2019 Volume 67 Issue 4 Pages 308-315
2019 Volume 67 Issue 4 Pages 308-315
Lipid bilayer membranes are soft, fluid, and dynamic architecture where molecules are constantly moving and thermally fluctuating under physiological conditions. In this review, a strategy to quantify molecular dynamics in membranes is introduced by utilizing solution-state NMR spectroscopy as a versatile, noninvasive technique. The dynamics involves lateral diffusion and protrusion motion, in parallel and vertical direction to the membrane surface. Dynamical behavior of small-sized drugs, chemicals, and peptides is also reviewed in relation to the lipid movements in membranes, on the basis of recent multinuclear NMR in combination with the pulsed field gradient technique. Finally, in-cell NMR method is introduced as a promising technique to capture drug transport processes in real time, to shed light on mechanisms of deliveries to living cells without perturbation of the system.
Lipid bilayer membranes are characterized by fluid, soft, and dynamic architecture where molecules are constantly moving and thermally fluctuating under physiological conditions. Historically, Singer and Nicolson have defined membranes as two-dimensional liquid-like structures, as schematically pictured by “the fluid mosaic model” more than 40 years ago.1) Since then, the dynamic properties of molecules in membrane environments have been an issue of great attention and extensively studied. At present, biomembranes are generally considered as complex and inhomogeneous structures. The roles of specialized microdomains such as lipid rafts2) are important and protein/glycoprotein complexes are emphasized in describing dynamics and functions of membranes.3) The presence of membrane-associated cytoskeletal fences and extracellular matrices also lowers the lateral diffusion and motional range of membrane components.3) Although updated concepts show the mosaic nature of biomembranes in which some of the membrane proteins and lipids are limited in their rotational and lateral motilities,3) the molecular movements and fluctuations still play a crucial role in vital activities of biomembranes.
NMR spectroscopy is a versatile, noninvasive technique to study molecular movements in membranes; it enables us to quantify molecular dynamics such as relaxation, lateral diffusion, and protrusion (the vertical direction to the membrane surface) without perturbing the system. Because water is abundant under physiological conditions, the solution NMR method is especially powerful to describe molecular movements in ‘aqueous’ membrane environments. Here, we review how to quantify molecular dynamics in model and cell membranes by solution NMR technique, to gain insights into dynamic behavior of real biological membranes. Dynamic aspects of small-sized drugs, chemicals, and peptides are also reviewed in relation to the lipid movements in membranes, on the basis of our recent NMR studies.
NMR spectroscopy not only enables us to distinguish dynamics inside and outside of membranes, but also focuses on the atomic site of interest within the membrane. The dynamic parameters obtained from NMR measurements involve the linewidth, Δν1/2, the longitudinal relaxation time, T1, the traverse relaxation time, T2, and the self-diffusion coefficient, D.4) Linewidth gives information about segmental motions and exchange rates. The dynamic and static broadenings of NMR signals are due to a decrease in the motional narrowing effect and an increase in inhomogeneity, respectively. Two relaxation times, T1 and T2, provide us with relaxation rates as well as segmental motions. The values of Δν1/2, T1, and T2 reflect motions of molecules and molecular assemblies to obtain correlation times relevant to the dynamics.5) Self-diffusion coefficient, D is also an index of translational mobility of molecules and/or molecular assemblies in the membrane.
Lipid motions in fluid membranes are categorized by a variety of dynamical modes.6–8) As illustrated in Fig. 1, the motions include lateral diffusion (i) and protrusion (ii). The former is the motion in parallel direction to the membrane surface, and the latter is that along the bilayer normal. The rotational diffusion (iii), the collective movements of membrane patches called undulation (iv), and flipflop (v) of lipid molecules between the two monolayers of the bilayer are also involved.9) The respective modes are characterized by the wide range of correlation time scale from picoseconds to hours.7) Drug transport mechanisms are thought to be closely related to such dynamic behavior of membrane lipids, the detail of which will be described in Section 4.
