2019 Volume 67 Issue 7 Pages 654-665
2019 Volume 67 Issue 7 Pages 654-665
Quassinoids, one kind of triterpenoids with multiple bioactivities such as anti-cancer, anti-malarial, anti-oxidative, anti-microbial, anti-diabetic, anti-viral, and anti-inflammatory effects, have drawn much attention in recent years. Between 2004 and 2018, the structural characteristics and plant sources of 190 quassinoids were reported. Herein, the structure–activity relationships (SARs) of quassinoids along with the anti-cancer mechanisms of four representative quassinoids, eurycomanone, bruceine D, dehydrobruceine B, and brusatol are discussed. This review might be useful for further research and development of quassinoids.
Quassinoids, one kind of derivatives degraded from triterpenoids,1) are widely distributed in the family Simaroubaceae. As one of the most significant classes of biologically active substances, quassinoids play an irreplaceable role in treating various diseases including cancer,2) parasitic infection,3) viral infection,4) inflammation,5) diabetes,6) fertility disability,7) gastro-dysfunction and hepato-dysfunction,8) and so on. By the year 2004, more than 200 natural quassinoids obtained from 34 species in 14 genera have been reported and reviewed.1,9) The chemical structure research reported by these comprehensive reviews covered almost all kinds of quassinoids, such as C18, C19, C20, C22, and C25 types. While, their known biological effects were limited to anti-cancer and anti-malarial activities.10) During the last few decades, plants rich in quassinoids have become indispensable natural resources for new drug discovery. For instance, bruceantin, which was first isolated from the Brucea antidysenterica, exhibited remarkable bioactivities not only on cell apoptosis, cell proliferation, and tumor angiogenesis but also on inflammation and metabolic diseases.11) However, although clinical trials using bruceantin in combination with anti-cancer drug failed to demonstrate superior efficacy,12) the development prospects for this compound remain hopeful.
In this review, the chemical structural characteristics and plant sources (especially B. javanica and Eurycoma longifolia) of 190 quassinoids reported between 2004 and 2018, as well as their structure–activity relationships (SARs) and proposed anti-cancer mechanisms of four representative quassinoids (eurycomanone, dehydrobruceine B, bruceine D, and brusatol) are discussed.
During the last 15 years, 21 species belonging to 14 genera of two families (Simaroubaceae and Ptaeroxylaceae) have been reported to contain 190 quassinoids. According to the information on 190 quassinoids listed in Table 1, quassinoids are widely distributed in roots, stems, leaves, branches, flowers, fruits, and root barks of various plants from the above mentioned two families. However, only cedphiline (183) was obtained from Ptaeroxylaceae family plant, Cedrelopsis grevei,13) suggesting that Simaroubaceae is almost a unique botanical origin of quassinoids.
| Genus | Plants | Parts | Compounds | Ref. |
|---|---|---|---|---|
| Cedrelopsis | C. grevei | Barks, stems | Cedphiline (183) | 13) |
| Ailanthus | A. altissima | Barks | 6α-Tigloyloxychaparrin (79) | 14) |
| Barks | Altissinol B (82) | 14) | ||
| Barks | 13,18-Dehydroglaucarubinone (91) | 14) | ||
| Barks | Altissinol A (92) | 14) | ||
| Barks | 6α-Tigloyloxychaparrinone (99) | 14) | ||
| Barks | (−)-Chaparrinone (83) | 14) | ||
| Roots, barks | Isoailanthone (98) | 2) | ||
| Stems | Shinjulactone A (80) | 5) | ||
| Stems | Ailanthone (90) | 15) | ||
| Stems | Shinjudilactone (104) | 5) | ||
| Stems | Shinjuglycoside B (109) | 5) | ||
