2019 Volume 67 Issue 4 Pages 372-381
2019 Volume 67 Issue 4 Pages 372-381
A series of meta-amido bromophenol derivatives were designed and synthesized. The compounds were found to potently inhibit the growth of Mycobacterium tuberculosis H37Ra. They also exhibited moderate inhibitory activity against Mycobacterium tuberculosis H37Rv and multidrug-resistant strains. The compounds did not show inhibitory activity against normal Gram-positive and Gram-negative bacteria. Moderate cytotoxicities and good metabolic stability were observed for the selected compounds. The results demonstrated meta-amido bromophenols as a new class of antitubercular agents with good potentials.
Tuberculosis (TB), a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb), has plagued people for thousands of years and remains a huge threat to public health. According to the report of the WHO, it is estimated that over 1.3 million people died from TB in 2017.1) One third of the population in the world carries latent TB, which can be activated upon immune system suppressed or disrupted, such as aging and human immunodeficiency virus (HIV) co-infection.2) Moreover, Mtb has developed the resistance to many first-line anti-TB drugs including rifampicin, isoniazid, streptomycin, ethambutol and pyrazinamide, etc.3,4) Rather limited drugs are applicable for the patients infected with multidrug-resistant (MDR) Mtb and extensive drug-resistant (XDR) Mtb.5) The treatment period is extremely long (typically 18–24 months) for MDR-TB and the cure rates are less than 50%.6) In recent four decades, only two new anti-TB drugs, bedaquiline7) and delamanid,8) were approved for the treatment of MDR-TB and XDR-TB. The two drugs are still beset by the side effects. For these reasons, anti-TB drugs with novel structures and action mechanisms are urgently desired.9)
In the recent study, we found that meta-amido phenol derivatives exhibit good inhibitory activity against Mtb in vitro.10) However, the privileged compound YZ-7 did not exert in vivo efficacy attributed to the fast clearance in the liver. The 3-benzyl group in compound YZ-7 was supposed to be liable to the oxidation catalyzed by CYP450. The further structural modifications were made to increase the metabolic stability (Chart 1). The replacement of 3-benzyl with a bromine led to the decreased activity. The shift of the bromine to 2-position resulted in the complete loss of the antitubercular activity. However, to our delight, the 4-bromo derivative 3 showed good inhibitory activity, which is comparable with YZ-7. In this paper, we report the design, synthesis and biological evaluation of a series of meta-amido bromophenols as new antitubercular agents.
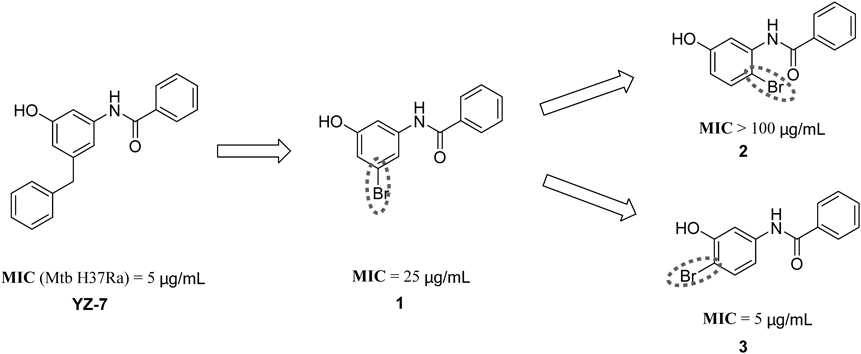
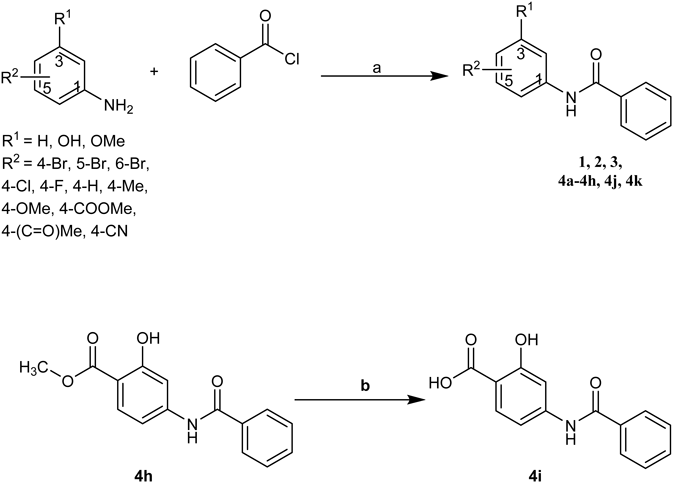
A series of meta-amido bromophenols were designed based on the structure of the lead compound 3. The structural modifications were made at the left-side bromophenol moiety, the amide linker, and the right-side phenyl group respectively (Chart 2). For the modification of bromophenol moiety, the hydroxyl was removed or methylated to provide compounds 4a, b. The bromine was replaced with chlorine, fluorine, methyl, methoxy, ester, acetyl, and cyano to afford derivatives 4c–k. For the modification of the amide linker, the shift of amide group to para- or ortho-position of the hydroxyl group led to compounds 5a and b. Furthermore, the transpositional analog 5c and N-benzyl analog 5d were prepared. For the structural modification of the right-side phenyl group, the replacement of the phenyl with alkyl, vinyl, heteroaryl, and the introduction of various substitutents on the phenyl ring led to the compounds 6a–u.

The synthesis of compounds 1–3 and 4a–k is outlined in Chart 3. The reaction of substituted anilines with benzoyl chloride provided the target compounds in good yields. Compound 4i was obtained after the hydrolysis of 4h in aqueous sodium hydroxide.
Reagents and conditions: (a) Et3N, THF, 0°C to r.t., 67–87%; (b) NaOH, CH3OH, H2O, 50°C, 73%.
The synthesis of compounds 5a–d is outlined in Chart 4. Compounds 5a and b were prepared via the reaction of 4-amino-2-bromophenol or 2-amino-6-bromophenol with benzoic acids in the presence of hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) and N,N-diisopropylethylamine (DIPEA). Compound 5c was obtained via the condensation of 4-bromo-3-methoxybenzoic acid and aniline. Compound 5d was prepared via the benzylation of 4-bromo-3-methoxyaniline and subsequent demethylation.
