2019 年 67 巻 5 号 p. 404-409
2019 年 67 巻 5 号 p. 404-409
The purpose of the study was to evaluate the ability of different beverages to mask the bitterness of zopiclone and eszopiclone in tablet formulations using the artificial taste sensor and human gustatory sensation testing. The beverages tested for bitterness-masking effects were: Mugicha, Sports beverage, Lactic acid drink, Orange juice and a diluted simple syrup (an 8.5% sucrose solution). The bitterness intensities estimated by the taste sensor of zopiclone or eszopiclone one-tablet solutions mixed with the various beverages, corresponded well with the observed bitterness intensities measured by gustatory sensation testing. The Sports beverage, Lactic acid drink and Orange juice significantly suppressed the bitterness intensity of both zopiclone and eszopiclone 1-tablet solutions compared with water when tested in the artificial taste sensor. Sports beverage, Lactic acid drink and Orange juice all contain citric acid as acidifier, so it was postulated that citric acid was involved in the mechanism of bitterness intensity suppression of zopiclone and eszopiclone 1-tablet solutions by these three beverages. It was then shown that citric acid suppressed the bitterness intensity of a zopiclone one-tablet sample solution in a dose-dependent manner. 1H-NMR spectroscopic analysis of mixtures of citric acid with zopiclone suggested that the carboxyl groups of citric acid interact with the amine group on zopiclone. This study therefore showed that the bitterness intensities of zopiclone and eszopiclone can be suppressed by citric-acid-contained beverages and suggests that this bitterness suppression is due to a direct electrostatic interaction between citric acid and the two drugs.
Taste is important for determining the acceptability of a pharmaceutical formulation. Many active pharmaceutical ingredients exhibit an unpleasant taste, making taste masking an important step in formulation development. The use of an ‘electronic tongue’ or taste sensor for pharmaceutical purposes is a useful innovation which reduces dependence on human gustatory sensation testing.
A quantitative analytical method for the evaluation of the bitterness of basic pharmaceutical products with a taste sensor has been reported by Uchida et al.1–10) The taste sensor was initially developed by Toko et al.11) and is an analytical sensor array system comprising various lipid–polymer membranes which are capable of transforming information about the substances which produce taste into electrical signals.12,13) The taste sensor output exhibits different patterns for chemical substances with different taste qualities (sweetness, saltiness, sourness, bitterness and umami), while exhibiting similar patterns for chemical substances with similar tastes.
Zopiclone and eszopiclone (Fig. 1) are stereoisomeric cyclopyrrolones which have recently become available in Japan for the treatment of insomnia.14–16) They are classified as non-benzodiazepines, and act on the γ-aminobutyric acid (GABA)–benzodiazepine receptor chloride channel complex. Non-benzodiazepine agents are much more selective for the benzodiazepine receptor subtypes (GABAA) than benzodiazepines and have reportedly fewer side-effects.17,18) However, the bitter unpleasant tastes of zopiclone and eszopiclone as those side effects have been reported.16,19–21) Roth et al. assessed the efficacy and safety of eszopiclone in patients with insomnia and reported that 21 patients out of every 77 patients complained about unpleasant taste of eszopiclone. Furthermore, one eszopiclone-treated patient (1.3%) discontinued because of unpleasant taste. Zopiclone tablet or eszopiclone tablet are designed to prevent disintegration in oral cavity with a coated film, whereas a half tablet and crushing are sometimes prescribed in consideration of the swallowing function of the patient. From these facts, taste masking of zopiclone and eszopiclone is urgent task.
The aim of this study was to evaluate the bitterness-masking effect of various beverages on zopiclone and eszopiclone tablets using the artificial taste sensor and human gustatory sensation testing.
Quinine hydrochloride was purchased from Sigma Chemical Co. (U.S.A.), zopiclone tablets (7.5 mg) (Amoban®) from Sanofi K.K. (Tokyo, Japan), eszopiclone tablets (2 mg) (Lunesta®) from Eisai Co., Ltd. (Tokyo, Japan). The beverages tested were Mugicha (Kenko mineral Mugicha, Ito En Ltd., Japan), Sports beverage (Aquarius, Coca-cola, Japan), Lactic acid drink (Calpis water, Asahi Soft Drinks Co., Ltd., Japan), Orange juice (Tropicana 100% Orange, Kirin Co., Ltd., Japan) and a diluted simple syrup (8.5% sucrose solution). Zopiclone was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), citric acid was purchased Nacalai Tesque, Inc. (Kyoto, Japan). All other reagents were special reagent grade.