| A. excelsa | Barks, stems | Ailanex A (27) | 16) | |
| Barks, stems | Ailanex B (28) | 16) | ||
| Barks, stems | Glaucarubin (77) | 16) | ||
| Brucea | B. javanica | Fruits | Bruceine J (112) | 3) |
| Fruits | Bruceine B (113) | 17) | ||
| Fruits | Brucein A (114) | 18) | ||
| Fruits | Brusatol (115) | 19) | ||
| Fruits | Bruceantin (116) | 4) | ||
| Fruits | Bruceantinol B (118) | 3) | ||
| Fruits | Bruceantinol (119) | 3) | ||
| Fruits | Bruceantarin (120) | 20) | ||
| Fruits | Bruceine L (123) | 21) | ||
| Fruits | Bruceine M (127) | 22) | ||
| Fruits | Yadanziolide A (132) | 23) | ||
| Fruits | Dehydrobruceine A (144) | 24) | ||
| Fruits | Bruceene (147) | 20) | ||
| Fruits | Bruceene A (148) | 20) | ||
| Fruits | Yadanzioside E (156) | 20) | ||
| Fruits | Javanicoside C (159) | 25) | ||
| Fruits | Yadanzioside F (160) | 20) | ||
| Fruits | Yadanzioside A (161) | 20) | ||
| Fruits | Bruceoside A (162) | 20) | ||
| Fruits | Bruceantinoside A (164) | 25) | ||
| Fruits | Yadanzioside C (165) | 20) | ||
| Fruits | Yadanzioside G (168) | 25) | ||
| Fruits | Yadanzioside M (170) | 20) | ||
| Fruits | Yadanzioside B (172) | 20) | ||
| Fruits | Bruceoside B (173) | 23) | ||
| Fruits | Yadanzioside P (174) | 20) | ||
| Fruits | Yadanzioside K (176) | 20) | ||
| Fruits | Bruceine K (181) | 21) | ||
| Roots | 15β-Hydroxyklaineanone (48) | 26) | ||
| Roots | 11-Dehydroklaineanone (52) | 26) | ||
| Seeds | Aglycone of yadanzioside D (122) | 27) | ||
| Seeds | Javanicolide D (124) | 27) | ||
| Seeds | Javanicolide H (139) | 17) | ||
| Seeds | Javanicolide E (140) | 23) | ||
| Seeds | Javanicolide F (141) | 23) | ||
| Seeds | Javanicolide C (142) | 27) | ||
| Seeds | Dehydrobruceine B (143) | 23) | ||
| Seeds | Dehydrobruceine C (146) | 28) | ||
| Seeds | Javanic acid A (149) | 17) | ||
| Seeds | Javanic acid B (150) | 17) | ||
| Seeds | Bruceanic acid E (151) | 17) | ||
| Seeds | Bruceanic acid F (152) | 17) | ||
| Seeds | Buruceanic acid E methyl ester (153) | 17) | ||
| Seeds | Javanicolide B (154) | 29) | ||
| Seeds | Yandanzigan (155) | 23) | ||
| Seeds | Javanicoside K (157) | 30) | ||
| Seeds | Javanicoside L (158) | 30) | ||
| Seeds | Javanicoside F (163) | 27) | ||
| Seeds | Javanicoside D (166) | 27) | ||
| Seeds | Javanicoside E (167) | 27) | ||
| Seeds | Yadanzioside O (169) | 27) | ||
| Seeds | Yadanzioside I (171) | 23) | ||
| Seeds | Yadanzioside L (175) | 23) | ||
| Seeds | Javanicoside J (177) | 30) | ||
| Seeds | Javanicoside B (178) | 27) | ||
| Seeds | Javanicoside I (179) | 30) | ||
| Seeds | Yadanzioside N (180) | 30) | ||
| Stems | Yadanziolide V (26) | 31) | ||
| Stems | Brujavanol B (44) | 26) | ||
| Stems | Brujavanol A (45) | 26) | ||
| Stems | Brujavanol D (46) | 32) | ||
| Stems | Brujavanol C (47) | 32) | ||
| Stems | Yadanziolide T (51) | 31) | ||
| Stems | 5α,14β,15β-Trihydroxyklaineanone (54) | 32) | ||
| Stems | Yadanziolide U (76) | 31) | ||
| Stems | Brucein C (117) | 3) | ||
| Stems | Bruceine F (121) | 32) | ||
| Stems | Bruceine E (125) | 17) | ||
| Stems | Bruceine D (131) | 17) | ||
| Stems | Bruceine H (133) | 17) | ||
| Stems | Yadanziolide B (137) | 31) | ||
| Stems | Dehydrobrusatol (145) | 24) | ||
| Castela | C. macrophylla | Leaves, twigs, thorns | (−)-Glaucaru-bolone (84) | 33) |
| Leaves, twigs, thorns | (−)-Holacanthone (85) | 33) | ||
| Seeds | (−)-Glaucaru-bolone-15-O-β-D-glucopyranoside (111) | 33) | ||
| Eurycoma | E. longifolia | Roots | Eurycolactone E (4) | 34) |
| Roots | Eurycomalactone (6) | 35) | ||
| Roots | 18-Dehydro-6α-hydroxyeurycomalactone (9) | 36) | ||
| Roots | Eurycomalide D (11) | 37) | ||
| Roots | Eurycomalide C (12) | 34) | ||