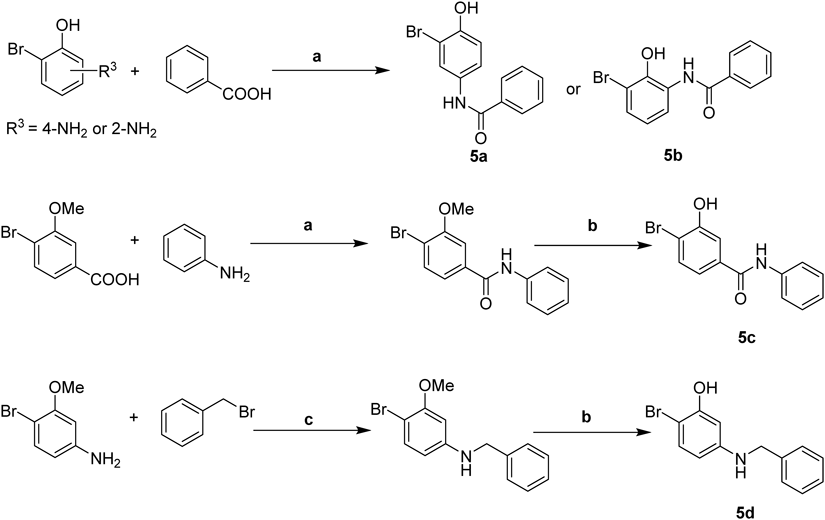
Reagents and conditions: (a) HATU, DIPEA, DMF, r.t., 71–90%; (b) BBr3, CH2Cl2, −20 to 0°C, 38–44%; (c) K2CO3, DMF, 50°C, 72%.
The synthesis of compounds 6a–u is outlined in Chart 5. 4-Bromo-3-methoxyaniline was treated with BBr3 to give 4-bromo-3-hydroxyaniline. The subsequent reaction with acyl chlorides or the direct condensation with acids in the presence of HATU and DIPEA provided compounds 6a–m.

Reagents and conditions: (a) BBr3, CH2Cl2, 0°C to r.t., 88%; (b) R4COCl, Et3N, THF, 0°C to r.t., 42–83%; (c) R4COOH, HATU, DIPEA, DMF, r.t., 76–80%.
Inhibitory activities of compounds 4a–k were evaluated against autoluminescent Mtb H37Ra strain.11) The bacteria growth was determined by the bioluminescence intensity and the results are summarized in Table 1. The existence of free hydroxyl is crucial for the antitubercular activity. Both the deletion and the methylation led to the complete loss of the inhibitory activity (Table 1, entries 2–3). The replacement of bromine with chlorine or fluorine resulted in the decreased activity (Table 1, entries 4–5). 4-Unsubstitutued derivative 4e is inactive (Table 1, entry 6). The results confirmed that the 4-substitution is indispensable. Non-halogen substituents such as methyl, methoxy, ester, carboxyl, acetyl, and cyano are incompatible (Table 1, entries 8–13). The bromine is demonstrated to be unique for the antitubercular activity, probably due to its suitably steric and electronic effect.
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | MIC (µg/mL) | Compound | R1 | R2 | MIC (µg/mL) |
| 3 | –OH | Br | 5 | 4f | –OH | Me | >100 |
| 4a | –H | Br | >100 | 4g | –OH | OMe | >100 |
| 4b | –OMe | Br | >100 | 4h | –OH | COOMe | >100 |
| 4c | –OH | Cl | 12.5 | 4i | –OH | COOH | >100 |
| 4d | –OH | F | 50 | 4j | –OH | COMe | >100 |
| 4e | –OH | H | >100 | 4k | –OH | CN | >100 |
| INH | 0.1 | EMB | 2 | ||||
INH: isoniazid; EMB: ethambutol.
The compounds 5a–d with the modifications of amide linker were investigated and the results are summarized in Table 2. The placement of amide group at the meta-position of hydroxyl group is crucial, since the compounds 5a and b almost lose antitubercular activities (Table 2, entries 1–2). The compounds 5c and d with the transposition of amide group or N-benzylation showed lower activity (Table 2, entries 3–4).
 |
Furthermore, the antitubercular activity of compounds 6a–u were determined and the results are summarized in Table 3. The replacement of phenyl with methyl, vinyl and heptyl led to the significant loss of the activity (Table 3, entries 2–4). However, the cyclohexyl derivative 6d showed certain antitubercular activity. The morpholine derivative 6e showed poor activity. The results suggested the strongly hydrophobic group is favorable herein. The lower activities of heteroaryl derivatives 6f–h (Table 3, entries 6–9) also support this hypothesis. The effect of the substitution on the phenyl group was examined (Table 3, entries 10–23). The substitution of chlorine at ortho- and meta-position (6i, j) afforded the comparable activity with compound 3, but para-chloro derivative 6k showed 4-folds increase in the activity. The para-fluoro derivative 6l showed slightly lower activity. The para-bromo and para-methyl analogs 6m, n exhibited the comparable activity with 6k. Other para-substitutents, such as MeO, CF3O, and dimethylamino also afforded good activity. The substitution with the electron-withdrawing and hydrophobic CF3 group (6r) showed superior activity. The minimum inhibitory concentration (MIC) value is 20-folds lower than compound 3. The para-nitro derivative 6s exhibited lower activity. The para-hydroxyl substitution (6t) led to the complete loss of activity. The replacement phenyl with a 2-naphthyl (6u) afforded a better activity. The results demonstrated that the hydrophobic property of the para-substitutent is favorable for antitubercular activity. On the other hand, the activity is not sensitive to the electronic property of the substitutent. Both electron-donating groups and electron-withdrawing groups provide good activity.
 |
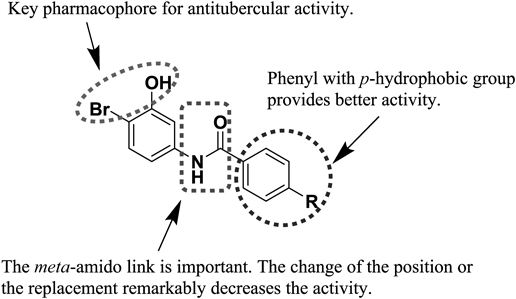
The SAR of meta-amido bromophenol derivatives is summarized in Chart 6. The ortho-bromphenol moiety is a key pharmacophore for antitubercyular activity. The meta-amido linker is also important. The transposition of amide group or the replacement by N-benzyl group decreases the activity. For the right side moiety, the hydrophobic and π–π interaction are crucial. The phenyl with 4-hydophobic substitutent provides the best activity.
Compounds 6p and r were selected for further evaluation against Mtb H37Rv and six clinically isolated MDR-TB strains. The results are summarized in Table 4. Both compounds exhibited moderate inhibitory activity against Mtb H37Rv and MDR-TB strains (MIC = 4–16 µg/mL). The activities are significantly lower than those against H37Ra. The reason is elusive at the present stage.
| Strains | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| 6p | 6r | RIF | INH | EMB | |
| H37Rv | 8 | 16 | 0.03 | 0.41 | 2 |
| V4 | 8 | 16 | <1 | >10 | <2 |
| K4 | 8 | 16 | >50 | >50 | — |
| K5 | 8 | 16 | >50 | >50 | — |
| K12 | 4 | 8 | >10 | >10 | >4 |
| K16 | 8 | 16 | >10 | >10 | >4 |
| K18 | 8 | 16 | >10 | >10 | >4 |
RIF: rifampicin.