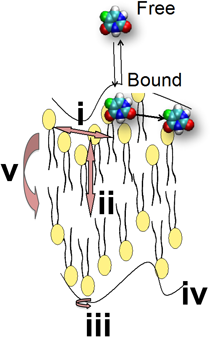
(Reprinted with permission from ref. 9. Copyright 2016 John Wiley and Sons.) (Color figure can be accessed in the online version.)
Lateral diffusion in the plane of the bilayer is a representative of molecular dynamics in membranes. To measure the molecular diffusion, the pulsed field gradient (PFG) NMR spectroscopy10) is used. It is informative to find out how the movements of membrane components are modulated without perturbing the system. The previous PFG NMR studies have observed signals of phospholipid/cholesterol molecules in bilayers that are oriented on a glass plate.11) The PFG signal of a hydrophobic drug has also been measured in multilamellar liposomes, together with phospholipids in the bilayer.12) Magic-angle spinning (MAS) technique has remarkably contributed to the attainment of well-resolved NMR spectra in viscous, anisotropic membranes.12)
Later, Okamura et al. have reported self-diffusion of lipids in micelles and vesicles as model fluid membranes.13) By controlling diameters of micelles and vesicles, they have demonstrated how the lipid diffusion is influenced by the surface curvature of the respective lipid aggregates. High-resolution solution NMR has been applied in combination with the PFG technique. The surface curvatures have been controlled by selecting average diameters of 3 nm (micelle), 30 nm (small unilamellar vesicle, SUV), and 100 and 400 nm (large unilamellar vesicle, LUV). For obtaining reliable NMR signal in viscous, anisotropic membranes, a high-field-gradient probe has been designed and used in combination with a solution NMR apparatus. The self-diffusion coefficient D is generally given by the slope of the Stejskal–Tanner plot,14) by using the relation between echo signal intensities and the field gradient strengths applied. The Stejskal–Tanner equation is written by
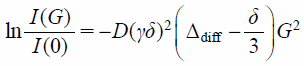 | (1) |
where I(G) and I(0) are the echo signal intensities in the presence and absence of the field gradient; γ, the gyromagnetic ratio; δ, the gradient pulse width; Δdiff, the diffusion time interval; and G, the field gradient strength, respectively. It is reasonable that lipid diffusion is more accelerated as the surface curvature becomes higher. Actually, the diffusion coefficient of phospholipid molecules is in the order of micelle > SUV > LUV,13) consistent with the surface curvature dependences of the dynamical structure in each lipid aggregate.15)
In view of surface curvature dependence of lipid dynamics in membranes, the hydrodynamic (HD) continuum model16) tells that the diffusion of lipid aggregate itself is limited in inverse proportion to the size of aggregates. However, the PFG NMR study by Okamura et al. has demonstrated that the slowdown of lipid motions in membranes is leveled off in 100- and 400-nm LUVs.13) Judging from almost no surface curvature effect of LUV in contrast to the highly-curved micellar surface,15) the result is thought to be reasonable. In fact, Yoshii et al. have demonstrated that the lateral diffusion coefficient of the lipid in 800-nm LUV is ca. 1 × 10−11 m2 s−1 in the fluid state,17) more than one order of magnitude faster than the rotational and the translational diffusion of LUV itself that is predicted by the HD continuum model. Notice that the rotational and the translational diffusion coefficients of 800-nm LUV are of the order of 10−13 m2 s−1. It is also found that even in a cell-sized giant vesicle (10–20 µm in diameter), the lateral diffusion coefficient of the lipid is as large as 0.8 × 10−11 m2 s−1 at relevant temperature.18,19) The value is not so much decreased as compared to LUV of 800-nm diameters, although the size of the giant vesicle is more than one order of magnitude as large as LUV. These findings show that lipid lateral diffusion is considerably enhanced in the giant vesicle, despite the rotational and translational diffusion as limited as the order of 10−14 m2 s−1 by the HD estimate.
