau-NHBr may react with MTX to generate 3′-Br-MTX, whereas Tau-NHCl could not react with MTX. Although the taurine concentration is low in plasma (38 µM), it is high in neutrophils (19 mM) and eosinophils (15 mM).25) The reaction of MTX with HOCl generated by myeloperoxidase might be suppressed by taurine, whereas the reaction with HOBr by eosinophil peroxidase may not be suppressed.
Several studies have examined the concerning biological activities of 3′-Br-MTX and 3′-Cl-MTX. 3′-Br-MTX, as well as MTX, suppressed uptake of tritium-labeled MTX (MTX-3H) by the choroid plexus of a rabbit brain in vitro.26) Bromine-77-labeled 3′-Br-MTX (3′-77Br-MTX) injected into the femoral artery of a tumor bearing rat leg together with MTX showed high initial retention activity by the tumor.27) These reports indicate that 3′-Br-MTX can be taken up in organs or cells comparable to MTX. A Yoshida lymphosarcoma line resistant to an alkylating agent showed collateral sensitivity against 3′-Br-MTX.28) The original Yoshida sarcoma was transplanted into rats, which were then treated with 3′-Br-MTX. Initially, the tumor volume reduced, but tumor regrowth commenced, and less response was seen to the second dose. Some of the 3′-Br-MTX treated tumors were then transplanted into other rats. Treatment of the retransplanted tumors with the alkylating agent reduced tumor successfully. This suggests that 3′-Br-MTX can work as an antitumor reagent as well as or stronger than MTX under certain conditions. 3′-Cl-MTX inhibited both cultured human leukemic lymphoblasts and the corresponding MTX-resistant subline at a similar efficiency to MTX,29) suggesting that 3′-Cl-MTX works as an antitumor reagent as well as MTX.
The present study showed that MTX reacts with HOBr and HOCl to generate 3′-Br-MTX and 3′-Cl-MTX, respectively. Free amino acids suppressed both the HOBr and HOCl reactions, while taurine suppressed the HOCl reaction but not the HOBr reaction. These results suggest that 3′-Br-MTX and 3′-Cl-MTX may be generated from MTX at inflammation sites, although the reactions will be suppressed by coexistent amino acids. If these halogenations occur on MTX administered to humans with inflammation, they may affect the medical efficacy of MTX.
L-(+)-Amethopterin (MTX) was purchased from Nacalai Tesque (Kyoto, Japan). NaBr (>99.99%) was purchased from Sigma-Aldrich (MO, U.S.A.). Other chemicals were obtained from Nacalai Tesque or TCI (Tokyo, Japan). Bromide-free hypobromous acid (HOBr) was prepared by the addition of silver nitrate and subsequent distillation, as previously reported.17,29) The concentration of HOBr was determined spectrophotometrically at 331 nm in 10 mM NaOH using a molar extinction coefficient of 315 M−1 cm−1.30) Chloride-free sodium hypochlorite (NaOCl) was prepared by the method previously reported.31) The concentration of NaOCl was determined spectrophotometrically at 290 nm using a molar extinction coefficient of 350 M−1 cm−1.32) Water was purified with a Millipore Milli-Q deionizer.
HPLC and MS ConditionsThe HPLC system included a UV-vis photodiode-array detector (SPD-M10Avp, Shimadzu, Kyoto, Japan) equipped with an octadecylsilane column of 4.6 × 250 mm and particle size 5 µm (Inertsil ODS-3, GL Sciences, Tokyo, Japan). The eluent was 20 mM ammonium acetate (pH 7.0) containing methanol. The methanol concentration was increased from 0 to 50% during 15 min in linear gradient mode, and maintained at 50% from 15 to 30 min. The column temperature was 40°C and the flow rate was 1 mL/min. MS measurements were performed on a ESI-TOF-MS spectrometer (MicroTOF, Bruker, Bremen, Germany) in negative mode. The sample isolated by RP-HPLC was directly infused into the MS system by a syringe pump without a column.
Spectrometric Data3′-Bromomethotrexate (3′-Br-MTX). ESI-TOF-MS: m/z 531, 533 (1 : 1). HR-ESI-TOF-MS: m/z 531.074154 obsd, 531.074552 calcd for C20H2179BrN8O5−. UV: λmax = 260, 372 nm (pH 7.0). ε260 nm = 26800 M−1 cm−1. 1H-NMR (500 MHz, dimethyl sulfoxide (DMSO)-d6): δ (ppm/tetramethylsilane (TMS)) 8.77 (s, 1H, H-7), 8.30 (d, J = 6.9 Hz, 1H, NH), 8.12 (d, J = 2.3 Hz, 1H, H-2′), 7.81 (dd, J = 8.6, 1.7 Hz, 1H, H-6′), 7.28 (d, J = 8.6 Hz, 1H, H-5′), 4.44 (s, 2H, H-9), 4.25 (m, 1H, H-α), 2.78 (s, 3H, CH3), 2.25 (m, 2H, H-γ), 1.92 (m, 2H, H-β). 13C-NMR (125 MHz, DMSO-d6): δ (ppm/TMS) 174.8 (γ-COOH), 172.3 (α-COOH), 163.5 (C=O), 162.8, 162.7, 155.1 (C-8a), 152.3 (C-4′), 149.8 (C-7), 145.2 (C-6), 132.5 (C-2′), 130.2 (C-1′),127.3 (C-6′), 121.7 (C-5′), 121.1, 117.7 (C-3′), 58.2 (C-9), 53.2 (C-α), 40.8 (CH3), 32.2 (C-γ), 27.7 (C-β). 3′-Chloromethotrexate (3′-Cl-MTX). ESI-TOF-MS: m/z 487, 489 (3 : 1). HR-ESI-TOF-MS: 487.125732 obsd, 487.125067 calcd for C20H2135ClN8O5−. UV: λmax = 261, 372 nm (pH 7.0). ε260 nm = 26500 M−1 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ (ppm/TMS) 8.74 (s, 1H, H-7), 8.28 (d, J = 6.9 Hz, 1H, NH), 7.93 (s, 1H, H-2′), 7.75 (d, J = 8.3 Hz, 1H, H-6′), 7.26 (d, J = 8.6 Hz, 1H, H-5′), 4.46 (s, 2H, H-9), 4.22 (m, 1H, H-α), 2.81 (s, 3H, CH3), 2.22 (m, 2H, H-γ), 1.92 (m, 2H, H-β). 13C-NMR (125 MHz, DMSO-d6): δ (ppm/TMS) 175.0 (γ-COOH), 173.7 (α-COOH), 163.6 (C=O), 162.8, 162.7, 155.1 (C-8a), 150.8 (C-4′), 149.6 (C-7), 145.2 (C-6), 129.4 (C-2′), 129.3 (C-3′), 126.6 (C-6′), 126.3 (C-1′), 121.1 (C-5′), 121.0, 57.8 (C-9), 53.4 (C-α), 40.3 (CH3), 32.5 (C-γ), 27.8 (C-β).
Quantitative ProceduresThe concentrations of the products were evaluated according to integrated peak areas on RP-HPLC chromatograms detected at 260 nm and by the molecular extinction coefficients at 260 nm (ε260 nm).
The ε260 nm value of 22700 M−1 cm−1 was used for MTX. The ε260 nm values of 3′-Br-MTX and 3′-Cl-MTX were determined from the integration of proton signals of NMR and the HPLC peak area detected at 260 nm relative to that of MTX in the mixed solution. The estimated ε260 nm values were 26800 M−1 cm−1 for 3′-Br-MTX and 26500 M−1 cm−1 for 3′-Cl-MTX.
The authors declare no conflict of interest.