The current treatment of TB requires the combinations of several antitubercular agents and a period of 6 months for drug-susceptible Mtb. Therefore, new antitubercular agents are expected to exclusively inhibit Mtb without influencing the normal intestinal flora.12) The antibacterial activities of compounds 6p and r were examined against Gram-positive bacteria S. aureus, E. faecalis and Gram-negative bacteria E. coli, P. aeruginosa. Amoxicillin was used as the positive control. The results are summarized in Table 5. Compounds 6p and r did not show inhibitory activity (MIC >50 µg/mL) against the tested bacteria. This result demonstrated that the inhibitory activity of meta-amido bromophenols against Mtb is highly exclusive.
| Bacteria | MIC (µg/mL) | ||
|---|---|---|---|
| 6p | 6r | Amoxicillin | |
| E. coli | >50 | >50 | 1.56 |
| S. aureus | >50 | >50 | 0.78 |
| E. faecalis | >50 | >50 | 6.25 |
| P. aeruginosa | >50 | >50 | —a) |
a) Unmeasured.
The cytotoxicities of compounds 6p, r against cell lines A549 and HepG2 were evaluated and the results are listed in Table 6. Low cytotoxicities (IC50 > 50 µg/mL) were observed against A549, but moderate cytotoxicities were determined against HepG2. The select indexs (SI = IC50/MIC) are in a range of 47–200 and are acceptable for the further research.
| Compound | IC50 (µg/mL) | MIC (H37Ra) | |
|---|---|---|---|
| A549 cells | HepG2 | ||
| 6p | >50 (SI > 100) | 23.4 (SI = 47) | 0.5 |
| 6r | 50 (SI = 200) | 23.3 (SI = 93) | 0.25 |
The stability of compounds 6r in rat liver microsome was also examined. In comparison with compound YZ-7 (T1/2 = 16.18 min, CLint = 85.7 mL/min/kg), 6r had a better metabolic stability (T1/2 = 30.0 min, CLint = 69.9 mL/min/kg). The result confirmed that the replacement of benzyl group in YZ-7 improves the metabolic stability.
We identified meta-amido bromophenols derivatives as a new class of antitubercular agents. Several compounds exhibited potent inhibitory activity against Mtb H37Ra. Furthermore, the selected compounds 6p and r showed moderate inhibitory activity against Mtb H37Rv and clinically isolated MDR-TB strains. The compounds did not inhibit the representative G+ and G− bacteria. Moderate cytotoxicities were determined and the selective indexes are acceptable. In addition, good metabolic stability in rat liver microsome was observed. Considering the simple structure and readily modular property, meta-amido bromophenols hold the good potentials for the further development as new antitubercular agents.
1H-NMR and 13C-NMR spectra were recorded on Bruker AVANCE 400 spectrometer. Chemical shifts of protons are reported in parts per million downfield from tetramethylsilane. Peaks are labeled as single (s), doublet (d), triplet (t), double doublet (dd), doublet of triplets (dt), multiplet (m). The high-resolution mass spectra were analyzed on a SHIMADZU LCMS-IT-TOF mass spectrometer. All chemicals were purchased from Sigma-Aldrich, Energy and Alfa Aesar Chemical Companies and were used without further purification.
Typical Procedure for the Synthesis of Compounds 1–3, 4a–kA solution of BBr3 in dichloromethane (1.0 M, 12 mL, 12 mmol) was added slowly to a solution of 4-bromo-3-methoxyaniline (800 mg, 3.96 mmol) in methylene chloride (15 mL) at 0°C. The resulting brown solution was warmed to room temperature and stirred for 24 h. After saturated aqueous NaHCO3 (30 mL) was added at 0°C, the solution was extracted with EtOAc (20 mL × 3). The combined organic layer was dried with anhydrous Na2SO4, filtered and concentrated in vacuum. The residue was purified by flash chromatography over silica gel (petroleum–EtOAc = 2 : 1) to give 5-amino-2-bromophenol (665 mg, 88%).
To a solution of 5-amino-2-bromophenol (55 mg, 0.29 mmol) and triethylamine (53 µL, 0.38 mmol) in tetrahydrofuran (THF) (3 mL) was added slowly benzoyl chloride (0.32 mmol) at 0°C. The reaction mixture was then stirred at room temperature for 30 min. After the reaction was quenched with water (10 mL), the solution was extracted with EtOAc (10 mL × 2). The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated in vacuum. The residue was purified by column chromatography to afford the product 3 (74 mg, 87%).
N-(4-Bromo-3-hydroxyphenyl)benzamide (3)White solid, mp 178.7–180.2°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.29 (s, 1H), 10.25 (s, 1H), 7.98–7.87 (m, 2H), 7.66 (d, J = 2.4 Hz, 1H), 7.61–7.56 (m, 1H), 7.52 (t, J = 7.3 Hz, 2H), 7.41 (d, J = 8.7 Hz, 1H), 7.12 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, dimethyl sulfoxide (DMSO)-d6) δ: 166.15, 154.42, 140.04, 135.33, 132.87, 132.11, 128.86, 128.15, 113.11, 108.68, 103.73; electrospray ionization (ESI)-MS m/z: 291.9944 (Calcd for C13H10NO2Br [M + H]+: 291.9968).
N-(3-Bromo-5-hydroxyphenyl)benzamide (1)The compound was synthesized via a similar procedure starting from 3-bromo-5-methoxyaniline. White solid, yield 80%. mp 184.3–186.2°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.25 (s, 1H), 9.96 (s, 1H), 7.94–7.89 (m, 2H), 7.62–7.57 (m, 1H), 7.55–7.50 (m, 2H), 7.49 (t, J = 1.8 Hz, 1H), 7.33 (t, J = 2.0 Hz, 1H), 6.68 (t, J = 2.0 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 166.24, 159.08, 141.93, 135.15, 132.23, 128.90, 128.16, 121.91, 113.96, 106.67; ESI-MS m/z: 291.9952 (Calcd for C13H10NO2Br [M + H]+: 291.9968).
N-(2-Bromo-5-hydroxyphenyl)benzamide (2)The compound was synthesized via a similar procedure starting from 2-bromo-5-methoxyaniline. White solid, yield 52%. mp 195.5–197.2°C; 1H-NMR (400 MHz, DMSO-d6) δ: 9.86 (d, J = 27.5 Hz, 2H), 7.97 (d, J = 6.9 Hz, 2H), 7.62–7.58 (m, 1H), 7.53 (dd, J = 10.8, 3.8 Hz, 2H), 7.45 (dd, J = 8.7, 2.9 Hz, 1H), 7.07 (s, 1H), 6.72–6.57 (m, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.32, 157.20, 136.99, 134.16, 132.91, 131.97, 128.65, 127.70, 115.26, 115.22, 108.45; ESI-MS m/z: 291.9922 (Calcd for C13H10NO2Br [M + H]+: 291.9968).
N-(4-Bromophenyl)benzamide (4a)The compound was synthesized via a similar procedure starting from 4-bromoaniline. White solid, yield 85%. mp 209.9–212.0°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.38 (s, 1H), 7.95 (d, J = 7.2 Hz, 2H), 7.77 (d, J = 8.8 Hz, 2H), 7.66–7.44 (m, 5H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.69, 138.60, 134.72, 131.75, 131.46, 128.45, 127.70, 122.21, 115.34; ESI-MS m/z: 297.9822 (Calcd for C13H10NOBr [M + Na]+: 297.9838).