3.2. Lateral Diffusion of Lipids Separated from Vesicle DiffusionThe diffusion in a lipid bilayer vesicle consists of three independent modes; (i) the lateral diffusion of lipid molecules on the vesicle surface, (ii) the rotational and (iii) the translational diffusion of the vesicle as a whole. In such situation, the problem is that the diffusion observed is the sum of these three modes and they are not separable in the experiment.
Recently, Yoshii et al. have proposed a strategy to separate lipid molecular diffusion from the rotational and the translational diffusion of a spherical large unilamellar vesicle from the PFG NMR measurement. The lateral diffusion coefficient of membrane lipids, DL can be obtained as a time-dependent part of the observed diffusion coefficient in 800-nm LUV, by systematically changing the diffusion time interval of the PFG NMR measurement.17)
For a lipid molecule diffusing within a vesicle17) (Fig. 2), it is thought that the lipid is moving laterally to the vesicle surface with the diffusion coefficient DL. In addition, the vesicle is also moving with the rotational and the translational diffusion coefficients DROT and DT. DL is considered to be larger than DROT and DT because DL reflects the motion of a single lipid molecule whereas DROT and DT reflect the movements of the large vesicle as a whole. If the time interval is sufficiently short, the influence of the rotational and the translational diffusion of the vesicle itself can be neglected. In such case, the observed diffusion coefficient is dominated by the lateral diffusion coefficient DL of lipids on the vesicle surface (Fig. 2a). In contrast, the diffusion coefficient is equal to the translational diffusion coefficient DT of the vesicle after the sufficiently long time interval17) (Fig. 2b). The diffusion coefficient Deff obtained by PFG NMR measurement is finally given by the sum of DL, DROT, and DT as
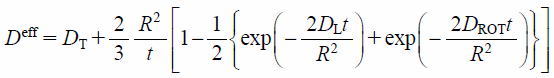 | (2) |
where R is the vesicle radius.17) Equation 2 indicates that, by using experimentally obtained Deff as a function of the time interval t, that is, the diffusion time Δdiff in the PFG NMR measurement, it is possible to estimate the precise value of lipid lateral diffusion coefficient, DL as the time-dependent part of Deff at a fixed vesicle radius, R.
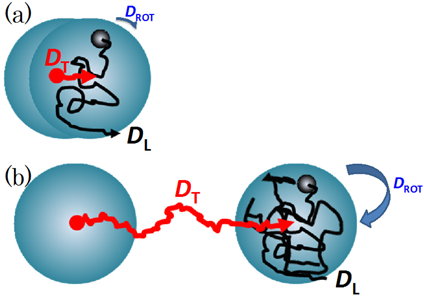
The lipid molecule is moving in the lateral direction on the vesicle surface (with the diffusion coefficient DL). The vesicle also changes the position in accordance with the translational (DT) and the rotational diffusion (DROT) after a short (a) and a long time interval (b). Reprinted from ref. 17, Copyright 2013, with permission from Elsevier. (Color figure can be accessed in the online version.)
Yoshii et al. have reported that the diffusion coefficient, DL of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine is as much as 1.5 × 10−11 m2 s−1 in the liquid-crystalline state at 45°C.17) This means that the lipid diffusion is more than one order of magnitude as fast as the rotational and the translational diffusion of the vesicle itself that is predicted by the HD continuum model,13)
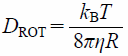 | (3) |
and
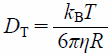 | (4) |
where kB, T, and η are the Boltzmann constant, the absolute temperature, and the viscosity coefficient for the solvent water, respectively. Such analysis is useful to quantify the lateral diffusion of lipids, peptides, and protein molecules in bilayer vesicles as model cell membranes.
