2019 年 67 巻 3 号 p. 271-276
2019 年 67 巻 3 号 p. 271-276
The different states of water incorporated in wet granules were studied by a low-field benchtop 1H-NMR time-domain NMR (TD-NMR) instrument. Wet granules consisting different fillers [cornstarch (CS), microcrystalline cellulose (MCC), and D-mannitol (MAN)] with different water contents were prepared using a high-speed granulator, and then their spin–spin relaxation time (T2) was measured using the NMR relaxation technique. The experimental T2 relaxation curves were analyzed by the two-component curve fitting, and then the individual T2 relaxation behaviors of solid and water in wet granules were identified. According to the observed T2 values, it was confirmed that the molecular mobility of water in CS and MCC granules was more restricted than that in the MAN granule. The state of water appeared to be associated with the drying efficiency and moisture absorption capacity of wet granules. Thus, it was confirmed that the state of water significantly affected the wet granulation process and the characteristics of the resultant granules. In the final phase of this study, the effects of binders on the molecular mobility of water in granulation fluids and wet granules were examined. The state of water in granulation fluids was substantially changed by changing the binders. The difference was still detected in wet granules prepared by addition of these fluids to the fillers. In conclusion, TD-NMR can offer valuable knowledge on wet granulation from the viewpoint of molecular mobility of water.
Granulation is the process in which small powder particles are processed to adhere to form larger multiparticle agglomerates called granules.1,2) It is one of the fundamental unit operations for manufacturing pharmaceutical solid dosage forms including tablets, granules, and capsules. The main purposes of granulation are to improve the flowability and the compaction characteristics of the powders, and to ensure constant uniformity of the active pharmaceutical ingredients (APIs) incorporated into the pharmaceuticals. Granulation methods can be divided into two types: wet granulation methods use a liquid in the process (in most cases, a binder dissolved in water), while dry granulation methods do not use any liquid. In the pharmaceutical industry, wet granulation is a more commonly used compared to dry granulation because the method is applicable to any APIs as long as they are stable in water. Wet granulation is performed by amassing a mix of dry powder with granulating fluids.
We assumed that the state of water in wet granules was significantly associated with the wet granulation process and characteristics of the resultant granules. In general, water molecules can be distinguished into bound water and free water in terms of molecular mobility.3,4) Compared with free water, the molecular mobility of bound water is substantially restricted. Because of these issues, this study conducted a detailed investigation about the state of water in wet granules using the NMR solvent relaxation technique.5–8) NMR relaxation is the process of recovery from an excited magnetic state to its equilibrium state.9) After a radiofrequency pulse, excited nuclear spins relax back to the equilibrium state. There are two mechanisms involving spin–lattice and spin–spin relaxations. The spin–spin relaxation is also referred to as transverse relaxation or T2 and describes the decay of the excited magnetization perpendicular to the applied magnetic field. The spin–lattice relaxation is also referred to as longitudinal relaxation or T1 and describes the return to equilibrium in the direction of the magnetic field. The T2 relaxation time (T2) is the time constant for the decay of transverse magnetization, whereas the T1 relaxation time (T1) is the time constant for the regrowth of longitudinal magnetization. T1 and T2 are widely used in evaluating the molecular mobility of compounds. For instance, the T1 and T2 values of bound water are supposed to be shorter than the values of free water because of the restriction on molecular mobility.