N-(4-Bromo-3-methoxyphenyl)benzamide (4b)The compound was synthesized via a similar procedure starting from 4-bromo-3-methoxyaniline. White solid, yield 89%. mp 134.6–136.5°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.35 (s, 1H), 7.96 (d, J = 7.3 Hz, 2H), 7.67 (d, J = 10.1 Hz, 1H), 7.63–7.48 (m, 4H), 7.40 (dd, J = 8.6, 2.1 Hz, 1H), 3.85 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 166.13, 155.75, 140.57, 135.18, 133.03, 132.20, 128.89, 128.11, 113.98, 105.26, 104.75, 56.45; ESI-MS m/z: 306.0110 (Calcd for C14H12NO2Br [M + H]+: 306.0124).
N-(4-Chloro-3-hydroxyphenyl)benzamide (4c)The compound was synthesized via a similar procedure starting from 5-amino-2-chlorophenol. White solid, yield 83%. mp 183.6–185.4°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.24 (d, J = 8.7 Hz, 2H), 7.92 (d, J = 7.2 Hz, 2H), 7.66 (d, J = 2.2 Hz, 1H), 7.56 (dt, J = 26.6, 7.2 Hz, 3H), 7.27 (d, J = 8.7 Hz, 1H), 7.17 (dd, J = 8.7, 2.2 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 166.09, 153.37, 139.39, 135.36, 132.07, 129.92, 128.84, 128.15, 114.59, 112.53, 108.88; ESI-MS m/z: 248.0462 (Calcd for C13H10NO2Cl [M + H]+: 248.0473).
N-(4-Fluoro-3-hydroxyphenyl)benzamide (4d)The compound was synthesized via a similar procedure starting from 5-amino-2-fluorophenol. Pale yellow solid, yield 83%. mp 180.1–182.3°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.18 (s, 1H), 9.93 (s, 1H), 7.99–7.90 (m, 2H), 7.63–7.56 (m, 2H), 7.53 (t, J = 7.3 Hz, 2H), 7.13 (m, 2H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.48, 147.45 (d, JCF = 237.4 Hz), 144.57 (d, JCF = 13.1 Hz), 135.67(d, JCF = 2.0 Hz), 135.03, 131.57, 128.42, 127.68, 115.70 (d, JCF = 20.2 Hz), 111.19 (d, JCF = 6.0 Hz), 110.09; ESI-MS m/z: 232.0748 (Calcd for C13H10NO2F [M + H]+: 232.0768).
N-(3-Hydroxy-4-methylphenyl)benzamide (4f)The compound was synthesized via a similar procedure starting from 5-amino-2-methylphenol. White solid, yield 81%. mp 213.4–216.0°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.07 (s, 1H), 9.36 (s, 1H), 7.96–7.89 (m, 2H), 7.60–7.48 (m, 3H), 7.43 (d, J = 1.8 Hz, 1H), 7.05 (dd, J = 8.1, 1.9 Hz, 1H), 6.99 (d, J = 8.2 Hz, 1H), 2.09 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.32, 155.22, 137.78, 135.26, 131.39, 130.18, 128.35, 127.66, 119.24, 111.08, 107.17, 15.64; ESI-MS m/z: 228.1086 (Calcd for C14H13NO2 [M + H]+: 228.1019).
N-(3-Hydroxy-4-methoxyphenyl)benzamide (4g)The compound was synthesized via a similar procedure starting from 5-amino-2-methoxyphenol. White solid, yield 80%. mp 176.5–178.5°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.02 (s, 1H), 9.07 (s, 1H), 7.99–7.87 (m, 1H), 7.60–7.47 (m, 1H), 7.35 (d, J = 2.4 Hz, 1H), 7.14 (dd, J = 8.7, 2.4 Hz, 1H), 6.88 (d, J = 8.7 Hz, 1H), 3.74 (s, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.09, 146.28, 144.14, 135.23, 132.73, 131.35, 128.36, 127.59, 112.26, 111.23, 108.92, 55.88; ESI-MS m/z: 244.0927 (Calcd for C14H13NO3 [M + H]+: 244.0968).
Methyl 4-Benzamido-2-hydroxybenzoate (4h)The compound was synthesized via a similar procedure starting from methyl 4-amino-2-hydroxybenzoate. White solid, yield 77%. mp 170.0–171.8°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H), 10.51 (s, 1H), 7.94 (dd, J = 7.4, 5.9 Hz, 2H), 7.77 (d, J = 8.8 Hz, 1H), 7.63–7.57 (m, 2H), 7.57–7.51 (m, 2H), 7.37 (dd, J = 8.8, 2.0 Hz, 1H), 3.88 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 169.09, 166.23, 161.11, 145.76, 134.57, 131.99, 130.63, 128.49, 127.87, 111.41, 107.77, 107.14, 52.29; ESI-MS m/z: 294.0727 (Calcd for C15H13NO4 [M + Na]+: 294.0737).
Synthesis of 4-Benzamido-2-hydroxybenzoic Acid (4i)To a solution of compound 4h (90 mg, 0.33 mmol) in THF (2 mL) was added aqueous NaOH (1.5 M, 8 mL). The mixture was stirred at 50°C for 2 h. After being cooled to room temperature, the solution was acidified with 2 M HCl to pH = 3–4. The precipitation was collected to give the compound 4h (62 mg, 73%). mp 263.1–264.7°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.48 (s, 1H), 8.05–7.89 (m, 2H), 7.76 (d, J = 8.7 Hz, 1H), 7.67–7.44 (m, 4H), 7.34 (dd, J = 8.7, 2.0 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 172.11, 166.64, 162.44, 146.08, 135.06, 132.40, 131.32, 128.93, 128.30, 111.59, 108.51, 107.38; ESI-MS m/z: 280.0563 (Calcd for C14H11NO4 [M + Na]+: 280.0580).
N-(4-Acetyl-3-hydroxyphenyl)benzamide (4j)The compound was synthesized via a similar procedure starting from 1-(4-amino-2-hydroxyphenyl)ethan-1-one. Pale yellow solid, yield 82%. mp 204.9–206.6°C; 1H-NMR (400 MHz, DMSO-d6) δ: 12.34 (s, 1H), 10.54 (s, 1H), 7.95 (d, J = 8.0 Hz, 2H), 7.90 (d, J = 8.8 Hz, 1H), 7.65–7.59 (m, 1H), 7.59–7.50 (m, 3H), 7.37 (d, J = 8.8 Hz, 1H), 2.60 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 203.27, 166.31, 162.34, 146.29, 134.50, 132.42, 132.07, 128.52, 127.90, 115.83, 111.06, 106.96, 26.96; ESI-MS m/z: 278.0792 (Calcd for C15H13NO3 [M + Na]+: 278.0788).