3.3. Protrusion and UndulationProtrusion, the movement of lipids or lipid segments in the vertical direction to the membrane surface (Fig. 1), is expected to correlate with the mechanism of drug transport across cell membranes. The vertical fluctuation or the out-of-plane vibration of lipid molecules in bilayer membranes has been studied by two-dimensional (2D)-NMR20–23) and incoherent quasielastic neutron scattering.6) The fluctuations of membrane thickness have also been observed by neutron spin echo spectroscopy, as an excess in the dynamics of undulation fluctuations.24)
Recently, Takechi et al. have applied the solution NMR to the cell sized giant vesicle (ca. 10 µm in diameter), to elucidate how lipid motions and fluctuations are modified in real cell membranes.25) 2D nuclear Overhauser enhancement (NOE) spectra and one-dimensional (1D) transient NOE spectra of giant vesicles have been compared to the results of LUV from 100- to 800-nm diameters. The spectra of the giant vesicle are characterized by broad band width of the 1H-NMR peaks and large chemical shift anisotropy of the 31P-NMR signal, although almost no difference is found in the chemical shifts and longitudinal relaxation times (T1) of lipid segments in giant vesicle and LUV.25) The result means that the lipid tumbling motion in the giant vesicle is remarkably slowed down. The rotational correlation time (τc) is estimated as large as sub-second to second order. According to the 1H–1H NOE enhancement (η) of lipid signal, there is a possibility that hydrophilic headgroups are in contact with hydrophobic alkyl chains in the giant vesicle.25) Such proximity between lipid headgroup and the alkyl chains is the result of large protrusion motion along the bilayer normal, regardless of less curvature of the membrane (Fig. 3). It is also reported that protrusion is limited by the presence of some hydrophobic molecules (Fig. 4, top), although little effect is found by adding cations around the membrane surface.26)
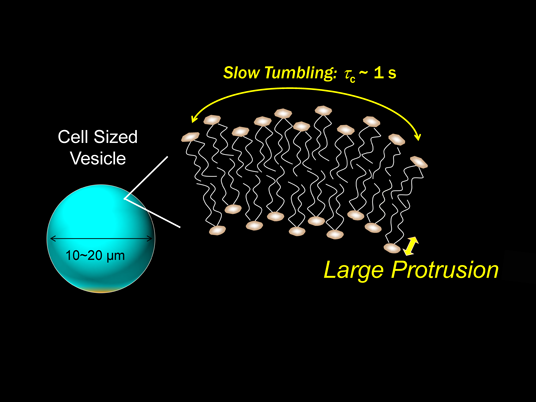
(Color figure can be accessed in the online version.)
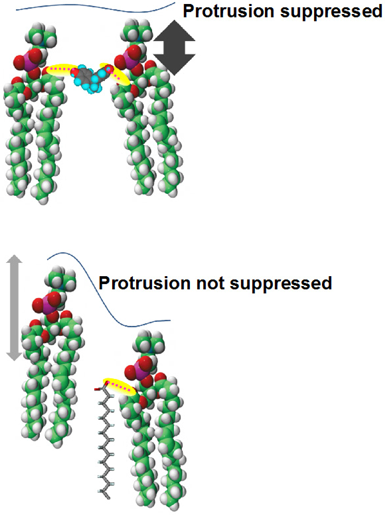
(Reprinted with permission from ref. 26. Copyright 2015 The Membrane Society of Japan.) (Color figure can be accessed in the online version.)
Drugs, especially small molecules, move to cell inside by passing through the plasma membrane. The movement is governed by the passive transport without the contribution of membrane transport proteins.27) In such case, drug transfer from water to lipid membranes is a key for the first step of bioactivities in the cell. Because lipid bilayer membranes are soft and dynamic architecture where the molecules are constantly moving and thermally fluctuating in a limited space, the mechanism of drug transport is associated with such dynamic properties of the membrane. Particularly, it is expected that drug permeation is closely related to the movement of drugs along the bilayer normal.
In this section, the dynamic aspects of drug transport mechanisms are reviewed. Lateral diffusion of drugs in the membrane, the kinetics of membrane binding and dissociation (or release), and the dynamic binding probability that quantifies the efficiency of drug transfer are involved. In every case, the solution NMR serves to quantify (i) how much, how fast, and how efficiently drugs are bound to the membrane, and (ii) how fast drugs move within the membrane.