Time-domain NMR (TD-NMR) is a low-field (20 MHz) benchtop 1H-NMR instrument that measures 1H-NMR relaxation time.10,11) It enables the rapid and easy measurement of T1 and T2 regardless of the phase of the samples (both solid and liquid samples are measurable). TD-NMR has been used in various research fields, in particular the chemical,12–14) food,15–17) plant,18,19) and material science fields.20–22) However, to date, its application to pharmaceutical research has been limited.10,11,23) Recently, we have demonstrated the usefulness of TD-NMR for the evaluation of physicochemical properties of pharmaceuticals. These studies include characterization of the crystalline state of APIs24) and the dispersion state of nanoparticles in concentrated suspensions.25)
In this study, wet granules were prepared using different fillers [cornstarch (CS), microcrystalline cellulose (MCC), and D-mannitol (MAN)], and then tested using TD-NMR. The present study employed T2 as an index to investigate the molecular mobility of the samples. In general, T2 measurement can be achieved with a shorter period compared to T1 measurement. Furthermore, the change behavior of T2 as a function of molecular mobility is simpler than that of T1; T2 becomes shorter monotonically with restriction on the molecular mobility. This study further assessed the drying process of the wet granules and the moisture absorption capacity of the prepared granules to assess the contribution of the state of water to the wet granulation process and the characteristics of the resultant granules. We also investigated the effects of binders on the state of water in the granulation fluids. Our findings confirmed that the state of water played a crucial role in wet granulation.
MCC (CEOLUS UF-711®) was purchased from Asahi Kasei Chemicals (Tokyo, Japan). CS (ST-C®) was purchased from Nippon Starch Chemical (Osaka, Japan). MAN (Mannit P®) was purchased from Mitsubishi Shoji Foodtech (Tokyo, Japan). Polyvinylpyrrolidone (PVP; Kollidon 90F®) was purchased from BASF Japan (Tokyo, Japan). Hydroxypropylcellulose (HPC; HPC-SL®) was purchased from Nippon Soda (Tokyo, Japan). Polyvinyl alcohol (PVA)–acrylic acid–methyl methacrylate (PVA copolymer; POVACOAT (F)®) was purchased from Daido Chemical (Osaka, Japan).
Wet GranulationPurified water and aqueous solutions dissolving 5% of each binder (HPC, PVP, and PVA copolymer) were used as granulation fluids. For preparation of wet granules, a designated amount of the granulation fluids was added to each filler (CS, MCC, and MAN), and then wet granulation was performed using a high-speed granulator (VG-mm®; Powrex, Hyogo, Japan) for 3 min at 800 rpm with the main blade and 3,000 rpm with a cross chopper.
Measurement of T2 Using TD-NMRThe 1H T2 relaxation behaviors of the samples were measured by TD-NMR using a Bruker minispec mq20® (Bruker BioSpin, Billerica, MA, U.S.A.) at a 1H frequency of 20 MHz at 25°C. The solid echo pulse sequence was used for the T2 measurement of wet granules. The following parameters were applied: number of scans = 8, recycle delay = 50 s. After the measurement, T2 was calculated using the TD-NMR Analyze Software® (Bruker BioSpin). In addition, the T2 measurement of granulation fluids (the binder solutions) was performed by the Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence. The acquisition parameters were as follows: number of scans = 10, the time between each pulse (τ spacing) = 2.5 ms, recycle delay = 11 s. The T2 was also calculated using the TD-NMR Analyze Software®. The measurement of T2 was triplicated.
Measurement of Drying Efficiency of Wet GranulesAfter preparation of the wet granules, they were incubated for 45 min in an oven at 50°C to dry the sample moderately. The remaining amount of water was calculated from the change in weight before and after incubation.
Measurement of Moisture Absorption of GranulesThe wet granules were completely dried for 8 h in an oven at 80°C. The resulting dry granules, in the size range of 250–355 µm, were obtained by sieving. After that, they were stored at 40°C and 75% relative humidity (RH) for 1 week in a stability chamber (CSH-110®; ESPEC, Osaka, Japan). Their moisture absorption was calculated from the change in weight before and after the experiment.