The Taste SensorThe taste sensor, SA402B (Intelligent Sensor Technology Inc., Atsugi, Japan) was used to determine the bitterness and sourness intensities of the sample solutions using sensor AC0, developed specifically to detect the bitterness of basic substances. In the first step of the procedure, reference solution (30 mM KCl solution containing 0.3 mM tartaric acid; corresponding to saliva) is measured and the electric potential obtained (mV) is defined as Vr. Then sample solution is measured and the electric potential obtained is defined as Vs. The relative sensor output (R), represented by the difference between the potentials of the sample and the reference solution (Vs − Vr), corresponds to the ‘taste immediately after putting in the mouth.’ The electrodes are subsequently rinsed with a fresh reference solution for 6 s. When the electrode is dipped into the reference solution again, the new potential of the reference solution is defined as Vr0. The difference (Vr0 − Vr) between the potentials of the reference solution before and after sample measurement is the ‘Change in membrane potential caused by adsorption’ (CPA) and corresponds to the so-called ‘aftertaste.’ The value obtained when CPA is divided by R is defined as the adsorption ratio.
In this study, the CPA of AC0 (CPAAC0) was taken as the predicted bitterness intensity of the basic drug tested. In a previous study,22) we showed that the bitterness intensity of solifenacin succinate could be evaluated using taste sensor AC0. Sensor AC0 is one of the most specific sensors for the evaluation of bitterness of basic substances. Quinine hydrochloride solution, at concentrations of 0.03, 0.1, 0.3 and 1.0 mM, were prepared as standards for the bitterness evaluation. Bitterness scores of 1, 2, 3 and 4 were allocated to these increasing concentrations of quinine hydrochloride. Zopiclone or eszopiclone tablet sample solutions were prepared at concentrations of 0.25, 0.5 and 1 tablet/sample.
Gustatory Sensation TestsFive healthy female subjects, 30 ± 9 years old, participated in the tests in which various sample solutions were evaluated. No subject reported having a cold or respiratory tract infection in the week prior to testing. The subjects were asked to refrain from eating, drinking, or chewing gum for at least 1 h prior to testing. All subjects were non-smokers and signed an informed consent before the experiments. The experimental protocol of this study (No. 16–43) was approved in advance, on July 23, 2016, by the ethical committee of Mukogawa Women’s University.
The gustatory sensation test to measure bitterness intensity was performed according to a modified previously described method.23,24) Quinine hydrochloride solution at concentrations of 0.01, 0.03, 0.1, 0.3 and 1.0 mM were prepared as the standards for bitterness evaluation. Bitterness scores of 0, 1, 2, 3 and 4 were allocated to these increasing concentrations of quinine hydrochloride. One tablet solutions of zopiclone or eszopiclone–40 mL water or beverages (Mugicha, Sports beverage, Lactic acid drink, Orange juice and Simple syrup) were prepared.
The sample concentrations tested were the same as those used in evaluations by the taste sensor. Before testing, the volunteers were asked to keep 2 mL of standard quinine hydrochloride solutions in their mouths for 5 s and were told the concentration and bitterness score of each solution. In the test, the volunteers were asked to keep 2 mL of sample solution in their mouths for 5 s and then to give each sample a bitterness score. After tasting each sample, subjects gargled well and waited for at least 20 min before tasting the next sample.
1H-NMR Spectroscopic AnalysisThe 1H-NMR spectra were measured on a JEOL 500 MHz spectrometer using dimethyl sulfoxide (DMSO)-d6 as a solvent and tetramethylsilane (TMS) as an internal standard. 1H-NMR spectra were acquired at 308 K (35°C) which is near ordinary temperature and capable of stably keeping probe contained sample solution in NMR equipment, with a 7 s relaxation delay. To evaluate interaction between zopiclone and citric acid underlying the mechanism of masking bitterness intensity of zopiclone by citric acid, zopiclone bulk was used for 1H-NMR measurement. Citric acid solution, zopiclone solution, zopiclone solution with citric acid (zopiclone–citric acid; 1 : 1, 1 : 2 and 1 : 8 M ratio) were prepared as sample solutions.