4.1. Lateral Diffusion of Drugs in MembranesAs described in Section 3, PFG NMR spectroscopy is a powerful method to directly monitor molecular diffusion of drugs as well as lipids in membranes. In the early 2000’s, Okamura et al. have simultaneously observed lateral diffusion of lipids and trapped drugs in membranes by high-field-gradient, high-resolution solution NMR at 600 MHz.13) Diffusion of an endocrine disruptor, bisphenol A (BPA, 4,4′-isopropylidenediphenol) has been directly measured, and compared with the diffusion of membrane lipids in size-controlled micelles and vesicles described in Section 3.1. It is found that BPA diffusion is limited in LUVs as compared with the diffusion in SUVs and micelles with high surface curvatures.13) Interestingly, the slowdown of BPA diffusion is also leveled off in 100- and 400-nm LUVs, in analogy with the lateral diffusion of phospholipid molecules in membranes. The situation is in contrast to the HD continuum model that gives the diffusion limited in inverse proportion to the lipid particle size.9,13) The result is quite reasonable because the surface curvature effect of LUV is almost negligible at the molecular level.
It is also interesting that BPA diffusion is controlled by lipid motion in the membrane. Okamura et al. have demonstrated that BPA mobility is related to the membrane lipid dynamics; the movement of BPA is synchronized or concerted with the lipid matrix motion.13) Considering that BPA is preferably bound to the membrane interface,28) the slowdown of BPA motion is due to the site-specific, strong binding to the membrane lipid. This is in contrast to the movements of benzene and toluene in membranes13); the movements show almost no dependences on the motion of membrane lipid matrices.
Later, Yoshii and Okamura have also analyzed lateral diffusion of a fluorinated BPA derivative, FBPA [(CF3)2C(C6H4OH)2] by 19F-NMR in combination with the PFG technique.29) They have reported that the mobility of FBPA in 100-nm LUV is two orders of magnitude as small as that in water, and corresponds to the movement of phospholipids in the membrane. The result is almost analogous to the case of non-fluorinated BPA.13) Okamura and Yoshii have also found that the mobility of an anticancer drug, 5-fluorouracil (5FU, 5-fluoro-1H-pyrimidine-2,4-dione) in phospholipid vesicle is quite analogous to the lipid movement, although 5FU size is considerably small as compared to the lipid molecule.30) This finding is due to the simultaneous detection of membrane-bound and unbound (free) components, together with the membrane lipid molecules. The application of multinuclear 19F- and 1H-NMR enables us to distinguish the respective components of 5FU and lipids simultaneously in situ. The PFG technique allows to determine the self-diffusion coefficients of membrane-bound and free drugs independently from the biexponential decay of the respective signal vs. the field gradient strength, G, in accordance with the mobility difference between the bound and the free components. Okamura and Yoshii have reported that the mobility of membrane-bound 5FU is limited by almost two orders of magnitude as compared with free 5FU30) (Fig. 5). Surprisingly, 5FU mobility in the membrane is almost analogous to the movement of membrane lipid. These results lead to the conclusion that molecular movements in membranes are in accordance with the intra-membrane fluidity, as described in the case of BPA. From the temperature dependences, the activation energy of 5FU diffusion motion is found to be 26 kJ/mol,30) very close to that of lipid diffusion motion, 27 kJ/mol.11,30)
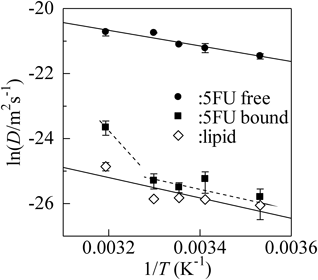
Reproduced from ref. 30, with the permission of AIP Publishing.