N-(4-Cyano-3-hydroxyphenyl)benzamide (4k)The compound was synthesized via a similar procedure starting from 4-amino-2-methoxy benzonitrile. Yellow brown solid, yield 67%. mp 151.3–153.6°C; 1H-NMR (400 MHz, DMSO-d6) δ: 8.19–8.10 (m, 2H), 7.78 (t, J = 7.4 Hz, 1H), 7.63 (t, J = 7.7 Hz, 2H), 7.48 (d, J = 9.1 Hz, 1H), 6.60–6.54 (m, 2H), 6.45 (s, 2H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.22, 155.14, 154.15, 134.99, 134.50, 130.38, 129.65, 128.57, 117.47, 111.90, 107.27, 90.59; ESI-MS m/z: 261.0582 (Calcd for C14H10N2O2 [M + Na]+: 261.0634).
Synthesis of Compounds 5a–cTo a solution of benzoic acid (37 mg, 0.30 mmol) and HATU (138 mg, 0.36 mmol) in N,N-dimethylformamide (DMF) (4 mL) was added DIPEA (63 µL, 0.36 mmol) and 4-amino-2-bromophenol (56 mg, 0.30 mmol) separately. The reaction mixture was stirred at room temperature for 4 h. After water (20 mL) was added, the mixture was extracted with EtOAc (10 mL × 2). The combined organic layer was dried over anhydrous Na2SO4 and concentrated under vacuum. The residue was purified by flash chromatography over silica gel (petroleum–EtOAc = 10 : 1 to 5 : 1) to give the compound 5a.
N-(3-Bromo-4-hydroxyphenyl)benzamide (5a)White solid, yield 90%. mp 198.8–200.2°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.16 (s, 1H), 10.11 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.92 (dd, J = 5.2, 3.3 Hz, 2H), 7.60–7.47 (m, 4H), 6.93 (d, J = 8.8 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.24, 150.44, 134.83, 131.86, 131.61, 128.48, 127.61, 124.95, 121.27, 116.03, 108.58; ESI-MS m/z: 291.9941 (Calcd for C13H10NO2Br [M + H]+: 291.9968).
N-(3-Bromo-2-hydroxyphenyl)benzamide (5b)The compound was synthesized via a similar procedure starting from 2-amino-6-bromophenol. White solid, yield 71%. mp 127.4–128.6°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.08 (s, 1H), 9.76 (s, 1H), 8.01 (d, J = 7.3 Hz, 2H), 7.62 (t, J = 7.3 Hz, 1H), 7.55 (t, J = 7.5 Hz, 2H), 7.44 (t, J = 6.7 Hz, 2H), 6.84 (t, J = 8.0 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 166.81, 148.04, 134.23, 132.43, 130.44, 128.91, 128.39, 127.91, 125.78, 121.05, 112.13; ESI-MS m/z: 291.9932 (Calcd for C13H10NO2Br [M + H]+: 291.9968).
4-Bromo-3-hydroxy-N-phenylbenzamide (5c)The compound was synthesized via a similar procedure starting from 4-bromo-3-hydroxybenzoic acid and aniline. White solid, yield 77%. mp 188.5–190.7°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.64 (s, 1H), 10.25 (s, 1H), 7.74 (d, J = 7.6 Hz, 2H), 7.64 (d, J = 8.2 Hz, 1H), 7.47 (d, J = 2.0 Hz, 1H), 7.38–7.30 (m, 3H), 7.10 (t, J = 7.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.85, 154.15, 139.05, 135.66, 132.81, 128.67, 123.79, 120.39, 119.36, 115.64, 113.05; ESI-MS m/z: 291.9819 (Calcd for C13H10NO2Br [M + H]+: 291.9968).
Synthesis of 5-(Benzylamino)-2-bromophenol (5d)To a solution of 4-bromo-3-methoxyaniline (80 mg, 0.40 mmol) and K2CO3 (110 mg, 0.80 mmol) in DMF (5 mL) was added (bromomethyl)benzene (75 mg, 0.44 mmol) at room temperature. The mixture was stirred at 55°C for 24 h. After water (20 mL) was added, the mixture was extracted with EtOAc (10 mL × 2). The combined organic layer was dried over anhydrous Na2SO4 and concentrated under vacuum. The crude product was purified by flash chromatography over silica gel (petroleum–EtOAc = 10 : 1 to 5 : 1) to give N-benzyl-4-bromo-3-methoxyaniline (84 mg, 72%).
The compound 5d was synthesized from N-benzyl-4-bromo-3-methoxyaniline via a procedure similar to “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k.” White solid, yield 38%. mp 77.2–78.3°C; 1H-NMR (400 MHz, DMSO-d6) δ: 9.65 (s, 1H), 7.38–7.27 (m, 4H), 7.25–7.17 (m, 1H), 7.04 (d, J = 8.6 Hz, 1H), 6.36 (t, J = 5.9 Hz, 1H), 6.19 (d, J = 2.6 Hz, 1H), 6.02 (dd, J = 8.7, 2.6 Hz, 1H), 4.19 (d, J = 5.9 Hz, 2H); 13C-NMR (101 MHz, DMSO-d6) δ: 154.70, 149.67, 140.37, 132.88, 128.76, 127.54, 127.13, 106.19, 100.62, 95.37, 46.91; ESI-MS m/z: 278.0149 (Calcd for C13H12NOBr [M + H]+: 278.0175).
Synthesis of Compounds 6a–u. N-(4-Bromo-3-hydroxyphenyl)acetamide (6a)The compound was synthesized via a procedure similar to “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using acetyl chloride. White solid, yield 54%. mp 215.7–216.5°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.22 (s, 1H), 9.93 (s, 1H), 7.47 (d, J = 2.4 Hz, 1H), 7.33 (d, J = 8.6 Hz, 1H), 6.94–6.79 (m, 1H), 2.01 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 168.41, 154.03, 139.74, 132.48, 111.25, 106.88, 102.39, 24.11; ESI-MS m/z: 229.9885 (Calcd for C8H8NO2Br [M + H]+: 229.9811).
N-(4-Bromo-3-hydroxyphenyl)acrylamide (6b)The compound was synthesized via a procedure similar to “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using acryloyl chloride. Brown solid, yield 63%. mp 183.6–186.0°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.30 (s, 1H), 10.16 (s, 1H), 7.54 (d, J = 2.3 Hz, 1H), 7.38 (d, J = 8.6 Hz, 1H), 7.00 (dd, J = 8.7, 2.3 Hz, 1H), 6.43 (dd, J = 17.0, 10.1 Hz, 1H), 6.25 (dd, J = 17.0, 2.0 Hz, 1H), 5.76 (dd, J = 10.1, 2.0 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 163.12, 154.06, 139.37, 132.56, 131.76, 127.01, 111.64, 107.25, 103.04; ESI-MS m/z: 241.9805 (Calcd for C9H8NO2Br [M + H]+: 241.9811).