The T2 relaxation curves of wet granules containing 20% of water to the total weight are shown in Fig. 1. The T2 measurement was performed using the solid echo–pulse sequence to evaluate the molecular mobility of wet granules in terms of both solid and liquid components. The relationship between relaxation times (T1 and T2) and molecular mobility is well understood.26) T2 monotonically shortens with restriction on the molecular mobility; thus, solids have much shorter T2 values than liquids. A biphasic T2 relaxation behavior was observed for all wet granules (Fig. 1a); first, a considerable amount of NMR signals rapidly decreased within a very short period, and after that, the remaining signal gradually decreased. The initial and second phases corresponded to the T2 relaxation behaviors of the solid and liquid components in the wet granules, respectively. To distinguish between the two T2 relaxation behaviors, a two-component analysis was conducted: the initial relaxation of the solid component was approximated by Gauss curve fitting, and the following relaxation of the liquid component was approximated by exponential curve fitting. As a result of the analysis, the T2 relaxation behaviors of solid and liquid components were successfully identified (Figs. 1b, c). The ability to evaluate both solid and liquid at the same time is a great advantage of TD-NMR. Although high-field NMR spectrometers (400 MHz and higher) also enable measurement of the relaxation times of the samples,27,28) they specifically measure either the solid or the liquid sample; solid-state NMR spectroscopy is aimed at measurement of solid samples.
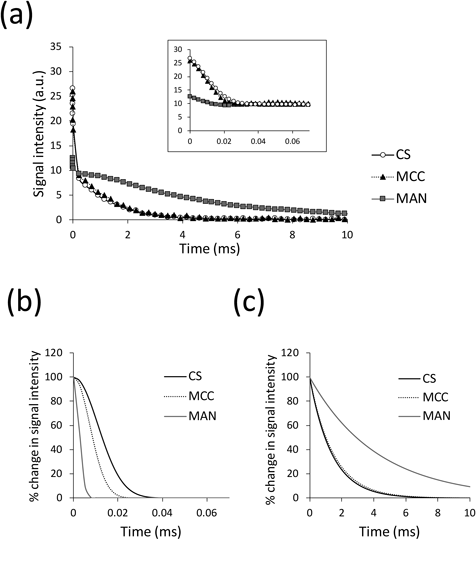
The test granules contained 20% by water weight. The T2 measurement was carried out by the solid–echo pulse sequence. The T2 relaxation behavior of the whole sample (a) showed biphasic behavior; numerous NMR signals rapidly decreased within a very short period (see inset graph), and then the remaining signal gradually decreased. They corresponded to the T2 relaxation of the solid and liquid components of the wet granules, respectively. The T2 relaxation values of the solid (b) and liquid (c) components of the wet granules were extracted by two-component curve fitting. The signal intensity of each T2 relaxation curve was normalized so that the initial values were 100%.
From the T2 values, we could characterize the wet test granules in terms of molecular mobility. Regarding the solid component, the T2 values calculated from the graphs in Fig. 1b were 0.0122, 0.0098, and 0.0072 ms for the CS, MCC, and MAN granules, respectively. Therefore, the inherent T2 values of the fillers were quite different from each other. The T2 values of water in wet granules calculated from the graphs in Fig. 1c were also different; they were 1.55, 1.33, and 4.02 ms for the CS, MCC, and MAN granules, respectively. This indicated that the state of water in wet granules was substantially affected by the fillers. The observed T2 values of water indicated that the molecular mobility of water in CS and MCC granules was more restricted than in the MAN granule. To investigate the state of water in granules in more detail, we measured the T2 of water in wet granules with different water contents. T2 values progressively increased with increasing water content (Fig. 2). The behaviors of the CS and MCC granules were clearly different from the MAN granule. The T2 values of water in the CS and MCC granules changed substantially with changing water content. For instance, the T2 of water in the MCC granule increased from 1.4 to 4.0 ms with increasing water content from 10 to 70%. As for the MAN granule, the T2 values hardly changed regardless of the water concentration: 4.2 and 4.7 ms of T2 for 10 and 30% of water, respectively. The T2 value of water was supposed to change according to the quantity of water interacting with the filler and the interaction affinity. The slopes of the plots in the figure can be used to compare the quantity of water interacting with fillers. That is, as long as the variation range of water content was the same, a larger change in T2 could be observed from the granules consisting of a filler that could interact with a larger amount of water. Thus, CS and MCC can interact with a larger amount of water than MAN. According to the findings, we concluded that CS and MCC had a more significant impact on water than MAN in terms of quantity and interaction affinity. It is known that MAN has not hygroscopic nature; thus, this result appears to be quite reasonable.