Although molecular motion is governed by intra-membrane fluidity, such situation is, however, not valid at high temperature. As shown in Fig. 5, the 5FU mobility in membranes tends to be enhanced. The enhancement is interpreted as the acceleration of 5FU movement within the lipid membrane, or rapid exchange of membrane-bound and free components of 5FU as a result of loose binding to lipid matrices at high temperature.30)
With regard to the intra-membrane fluidity, the previous solid-state NMR has demonstrated that the mobility of cholesterol and 1,2-dimyristoyl-sn-glycero-3-phosphocholine in the membrane is comparable with each other.31) The translational motion of cholesterol seems to be influenced by the intra-membrane fluidity. On the other hand, the solid-state MAS NMR has demonstrated that the mobility of indole and an anti-inflammatory drug, ibuprofen is enhanced as compared to the lipid matrices in the membrane,12,32) in analogy with the diffusivity of benzene and toluene in the membrane.13) An anesthetic, halothane also moves faster than the lipid matrices in membranes, as estimated by the molecular dynamics simulation.33)
4.2. Kinetics of Membrane Binding and Dissociation4.2.1. Binding of Small DrugIt is predicted that the drug translational motion along the bilayer normal is associated with the drug entry into the cell across the lipid membrane (Fig. 1). Yoshii and Okamura have demonstrated the drug motion in the normal direction to the lipid membrane, from the viewpoint of exchange kinetics of free and membrane-bound drug molecules between water and lipid membranes. Using high resolution 19F- and 1H-NMR in combination with the PFG technique, they have quantified the rate constants of membrane binding kFB and dissociation (or release) kBF of drugs.29,34,35)
Let us consider that drug exchange motion between water and lipid membrane is under equilibrium as
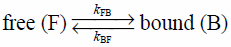 | (5) |
In such case, the analytical formulas of NMR signal intensities are given by Bloch equation in combination with diffusion and exchange terms as36)
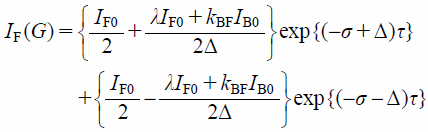 | (6) |
and
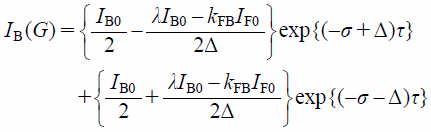 | (7) |
where IF(G) and IB(G) are the PFG NMR signal intensities of free (F) and membrane-bound (B) components. λ, σ, and Δ are
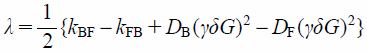 | (8) |
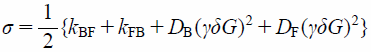 | (9) |
and
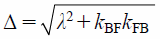 | (10) |
where DF and DB are the diffusion coefficients of free and membrane-bound 5FU. The exchange rate constants, kFB and kBF, can be finally quantified by fitting Eqs. 6 and 7 to the PFG signal intensities, IF(G) and IB(G).34)
Yoshii and Okamura have reported, at 303 K where the membrane lipids are in the fluid liquid-crystalline state, that the rate constants of 5FU binding kFB and dissociation (release) kBF are 0.2 ± 0.1 and 4.1 ± 1.5 s−1, respectively.34) This means that the half-value periods of 5FU binding and dissociation (release) are 3.5 and 0.17 s. The time span of the membrane binding and dissociation of 5FU is from second to sub-second timescale. The activation energy of the exchange motion is ca. 57 kJ/mol,34) the energy which is more than twice as high as lateral diffusion in membranes (26 kJ/mol) described in Section 4.1. It is found that 5FU motion along the bilayer normal is found to be limited as compared to the lateral diffusion in membranes.
4.2.2. Binding of PeptideThe dynamic behavior of peptide binding to membranes is of significance as a primary stage of biologically-relevant functions. Recently, Yoshii et al. have quantified the kinetics of binding of a neuropeptide, leucine-enkephalin (L-Enk, Tyr-Gly-Gly-Phe-Leu) to lipid bilayer membranes by PFG 1H-NMR in situ.35) The attenuation of the peptide PFG signal has been analyzed by using the analytical formula of the Bloch equation with diffusion and exchange terms in the presence of LUV as model cell membranes. Although free and membrane-bound components are overlapped in the 1D 1H-NMR spectrum, the PFG method has unveiled the membrane-bound L-Enk after the preferential decay of the free component of L-Enk at high field gradient.35) It has been reported that the rate constants of the peptide binding and dissociation are 0.040 and 0.40 s−1, respectively, at 303 K.35) From these, the lifetime of the peptide binding and dissociation is estimated as the value from second to ten-second timescale. It is noted that both of the rate constants, kFB and kBF, of L-Enk are reduced by cholesterol in membranes (unpublished data). Because cholesterol tends to rigidify the membrane, the loss of membrane fluidity by cholesterol suppresses the drug mobility and takes much time for binding and dissociation.