ExperimentsFirstly, different concentrations of zopiclone and eszopiclone tablets (sample solutions containing 0.25, 0.5 or 1 tablet/sample) were evaluated in the taste sensor using membrane AC0. Then one-tablet samples of each drug were mixed with each of the five beverages (Mugicha, Sports beverage, Lactic acid drink, Orange juice and Simple syrup) and this combination was evaluated in the taste sensor and by gustatory sensation testing.
Statistical AnalysisBellCurve for Excel® (Social Survey Research Information Co., Ltd., Tokyo, Japan) was used for statistical analysis. The Tukey test was used for multiple comparisons. Correlation was examined using Spearman’s correlation test. The 5% level of probability was considered significant.
Figure 2 shows the relationship between bitterness intensity and sensor output (CPAAC0) using quinine hydrochloride solutions of increasing concentrations. CPA, the change in sensor membrane potential caused by adsorption of bitter substance to the sensor membrane, was evaluated in reference solution under constant pH and electric conductivity and as correlative factor to bitterness intensity. Figure 3 shows the CPAAC0 of solutions of zopiclone and eszopiclone tablets. The mean value of CPAAC0 for a quinine solution with τ = 1, the bitterness threshold, was 4.85. Sample solutions of zopiclone or eszopiclone containing 0.25, 0.5 or 1 tablet/sample all had CPAAC0 values above the bitterness threshold. The bitterness intensities of one-tablet solutions of zopiclone and eszopiclone were about τ = 4 and τ = 3, respectively. Additives of zopiclone and eszopiclone tablet in water were confirmed that they had no response to bitterness sensor (data not shown). It was suggested that taste sensor outputs of zopiclone or eszopiclone tablet were caused by zopiclone or eszopiclone themselves.
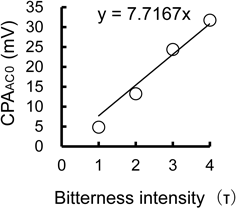
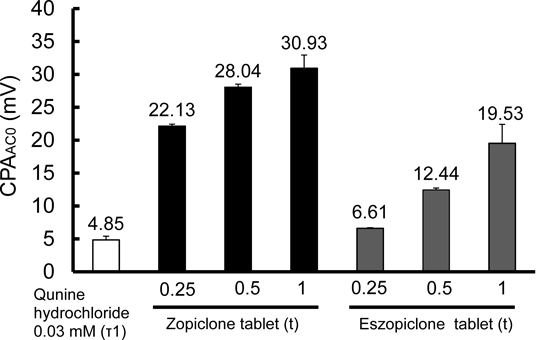
n = 3, mean ± standard deviation (S.D.).
Figure 4 shows the influence of beverages (Water, Mugicha, Sports beverage, Lactic acid drink, Orange juice and Simple syrup) on CPAAC0 values of zopiclone or eszopiclone (1-tablet solutions) as evaluated by the taste sensor. The CPAAC0 values of zopiclone and eszopiclone in Sports beverage, Lactic acid drink and Orange juice were significantly lower than those in water (Tukey test, p < 0.001).
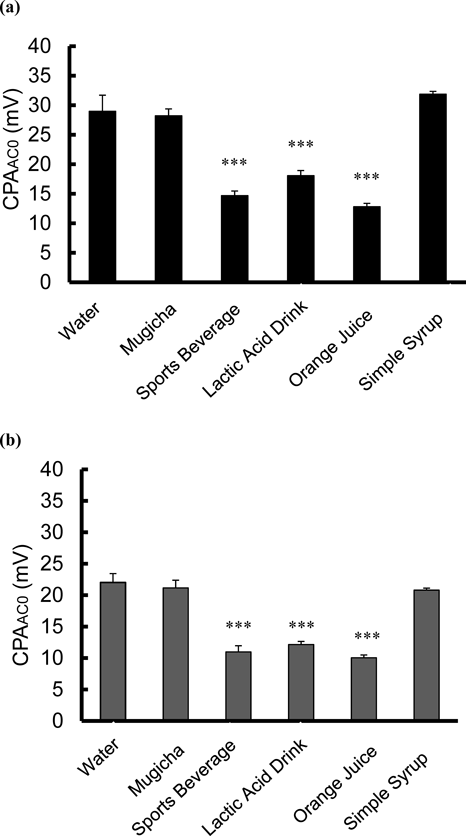
n = 3, mean ± S.D., *** p < 0.001 vs. water (Tukey test).