After preparation of the wet granules with different water content, the T2 values of water in granules were measured as shown in Fig. 1. Each value represents the mean ± S.D. (n = 3). S.D. is standard deviation.
This study further investigated the hydration of the solid component in wet granules using T2 (Fig. 3). In this experiment, the dry powdered fillers were measured as well as the wet granules, and their T2 values were compared. The T2 values of CS and MCC in wet granules were significantly increased by the addition of water. In particular, the T2 value of CS went up by 28% by the addition of water. By contrast, the T2 value of MAN did not change with water addition. This result was probably caused by the difference in the hydration properties of fillers. CS was proved to have the most obvious hydration property; CS can possess the largest amount of hydrated water among the test fillers. The hydrated water penetrated the filler particle, and then weakened the interaction between fillers, resulting in an increase in T2. We also believe that the molecular mobility of the hydrated water was much more restricted than that of water interacting with the surface of filler particle: the state of the hydrated water was thought to be closer to the solid phase rather than liquid phase. Thereby, the rapid T2 relaxation behavior (Fig. 1b) contained information about not only solid components but also hydrated water. By contrast, MAN was hardly hydrated because the T2 value of MAN was unchanged after the addition of water. From these findings, we fully characterized the state of water in the wet test granules.

After acquisition of the T2 of dry fillers (CS, MCC, and MAN), wet granules were prepared by adding 20% of water, and then the T2 values of fillers were obtained as shown in Fig. 1. Each value represents the mean ± S.D. (n = 3). ** p < 0.01 vs. dry fillers.
The state of water in wet granules is thought to exert a significant effect on the granulation process. First, there is a large possibility that the drying efficiency of wet granules is affected by the state of water. Some studies reported that bound water evaporates with more difficulty than free water because of the restriction of the molecular mobility by the drying process.29,30) Based on these reports, we investigated the drying efficiency of wet granules after the incubation for 45 min at 50°C. As a result of the experiment, the CS and MCC granules showed more remaining water than the MAN granule, 16%, and 21% respectively (Fig. 4a). A significant difference in the remaining water level was observed between the CS and MAN granules. This result represents the contribution of the state of water in wet granules to the drying efficiency. As clarified above, the molecular mobility of water in CS and MCC granules were restricted more tightly than that in MAN granule. The restriction on the molecular mobility is supposed to prevent water from evaporating. Furthermore, the remaining water level of CS granule seemed to be higher than that of MCC granule (Fig. 4a). That was probably because of the difference in hydrated water amount incorporated within the filler particle. The hydrated water was strongly resistant to evaporation. According to Fig. 3, CS granule possessed a larger amount of hydrated water than MCC granule. Owing to the high hydration property, the remaining water level of CS granule was higher than that of MCC granule (Fig. 4a).

(a) The remaining water level (in % from the initial amount) was measured after drying at 50°C for 45 min. (b) The moisture absorption levels of the resultant granules (% increase relative to granule weight) were measured by the change in sample weight after storage at 40°C, 75% RH, for 1 week. Each value represents the mean ± S.D. (n = 3). * p < 0.05 and ** p < 0.01 (the Tukey–Kramer method).
This study further assessed the moisture absorption capacity of granules to discuss the effect of the state of water on the characteristics of resultant granules. After complete drying of the wet granules, the resultant granules were stored at 40°C and 75% RH for one week. As shown in Fig. 4b, the CS granules showed the largest moisture absorption (9.8 ± 0.3%), but no moisture absorption was detected in the MAN granule. The rank order of higher water absorption capacity was consistent with that of the higher remaining water level shown in Fig. 4a. The water absorption capacity could also be explained by the hydration property. That is, as far as the test fillers were concerned, the filler having a higher capacity of hydration water could form granules having a larger moisture capacity. Taken together, it was confirmed that the state of water in wet granules had a substantial impact on the granulation process and the characteristics of the resultant granules.