4.2.3. Binding of Hydrophobic DrugIn case of 5FU and L-Enk, the membrane-bound and the free states are distinguishable because of relatively slow exchange of rather hydrophilic drugs between bound and free states.34,35) However, the membrane binding of small hydrophobic drugs is generally so fast that we cannot distinguish the membrane-bound and free components easily in the NMR spectrum. In such case, the drug signal is observed as a singlet even in the presence of LUV. Typical example is the case of hydrophobic FBPA molecule. It has been found that the 19F-NMR signal of FBPA is observed as a singlet even in the presence of LUV.29) Such result is generally interpreted by the fast exchange motion between membrane-bound and free components of FBPA that is too rapid to distinguish on the NMR time scale. In a rapid exchange between membrane-bound and free states, the spectra consist of a single set of peaks whose chemical shifts are averaged between these the two.4) Yoshii and Okamura have found that the exchange rate constants can be nonetheless quantified by analyzing the averaged 1D NMR spectra.29) They have relied on the 1D-NMR signal intensity that is expressed by a set of Bloch equations with exchange terms as,
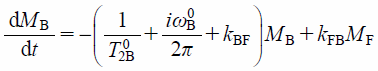 | (11) |
and
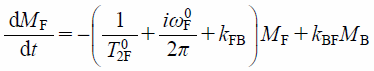 | (12) |
where MB and MF are the signal intensities of membrane-bound (B) and free (F) components, and T02i and ωi0 (i = B or F) are the transverse relaxation time and the resonance frequency of the pure B or F state of FBPA.29) In such case, the observed 1D NMR signal intensity is, finally, given as the sum of the analytical solution of B and F components expressed as a function of frequency ω under the equilibrium condition kBFfB=kFBfF as,
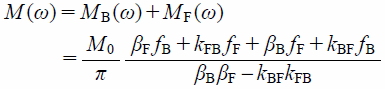 | (13) |
where
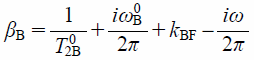 | (14) |
and
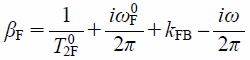 | (15) |
and fj is the fraction of the j (=B or F) state.29) As the transverse relaxation times T02B and T02F and the frequencies ω0B and ω0F are evaluated from the 1D 19F-NMR spectra in which LUVs are present and absent, the parameters kBF, kFB, fB, and fF can be determined by fitting Eq. 13 to the observed NMR signal intensity and reduced by using the relations fB+fF=1 and kBFfB=kFBfF.
Yoshii and Okamura have reported that the rate constant of FBPA binding kFB is enhanced from 20 to almost 300 s−1 with increasing the lipid concentration in LUV.29) The rate constant of membrane binding, kFB is much larger than that of hydrophilic 5FU (0.2 s−1).34) On the other hand, the dissociation rate constant, kBF of FBPA is in the range from 68 to 107 s−1.29) The half-value periods of the FPBA binding and dissociation (release), tFB and tBF, are calculated to be 3–32 and 5–10 ms, respectively.29) For hydrophobic FBPA, the time span of the membrane binding and dissociation (release) is a 10−3 to 10−2 s timescale, that is 2–3 orders of magnitude faster than the hydrophilic 5FU binding with sub-second to second timescale.