Figure 5 shows the correlation between estimated bitteness intensity derived from CPAAC0 and observed bitterness intensity by gustatory sensation testing of one-tablet solutions of zopiclone or eszopiclone mixed with water, Mugicha, Sports beverage, Lactic acid drink, Orange juice and Simple syrup. Estimated bitteness intensity derived from taste sensor output (CPAAC0) was calculated from the relationship between bitterness intensity and CPAAC0 using quinine hydrochloride solutions of increasing concentrations (CPAAC0 = 7.7167 × estimated bitteness intensity). There was a good correlation between estimated bitteness intensity derived from CPAAC0 and bitterness intensity measured by gustatory sensation test for all mixtures (r = 0.93, Spearman’s correlation test, p < 0.001).
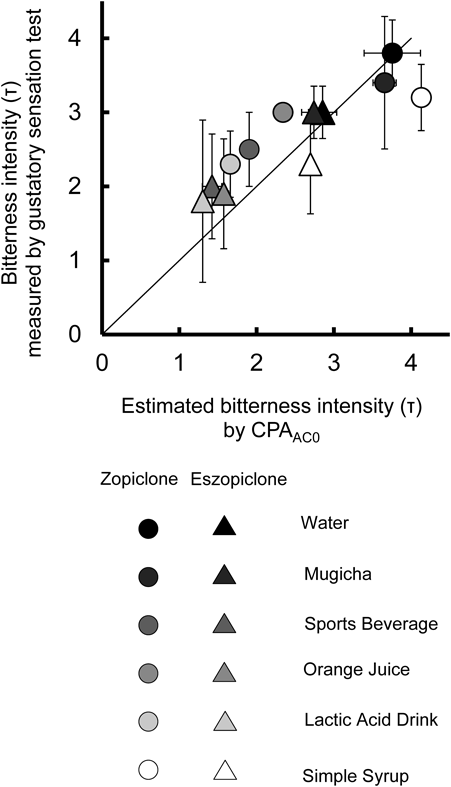
n = 5, mean ± S.D., r = 0.93, p < 0.001 (Spearman’s correlation test).
Figure 6 shows the relation between the pH of the mixtures of one-tablet solutions of zopiclone or eszopiclone with each of the five beverages and their CPAAC0 as measured by the taste sensor. The pH of zopiclone or eszopiclone one-tablet solutions with Sports beverage, Lactic acid drink and Orange juice, all of which had lower CPAAC0 values than the mixture with water, were about 4. CPAAC0 values of zopiclone or eszopiclone one-tablet solutions with Sports beverage, Lactic acid drink and Orange juice were significantly lower than the mixture with water (Fig. 4). Furthermore, the pH of zopiclone or eszopiclone one-tablet solutions with Sports beverage, Lactic acid drink and Orange juice, all of which had lower CPAAC0 values than the mixture with water, were about 4 and lower than those of zopiclone or eszopiclone one-tablet solutions with water, Mugicha and Simple syrup. As for the composition of beverages, sports beverage contains high fructose corn syrup, sodium chloride, citric acid, flavoring agent, sodium citrate, arginine, potassium chloride, magnesium sulfate, antioxidant (ascorbic acid), sweetening agent (sucralose), isoleucine, leucine and valine, lactic acid drink contains saccharide (high fructose corn syrup, sugar), powdered skim milk, lactic acid bacterium drink, acidifier, flavouring, stabilizer (soybean polysaccharides), Orange juice contains protein, carbohydrates, sodium chloride equivalent, potassium, vitamin C, folic acid, fructose, saccharide, citric acid, according to those homepages. A common component of Sports beverage, Lactic acid drink and Orange juice is citric acid as acidifier. It has been reported that the sourness can suppress bitterness.25) It was therefore postulated that citric acid may be involved in the mechanism of bitterness intensity suppression of zopiclone or eszopiclone.