N-(4-Bromo-3-hydroxyphenyl)nonanamide (6c)The compound was synthesized via a similar procedure “Synthesis of Compounds 5a–c” using octanoic acid. White solid, yield 76%. mp 181.8–183.3°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.18 (s, 1H), 9.84 (s, 1H), 7.47 (d, J = 2.3 Hz, 1H), 7.32 (d, J = 8.6 Hz, 1H), 6.89 (dd, J = 8.7, 2.4 Hz, 1H), 2.26 (t, J = 7.4 Hz, 2H), 1.61–1.49 (m, 2H), 1.26 (dd, J = 5.7, 2.5 Hz, 8H), 0.85 (d, J = 7.0 Hz, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 171.36, 154.00, 139.74, 132.43, 111.33, 106.97, 102.31, 36.47, 31.19, 28.65, 28.48, 25.10, 22.09, 13.97; ESI-MS m/z: 314.0734 (Calcd for C14H20NO2Br [M + H]+: 314.0750).
N-(4-Bromo-3-hydroxyphenyl)cyclohexanecarboxamide (6d)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using cyclohexanecarbonyl chloride. White solid, yield 80%. mp 194.9–197.2°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.19 (s, 1H), 9.79 (s, 1H), 7.46 (d, J = 2.3 Hz, 1H), 7.31 (d, J = 8.7 Hz, 1H), 6.90 (dd, J = 8.7, 2.4 Hz, 1H), 2.33–2.24 (m, 1H), 1.82–1.69 (m, 4H), 1.43–1.32 (m, 2H), 1.31–1.09 (m, 4H); 13C-NMR (101 MHz, DMSO-d6) δ: 174.44, 153.98, 139.90, 132.42, 111.49, 107.12, 102.33, 44.91, 29.13, 25.42, 25.25; ESI-MS m/z: 298.0391 (Calcd for C13H16NO2Br [M + H]+: 298.0437).
N-(4-Bromo-3-hydroxyphenyl)morpholine-4-carboxamide (6e)To a solution of 5-amino-2-bromophenol (55 mg, 0.29 mmol) and triethylamine (53 µL, 0.38 mmol) in THF (3 mL) was added slowly morpholine-4-carbonyl chloride (0.32 mmol) at 0°C. The mixture was stirred at 45°C for 12 h. The reaction was quenched with water (10 mL) and the mixture was extracted with EtOAc (10 mL × 2). The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography to afford the product 6e as a white solid (60 mg, 69%). mp 193.0–194.8°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.08 (s, 1H), 8.54 (s, 1H), 7.31 (d, J = 2.4 Hz, 1H), 7.27 (d, J = 8.7 Hz, 1H), 6.82 (dd, J = 8.7, 2.4 Hz, 1H), 3.62–3.56 (m, 4H), 3.42–3.38 (m, 4H); 13C-NMR (101 MHz, DMSO-d6) δ: 155.36, 154.22, 141.39, 132.48, 112.31, 107.88, 101.65, 66.44, 44.62; ESI-MS m/z: 301.0182 (Calcd for C11H13N2O3Br [M + H]+: 301.0143).
N-(4-Bromo-3-hydroxyphenyl)picolinamide (6f)The compound was synthesized via a similar procedure “Synthesis of Compounds 5a–c” using picolinic acid. White solid, yield 80%. mp 202.5–204.4°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.61 (s, 1H), 10.33 (s, 1H), 8.79–8.69 (m, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.10–8.02 (m, 1H), 7.80 (d, J = 2.4 Hz, 1H), 7.71–7.63 (m, 1H), 7.42 (d, J = 8.7 Hz, 1H), 7.21 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 162.57, 154.03, 149.84, 148.45, 138.74, 138.16, 132.48, 126.99, 122.46, 112.79, 108.18, 103.67; ESI-MS m/z: 292.9910 (Calcd for C12H9N2O2Br [M + H]+: 292.9920) .
N-(4-Bromo-3-hydroxyphenyl)thiophene-2-carboxamide (6g)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using thiophene-2-carbonyl chloride. White solid, yield 66%. mp 222.7–224.5°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.31 (s, 1H), 10.21 (s, 1H), 8.01 (d, J = 3.1 Hz, 1H), 7.84 (d, J = 4.8 Hz, 1H), 7.57 (d, J = 2.2 Hz, 1H), 7.41 (d, J = 8.7 Hz, 1H), 7.24–7.16 (m, 1H), 7.09 (dd, J = 8.7, 2.2 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 159.88, 154.01, 139.91, 139.16, 132.50, 132.04, 129.29, 128.09, 112.60, 108.20, 103.40; ESI-MS m/z: 297.9494 (Calcd for C11H8NO2SBr [M + H]+: 297.9532).
N-(4-Bromo-3-hydroxyphenyl)furan-2-carboxamide (6h)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using furan-2-carbonyl chloride. White solid, yield 53%. mp 207.2–209.7°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.32 (s, 1H), 10.19 (s, 1H), 7.93 (dd, J = 1.6, 0.7 Hz, 1H), 7.61 (d, J = 2.4 Hz, 1H), 7.43–7.31 (m, 2H), 7.09 (dd, J = 8.7, 2.4 Hz, 1H), 6.70 (dd, J = 3.5, 1.7 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 156.22, 154.00, 147.38, 145.90, 139.00, 132.49, 114.96, 112.66, 112.22, 108.21, 103.43; ESI-MS m/z: 281.9753 (Calcd for C11H8NO3Br [M + H]+: 281.9760).
N-(4-Bromo-3-hydroxyphenyl)-2-chlorobenzamide (6i)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 2-chlorobenzoyl chloride. White solid, yield 79%. mp 204.4–206.7°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.51 (s, 1H), 10.33 (s, 1H), 7.61 (d, J = 2.3 Hz, 1H), 7.56 (dd, J = 7.8, 2.5 Hz, 2H), 7.50 (m, J = 7.7, 1.7 Hz, 1H), 7.44 (m, J = 7.3, 1.1 Hz, 1H), 7.40 (d, J = 8.6 Hz, 1H), 7.01 (dd, J = 8.6, 2.3 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.02, 154.16, 139.30, 136.90, 132.66, 131.20, 129.91, 129.69, 128.95, 127.33, 111.93, 107.52, 103.43; ESI-MS m/z: 325.9586 (Calcd for C13H9NO2ClBr [M + H]+: 325.9578).
N-(4-Bromo-3-hydroxyphenyl)-3-chlorobenzamide (6j)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 3-chlorobenzoyl chloride. White solid, yield 74%. mp 184.2–186.1°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.35 (d, J = 3.6 Hz, 2H), 7.98 (t, J = 1.7 Hz, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.70–7.61 (m, 2H), 7.56 (t, J = 7.9 Hz, 1H), 7.42 (d, J = 8.7 Hz, 1H), 7.11 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.17, 154.03, 139.33, 136.86, 133.22, 132.50, 131.49, 130.44, 127.48, 126.58, 112.67, 108.24, 103.56; ESI-MS m/z: 325.9562 (Calcd for C13H9NO2ClBr [M + H]+: 325.9578).