In the next phase of this study, we investigated the effect of binders on the state of water in granulation fluids and granules. Various hydrophilic polymers are now used as binders for wet granulation.2) This study examined HPC, PVP, and PVA copolymer as test binders. For the measurement of the T2 values of granulation fluids, the CPMG pulse sequence was used. The pulse sequence is the most commonly used method for measuring the T2 of liquid samples. The water states were significantly changed with changing binders (Fig. 5a). Among them, the PVA copolymer solution showed the lowest T2 value, 587.2 ± 3.8 ms, indicating that this polymer could most significantly restrict the molecular mobility of water. As for the PVP solution, the T2 value, 2177 ± 23 ms, was equal to that of purified water, 2137 ± 4 ms. The T2 value of the HPC solution, 1676 ± 2 ms, was much shorter than that of purified water but was longer than that of the PVA copolymer. A similar result was obtained in our previous study.31) In the course of this study, we conducted a comparative study about the disintegration effects of various disintegrants to evaluate the interaction between each disintegrant and water. Suspensions dispersing 10% of disintegrant powders in purified water were prepared, and their T2 values were examined. The test disintegrants contained PVP and cellulose derivatives. The T2 of the PVP suspension was clearly longer than the T2 values of the suspensions of low-substituted HPC (L-HPC) and croscarmelloses.

As a test granulation fluid, aqueous solutions containing 5% of hydroxypropylcellulose (HPC), polyvinylpyrrolidone (PVP), and polyvinyl alcohol (PVA) copolymer were prepared. The T2 measurement of granulation fluids was carried out by the CPMG pulse sequence. The wet granules were prepared by adding the granulation fluids at a water content of 45%, and then the T2 measurement was achieved by the solid echo pulse sequence. The T2 of water in wet granules was measured by two-component curve fitting (as in Fig. 1). Each value represents the mean ± S.D. (n = 3). * p < 0.05 and ** p < 0.01 (the Tukey–Kramer method).
This study further investigated the molecular mobility of water added to CS as binder solutions. The T2 of wet CS granules prepared by the addition of different binder solutions is shown in Fig. 5b. The water content was fixed at 45% of the total weight. Although the difference was not very significant, a similar ranking order of T2 was observed from the wet granules; a significant difference in the T2 value of water between the PVA copolymer and water is still observed from Fig. 5b. Therefore, it was confirmed that the effect of binders on water in granulation fluids remained after the preparation of wet granules.
From these findings, it was determined that the state of water could substantially change by changing the components of granules, and such a difference has a significant impact on the granulation process, including the drying efficiency of wet granules. These findings could offer a comprehensive understanding of the wet granulation process.
The results showed that TD-NMR can provide detailed information about the state of water incorporated in wet granules consisting of different fillers. The molecular mobility of water in wet granules was substantially changed depending on the fillers. CS could hydrate with the largest amount of water, whereas the interaction between MAN and water was very weak. The state of water was proved to have a significant impact on the drying efficiency of wet granules and the moisture absorption capacity of the resultant granules. In the final phase of this study, the effect of binders on the state of water of granulation fluids and wet granules was identified. TD-NMR is a powerful technique for investigating the state of water in wet granules. This study could offer a profound insight into wet granulation.
This work was supported by JSPS KAKENHI Grant Number JP16K08192 and JSPS Core-to-Core Program, B. Asia-Africa Science Platforms.
The authors declare that they have no financial or competing interests concerning this manuscript. The Department of Pharmaceutical Technology, University of Toyama, is an endowed department supported by an unrestricted Grant from Nichi-Iko Pharmaceutical Co. (Toyama, Japan).