The mechanism underlying attenuation of bitterness intensities of zopiclone or eszopiclone tablets solutions by citric-acid-containing-beverages was evaluated by testing the bitterness-suppressing effect of citric acid solutions (0.1, 0.5 and 1 mM) on zopiclone one-tablet solutions in the taste sensor, using membrane AC0. It was shown that citric acid suppressed the bitterness intensity of zopiclone one-tablet solutions in a dose-dependent manner (Fig. 7, p < 0.001, Tukey test).

n = 3, mean ± S.D., ** p < 0.001 vs. 0 mM, †† p < 0.001 vs. 0.1 mM, ## p < 0.001 vs. 0.5 mM (Tukey test).
1H-NMR was used to evaluate the interaction between zopiclone and citric acid. Bitter basic drugs are adsorbed on the positively charged and hydrophobic part of the taste sensor membrane and probably cause a change in membrane potential by changing the charge density of the taste sensor output.24) Ogata et al. reported that the taste-masking effect of a combination of basic propiverine and acidic compounds is caused by an interaction between the nitrogen of propiverine and the acids.26) In our previous study, 1H-NMR analysis suggested that an electrostatic interaction between the amine group of diphenhydramine and the carboxyl group of chlorogenic acid reduced the membrane adsorption of diphenhydramine hydrochloride in the taste sensor and binding with the bitterness receptor in gustatory sensation tests.27)
The structure of zopiclone is shown in Fig. 1(a). We proposed that an interaction between the amine group of zopiclone and the carboxyl group of citric acid might reduce the membrane adsorption of zopiclone in the taste sensor and binding with the bitterness receptor in gustatory sensation tests. Particular attention was paid to the methyl proton in the vicinity of the amine group of zopiclone. 1H-NMR was used to evaluate the interaction between zopiclone and citric acid in order to understand the mechanism underlying bitterness attenuation of zopiclone by citric acid. 1H-NMR data of zopiclone with/without 1 M ratio, 2 M ratio, or 8 M ratio of citric acid are shown in Fig. 8. Firstly, the pH of zopiclone solution with/without 1 M ratio, 2 M ratio, or 8 M ratio of citric acid was measured and resulted as 6.6, 6.0 or 4.2, respectively. Secondly, ionization of the amine group in the vicinity of the methyl proton in zopiclone was predicted from pH profile of zopiclone using Marvin sketch software. Ionization of the amine group in the vicinity of the methyl proton in zopiclone was 67.66% (molar ratio of citric acid to zopiclone; 1, pH 6.6), 89.29% (molar ratio of citric acid to zopiclone; 2, pH 6.0), and 99.81% (molar ratio of citric acid to zopiclone; 8, pH 4.2) consequently. The signals of methyl proton of zopiclone (proton 1; proton number shows in Fig. 8) in the 1H-NMR spectrum of the mixture of zopiclone and citric acid were shifted slightly downfield in citric acid dose-dependent manner. Methyl proton of zopiclone shifted from 2.103 ppm (zopiclone only) to 2.302 ppm (molar ratio of citric acid to zopiclone; 1) (Δ0.199 ppm), to 2.333 ppm (mixing ratio of citric acid to zopiclone; 2) (Δ0.230 ppm), to 2.381 ppm (molar ratio of citric acid to zopiclone; 8) (Δ0.278 ppm). This suggests that the electron density was decreased at this location, and that the downfield shift was due to a deshielding effect. Ikeda et al. reported that the electron density near to the nitrogen atom was decreased by interaction.28) It is therefore suggested that zopiclone interacts with citric acid in the vicinity of the nitrogen atom of zopiclone. The signals of other protons in zopiclone were not shifted.