4.3. Dynamic Binding ProbabilityThe efficiency of drug transfer to membranes is one of the key factors to improve drug delivery processes. To quantify the efficiency, Yoshii and Okamura have introduced a dynamic binding probability, P, that is defined by a ratio, tR/tB29,37) (Fig. 6). Here, tR is defined as the mean time interval for a drug to reach at first to the membrane surface, and tB is the interval for the drug to bind to the membrane. The time interval, tR is obtained by the analytical formula of the reaction time of diffusion-controlled reaction,38) and tB is calculated from the observed kFB as tB = kFB−1. It has been reported that the dynamic binding probability P (=kFBtR) of FBPA is ca. 0.030.29) This means that the ratio, (the number of FBPA to reach to the membrane)/(the number of FBPA to bind to the membrane), is about 40. The binding probability of hydrophilic 5FU is, in contrast, much less than FBPA.29) The difference in hydrophobicity between FBPA and 5FU modulates the efficiency of drug transfer to the membrane.
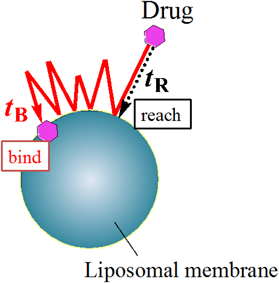
(Reprinted with permission from ref. 29. Copyright 2011 American Chemical Society.) (Color figure can be accessed in the online version.)
The final goal of in situ NMR method is an application to the living system. The mechanism of drug permeation across cell membranes is a key not only for a better understanding of the primary stage of bioactivities, but also for the purpose of rational drug design. So far, a number of studies have demonstrated that endocytosis is a typical mechanism for drug entry into the cell.39) However, it is still unknown, in some case, how the passive transport of drugs is going on and what governs the passive transport mechanism via the biological membrane.
Most recently, Takechi-Haraya et al. have accomplished real-time in-cell NMR method to study molecular mechanism of non-endocytic membrane translocation into living cells.40) Cell-permeable octaarginine (R8), a potential cationic peptide to transport membrane-impermeable oligonucleotides, plasmids, peptides, and proteins,41–45) has been used. By introducing 4-trifluoromethyl-L-phenylalanine to the N terminus, the energy-independent, non-endocytic transport of 19F-tagged R8 (19F-R8) to a human myeloid leukemia cell line has been observed at 4°C with a time resolution in the order of minutes. The real time evolution of the 19F-NMR spectra has demonstrated the processes of R8 translocation: (i) the binding to anionic glycosaminoglycans at the cell surface, (ii) the penetration into cell membrane, and (iii) the entry into cytosol through the membrane40) (Fig. 7). Utilizing NMR concentration analysis, Takechi-Haraya et al. have quantified in what amount of R8 stays in the translocation processes (i), (ii), and (iii) without perturbing the system. The study shows a potential for elucidating mechanisms of direct membrane transport of cell penetrating peptides with minimal perturbation. Although fluorescence labeling is used in many cases, it sometimes modulates cell entry processes; enhances the interaction with lipid membrane,46,47) induces photodamage of membranes,48) facilitates the uptake,49) modifies the cellular distribution,50,51) and changes the structural flexibility and conformation of the peptide.52) The label-free, in-cell NMR is advantageous, and in situ method is effective to observe cellular uptake of drugs in real time without any perturbation of the system. It is expected that in-cell NMR spectroscopy sheds light on the kinetics of drug transport to natural living cells, in relation to the cell membrane dynamics in real time.
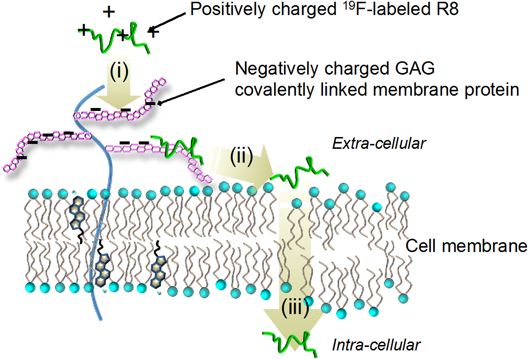
(Reprinted from ref. 40.) (Color figure can be accessed in the online version.)
The author is financially supported by Japan Society for the Promotion of Science and Hyogo Science and Technology Association. The author also thanks the editor, Prof. H. Saito of Kyoto Pharmaceutical University, for inviting to write this review.
The author declares no conflict of interest.