N-(4-Bromo-3-hydroxyphenyl)-4-chlorobenzamide (6k)The compound was synthesized via the procedure similar to compound 6e. White solid, yield 52%. mp 195.0–197.3°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.32 (s, 1H), 10.19 (s, 1H), 7.93 (dd, J = 1.6, 0.7 Hz, 1H), 7.61 (d, J = 2.4 Hz, 1H), 7.43–7.31 (m, 2H), 7.09 (dd, J = 8.7, 2.4 Hz, 1H), 6.70 (dd, J = 3.5, 1.7 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 156.22, 154.00, 147.38, 145.90, 139.00, 132.49, 114.96, 112.66, 112.22, 108.21, 103.43; ESI-MS m/z: 325.9565 (Calcd for C13H9NO2ClBr [M + H]+: 325.9578).
N-(4-Bromo-3-hydroxyphenyl)-4-fluorobenzamide (6l)The compound was synthesized via the procedure similar to compound 3. White solid, yield 83%. mp 181.9–183.4°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.29 (d, J = 18.2 Hz, 2H), 8.11–7.95 (m, 2H), 7.64 (s, 1H), 7.38 (dt, J = 16.4, 8.7 Hz, 3H), 7.11 (d, J = 8.7 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.54, 164.12 (d, JCF = 249.5 Hz), 154.01, 139.52, 132.46, 131.32, 130.50 (d, JCF = 9.1 Hz), 115.36 (d, JCF = 22.2 Hz), 112.65, 108.22, 103.35; ESI-MS m/z: 309.9838 (Calcd for C13H9NO2FBr [M + H]+: 309.9873).
4-Bromo-N-(4-bromo-3-hydroxyphenyl)benzamide (6m)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-bromobenzoyl chloride. White solid, yield 81%. mp 208.5–210.1°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.31 (s, 2H), 7.93–7.81 (m, 2H), 7.80–7.68 (m, 2H), 7.63 (d, J = 2.4 Hz, 1H), 7.41 (d, J = 8.7 Hz, 1H), 7.10 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.66, 154.02, 139.41, 133.94, 132.48, 131.43, 129.88, 125.44, 112.65, 108.23, 103.47; ESI-MS m/z: 369.9088 (Calcd for C13H9NO2Br2 [M + H]+: 369.9073).
N-(4-Bromo-3-hydroxyphenyl)-4-methylbenzamide (6n)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-methylbenzoyl chloride. White solid, yield 62%. mp 195.4–197.2°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.30 (s, 1H), 10.17 (s, 1H), 7.85 (d, J = 8.2 Hz, 2H), 7.66 (d, J = 2.4 Hz, 1H), 7.40 (d, J = 8.7 Hz, 1H), 7.33 (d, J = 8.0 Hz, 2H), 7.11 (dd, J = 8.7, 2.4 Hz, 1H), 2.38 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.47, 153.98, 141.70, 139.71, 132.40, 132.01, 128.94, 127.78, 112.63, 108.20, 103.14, 21.07; ESI-MS m/z: 306.0111 (Calcd for C14H12NO2Br [M + H]+: 306.0124).
N-(4-Bromo-3-hydroxyphenyl)-4-methoxybenzamide (6o)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-methoxybenzoyl chloride. White solid, yield 81%. mp 207.6–208.9°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.26 (s, 1H), 10.09 (s, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 2.3 Hz, 1H), 7.39 (d, J = 8.7 Hz, 1H), 7.11 (dd, J = 8.7, 2.3 Hz, 1H), 7.06 (t, J = 5.8 Hz, 2H), 3.83 (s, 3H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.96, 161.95, 153.93, 139.80, 132.34, 129.67, 126.88, 113.60, 112.60, 108.16, 102.96, 55.46; ESI-MS m/z: 322.0041 (Calcd for C14H12NO3Br [M + H]+: 322.0073).
N-(4-Bromo-3-hydroxyphenyl)-4-(trifluoromethoxy)benzamide (6p)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-(trifluoromethoxy)benzoyl chloride. White solid, yield 80%. mp 189.1–190.1°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.35 (d, J = 10.6 Hz, 2H), 8.13–8.01 (m, 2H), 7.64 (d, J = 2.4 Hz, 1H), 7.52 (d, J = 8.0 Hz, 2H), 7.42 (d, J = 8.7 Hz, 1H), 7.11 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.49, 154.04, 139.41, 134.07, 132.51, 130.16, 120.75, 112.63, 108.21, 103.50, 13C-NMR (101 MHz, DMSO-d6) δ: 164.49, 154.04, 150.50, 139.41, 134.07, 132.51, 130.16, 120.75, 112.63, 108.21, 103.50; ESI-MS m/z: 375.9757 (Calcd for C14H9NO3F3Br [M + H]+: 375.9791).
N-(4-Bromo-3-hydroxyphenyl)-4-(dimethylamino)benzamide (6q)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-(dimethylamino)benzoyl chloride. White solid, yield 71%. mp 238.3–240.7°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.22 (s, 1H), 9.87 (s, 1H), 7.85 (d, J = 8.9 Hz, 2H), 7.67 (d, J = 2.3 Hz, 1H), 7.37 (d, J = 8.7 Hz, 1H), 7.11 (dd, J = 8.7, 2.3 Hz, 1H), 6.74 (d, J = 9.0 Hz, 2H), 2.99 (s, 6H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.28, 153.89, 152.44, 140.19, 132.25, 130.96, 129.24, 120.93, 112.55, 110.76, 108.10, 102.52; ESI-MS m/z: 355.0345 (Calcd for C15H15N2O2Br [M + H]+: 335.0390).
N-(4-Bromo-3-hydroxyphenyl)-4-(trifluoromethyl)benzamide (6r)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-(trifluoromethyl)benzoyl chloride. White solid, yield 73%. mp 205.1–206.6°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.47 (s, 1H), 10.35 (s, 1H), 8.12 (d, J = 8.1 Hz, 2H), 7.90 (d, J = 8.3 Hz, 2H), 7.65 (d, J = 2.4 Hz, 1H), 7.43 (d, J = 8.7 Hz, 1H), 7.13 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.95, 154.50, 139.70, 139.16, 132.96, 131.85 (d, JCF = 32.3 Hz), 129.10, 125.83 (d, JCF = 3.0 Hz), 113.12, 108.72, 104.13; ESI-MS m/z: 359.9803 (Calcd for C14H9NO2F3Br [M + H]+: 359.9842).
N-(4-Bromo-3-hydroxyphenyl)-4-nitrobenzamide (6s)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 4-nitrobenzoyl chloride. Yellow solid, yield 48%. mp 237.0–239.1°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.58 (s, 1H), 10.38 (s, 1H), 8.37 (d, J = 8.8 Hz, 2H), 8.16 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 2.2 Hz, 1H), 7.45 (d, J = 8.7 Hz, 1H), 7.14 (dd, J = 8.7, 2.3 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 164.45, 154.52, 149.62, 141.00, 139.58, 133.02, 129.74, 124.01, 113.11, 108.69, 104.28. ESI-MS m/z: 336.9884 (Calcd for C13H9N2O4Br [M + H]+: 336.9818).