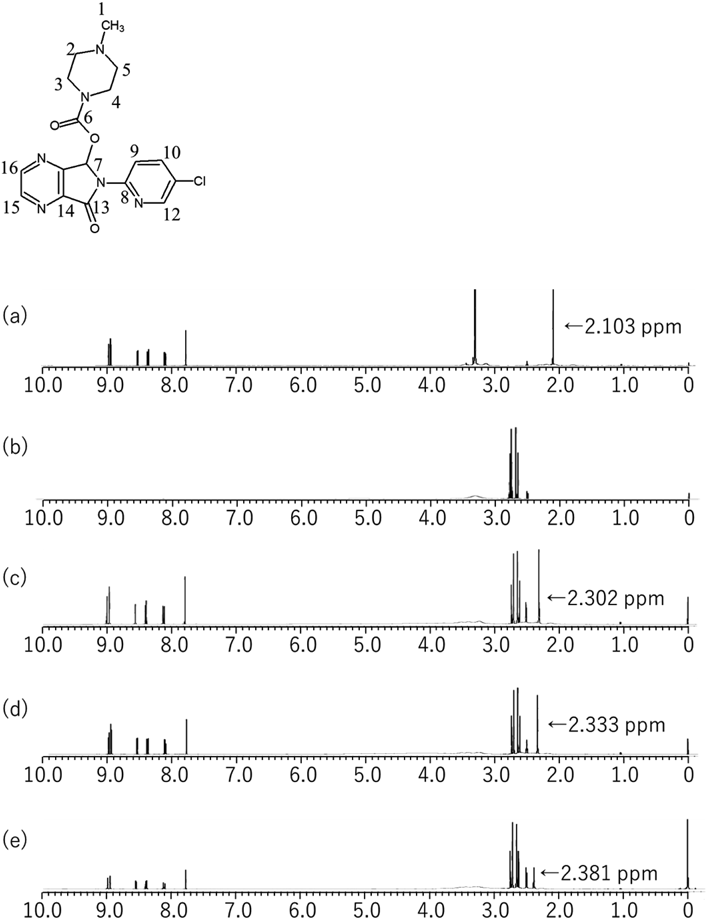
From 1H-NMR data and predictions about the ionization state of each substance using Marvin sketch software, an electrostatic interaction between the positive charge on the amine group of zopiclone and the negative charges on the carboxyl groups of citric acid is indicated. Eszopiclone is a stereoisomer of zopiclone. Considering its similar structure, the mechanism underlying attenuation of CPAAC0 and bitterness intensity of eszopiclone solutions with acidic beverages is considered to be the same as with zopiclone.
In our study, zopiclone (or eszopiclone) mixed with citric acid is a combination of a basic and an acidic compound. It is postulated that citric acid suppresses the bitterness of bitter substances with negative ion-charged groups by electrostatic interaction. It has been reported that zopiclone and eszopiclone are transferred from the blood into the saliva after oral administration.29,30) This shift may be assisted by acidification (by citric acid) in the oral cavity, which reduces the bitterness intensity of zopiclone or eszopiclone.
The influence of different beverages (water, Mugicha, Sports beverage, Lactic acid drink, Orange juice and a simple syrup) on the CPAAC0 of a one-tablet solution of zopiclone or eszopiclone was examined using the taste sensor; the bitterness intensity of the solutions was also examined by gustatory sensation testing. Sports beverage, Lactic acid drink and Orange juice suppressed CPAAC0 and the bitterness intensities of the zopiclone or eszopiclone solution compared with water. Citric acid, which is present as an acidifier in Sports beverage, Lactic acid drink and Orange juice, suppressed the bitterness intensity of a zopiclone one-tablet solution in a dose-dependent manner and 1H-NMR spectroscopic analysis of a mixture of citric acid with zopiclone suggested that the carboxyl groups on citric acid interact with the amine group of zopiclone. This study suggests that bitterness intensity of zopiclone and eszopiclone can be suppressed by citric-acid-containing beverages by direct electrostatic interaction between citric acid and zopiclone or eszopiclone. It was suggested that the beverages including citric acid such as orange juice would support to decrease bitterness intensity of zopiclone or eszopiclone when those were taken.
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for Promotion of Science 16K08426 (to Takahiro Uchida) and 16K08425 (to Miyako Yoshida).
The authors declare no conflict of interest.