N-(4-Bromo-3-hydroxyphenyl)-4-hydroxybenzamide (6t)The compound was synthesized by compound 6o as the material and then the procedure similar to “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k.” White solid, yield 32%. mp 215.2–216.7°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.19 (d, J = 47.7 Hz, 2H), 9.99 (s, 1H), 7.82 (d, J = 8.3 Hz, 2H), 7.64 (s, 1H), 7.38 (d, J = 8.7 Hz, 1H), 7.09 (d, J = 8.7 Hz, 1H), 6.85 (d, J = 8.3 Hz, 2H); 13C-NMR (101 MHz, DMSO-d6) δ: 165.62, 161.06, 154.36, 140.37, 132.76, 130.25, 125.77, 115.36, 113.00, 108.56, 103.25; ESI-MS m/z: 307.9882 (Calcd for C13H10NO3Br [M + H]+: 307.9917).
Synthesis of N-(4-Bromo-3-hydroxyphenyl)-2-naphthamide (6u)The compound was synthesized via a similar procedure “Typical Procedure for the Synthesis of Compounds 1–3, 4a–k” using 2-naphthoyl chloride. White solid, yield 73%. mp 207.6–209.9°C; 1H-NMR (400 MHz, DMSO-d6) δ: 10.43 (s, 1H), 10.32 (s, 1H), 8.56 (s, 1H), 8.11–7.97 (m, 4H), 7.72 (d, J = 2.4 Hz, 1H), 7.68–7.58 (m, 2H), 7.43 (d, J = 8.7 Hz, 1H), 7.17 (dd, J = 8.7, 2.4 Hz, 1H); 13C-NMR (101 MHz, DMSO-d6) δ: 166.17, 154.48, 140.14, 134.75, 132.92, 132.67, 132.51, 129.44, 128.49, 128.34, 128.16, 127.35, 124.96, 113.11, 108.67, 103.76; ESI-MS m/z: 342.0097 (Calcd for C17H12NO2Br [M + H]+: 342.0124).
Biological AssaysEvaluation of Inhibitory Activity against Mtb H37Ra in VitroSelectable marker-free autoluminescent Mtb H37Ra (UAlRa)13) broth culture in Middlebook 7H9 medium plus 0.05% Tween80 and 10% oleic acid albumin dextrose catalase (OADC) (7H9-Tw-OADC). Upon reaching an OD600 of 0.5–0.8, relative light unit (RLU) value was determined by luminometer. When the RLU of 200 µL broth culture reached to over 1 million, the test compounds were prepare with two-fold serial dilutions in a range of final concentration from 100 to 0.0625 µg/mL per 200 µL UAlRa of broth culture (RLU were diluted to 2000–5000 per 200 µL). On the other hand, DMSO final concentration of 2% was used as negative control while the compounds (2-fold decreasing concentration from 100 to 0.0625 µg/mL) as well as isoniazid (0.1 µg/mL) and ethambutol (2 µg/mL) were used as positive controls. The RLU values were determined once daily until the 6th day. Data were analysed as MIC90 values which is basically the lowest drug concentration that achieved a RLUdrug/RLUDMSO of less than 10% after incubation.
Evaluation of Inhibitory Activity against Mtb H37Rv and MDR-TB StrainsThe MICs of the test compounds were determined by the microplate alamar blue assay (MABA)14) against Mtb H37Rv and clinically isolated MDR-TB strains isolated from the Guangzhou Chest Hospital. Mtb H37Rv and MDR-TB strains were cultured in 7H9-Tw-OADC. The test compounds were prepared with two-fold serial dilutions in a range of final concentration from 0.25 to 128 µg/mL, two-fold dilutions of compounds were prepared in 7H9-OADC medium in a 100 µL volume in 96-well microplates. INH, RIF and EMB were used as the positive controls. Bacterial suspension of inoculum of 2 × 105 CFU/mL was added 100 µL per well into the microplates, and the plates incubated 6 d at 37°C. On the 7th day, 12.5 µL of 20% Tween 80 and 20 µL of alamar Blue (Bio-Rad) were added to the test plate, and incubated another 24 h at 37°C, and then observed the color change. If a blue color in the well was deemed to no growth, and the blue color change to pink or violet was interpreted as growth. The MIC was defined as the lowest drug concentration which prevented a color change from blue to pink.
Determination of the Antibacterial ActivityTo evaluated compounds exclusive of against Mtb, we selected 6p and r for determination of antibacterial activity against Gram-positive and Gram-negative bacteria. Initially, the test compounds were dissolved in DMSO to prepare the stock solutions (10 mg/mL), and then prepared a range of concentrations from 50 to 0.4 µg/mL by means of the standard two fold serial dilution method in 96-well microtest plates. Amoxicillin was used as the positive controls. The bacterial suspension was adjusted with sterile saline to a concentration of 1 × 105 CFU. One hundred microliters the bacterial suspension per well was added into the plates and incubated at 37°C for 24 h. The MIC of compound was defined as the lowest concentration (the highest dilution) that completely inhibited the growth of bacteria after incubation at 37°C for 18–24 h.15)
Determination of the CytotoxicityA549 cell and HepG2 cell were used for determination of the cytotoxicity of compounds. This two cell lines both were culture in RPMI 1640 Medium (with 5% fetal bovine serum (FBS) and 1% penicillin–streptomycin), and incubated at 37°C in 5% CO2 until they reach log phage. The test compounds were diluted with RPMI 1640 Medium by 2-fold dilution and the concentrations from 100 to 3.125 µg/mL. The cells suspension (1 × 105 cell/mL) of 100 µL were seeded in 96-well plates and then incubated at 37°C in 5% CO2 overnight.16) One hundred microliters different concentrations of the test compound were added to the plate by the form of replace the cell medium and 100 µL RPMI 1640 Medium was used as the 0% inhibitor control. Each experiment was repeated three times. After being incubated for 48 h, 10 µL/well CCK8 was added in the plates, and another 1–2 h incubation, the plates were read in the Envision MultilabelReader at 450 nm. Cell survival rate (activity%) was calculated as follow: activity% = 100 × (ODcompound − ODblank)/(ODcontrol − ODblank). The cytotoxicities were reported as IC50 values, which were calculated by GraphPad Prism Software version 5.23.
Determination of the Liver Microsome StabilityThe rat liver microsome was purchased from Xenotech Company. The final incubation volume was set at 45 µL, contained reduced nicotinamide adenine dinucleotide phosphate (NADPH) (2 mM), microsomes (0.5 mg/mL) and the tested compound (1 µM). The incubation times were 0, 5, 15, 30, 45 and 60 min. Ice-cold acetonitrile was added to terminate the reaction and then centrifuged at 4°C for 15 min at 4000 rpm. The concentration of the tested compound in supernatant was determined by LC-MS.17)
We thank the National Natural Science Foundation of China (No. 21472248, 21772240), the Chinese Academy of Sciences Grants (154144KYSB20150045), the National Mega-project of China for Innovative Drugs (2018ZX09721001-003-003), and Science and Technology Innovation Leader of Guangdong Province (2016TX03R095) for the financial support of this study.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.