| Roots | Eurycomalide B (15) | 38) | ||
| Roots | Eurycomalide A (16) | 38) | ||
| Roots | Eurylactone F (22) | 37) | ||
| Roots | Eurylactone E (23) | 37) | ||
| Roots | Eurylactone G (24) | 37) | ||
| Roots | Eurylactone A (25) | 37) | ||
| Roots | 2,3-Dehydro-4α-hydroxylongilactone (29) | 39) | ||
| Roots | Longilactone (30) | 37) | ||
| Roots | 14,15β-Dihydroxyklaineanone (49) | 37) | ||
| Roots | 15β-O-Acetyl-14-hydroxyklaineanone (50) | 26) | ||
| Roots | Eurycomanol (81) | 35) | ||
| Roots | Eurycomanone (93) | 37) | ||
| Roots | 13β-Methyl-21-dihydroeurycomanone (94) | 37) | ||
| Roots | Pasakbumin C (95) | 38) | ||
| Roots | Δ4,5,14-Hydroxyglaucarubol (101) | 40) | ||
| Roots | 13β,21-Dihydroxyeurycomanone (102) | 40) | ||
| Roots | Pasakbumin B (103) | 38) | ||
| Roots | (1S,5S,7R,8S,9R,10R,11S,13S,14S,15R)-5-iso-Eurycomadilactone (105) | 40) | ||
| Roots | (1S,5R,7R,8S,9R,10R,11S,13S,14S,15R)-Eurycomadilactone (106) | 40) | ||
| Roots | (1S,5S,7R,8S,9R,10R,11S,13R,14S,15R)-13-epi-Eurycomadilactone (107) | 40) | ||
| Roots | Eurycomalide E (108) | 37) | ||
| Roots | Eurycomanol-2-O-β-D-glucopyranoside (110) | 38) | ||
| Stems | 6α-Hydroxyeury-colactone E (5) | 41) | ||
| Stems | 5α-Hydroxyeury-comalactone (7) | 41) | ||
| Stems | Δ4(18)-Isomer of eurycolactone E (8) | 41) | ||
| Stems | 5-Dehydro-3-hydro-7β-hydroxy-6-oxoeuryco-lactone E (10) | 41) | ||
| Stems | 3α,4α-Epoxyeurycomalide B (13) | 41) | ||
| Stems | Epoxy-5,6-dehydroeurycomalactone (14) | 41) | ||
| Stems | 6α,14,15β-Trihydroxyklaineanone (53) | 41) | ||
| Stems | 14-epi-13,21-Dihydroeurycomanone (96) | 41) | ||
| Stems | 12,15-O-O-Diacetyl-13,21-dihydroeurycomanone (97) | 41) | ||
| Harrisonia | H. perforata | Stems, twigs | Perforalactone C (69) | 42) |
| Stems, twigs | Perforalactone B (189) | 42) | ||
| Stems, twigs | Perforalactone A (190) | |||
| Laumoniera | L. bruceadelpha | Barks | Delaumonone A (134) | 43) |
| Barks | Delaumonone B (136) | 43) | ||
| Nothospondias | N. staudtii | Barks, stems | Nothospondin (39) | 44) |
| Odyendyea | O. gabonensis | Barks | 2′-(R)-O-Acetylglaucarubin (78) | 45) |
| Barks | Ailanthinone (86) | 45) | ||
| Barks | 2′-(S)-O-Acetylglaucarubinone (88) | 45) | ||
| Barks | 2′-(R)-O-Acetylglaucarubinone (89) | 45) | ||
| Fruits | (−)-Odyendane (184) | 46) | ||
| Fruits | (−)-Odyendene (185) | 46) | ||
| Fruits | (−)-Odyendanol (186) | 46) | ||
| Picrasma | P. amara | Leaves | Neoquassin (67) | 47) |
| Leaves | Quassin (68) | 47) | ||
| P. javanica | Barks | Picrajavanicin B (35) | 48) | |
| Barks | Picrajavanicin D (36) | 48) | ||
| Barks | Picrajavanicin I (37) | 48) | ||
| Barks | Picrajavanicin C (40) | 48) | ||
| Barks | Javanicin I (61) | 48) | ||
| Barks | Picrajavanicin G (62) | 48) | ||
| Barks | Picrajavanicin A (63) | 48) | ||
| Barks | Picrajavanicin H (64) | 48) | ||
| Barks | Javanicin B (65) | 48) | ||
| Barks | Javanicin F (66) | 48) | ||
| Barks | Picrajavanicin L (70) | 49) | ||
| Barks | Picrajavanicin M (71) | 50) | ||
| Barks | Picrajavanicin K (72) | 48) | ||
| Barks | Picrajavanicin E (73) | 48) | ||
| Barks | Picrajavanicin F (74) | 48) | ||
| Barks | Picrajavanicin J (75) | 48) | ||
| Barks | Picrasin A (187) | 50) | ||
| Barks | 2′-Isopicrasin A (188) | 50) | ||
| P. quassioides | Stems | Desbenzoyl-picrajavanin A (31) | 51) | |
| Stems | Nigakilactone O (32) | 51) | ||
| Stems | Nigakilactone P (33) | 51) | ||
| Stems | Picrasin B (38) | 51) | ||
| Stems | Nigakilactone K (55) | 51) | ||
| Stems | Nigakilactone Q (56) | 51) | ||
| Stems | Picraqualide F (57) | 51) | ||
| Stems | Nigakilactone A (58) | 51) | ||
| Stems | Nigakilactone B (59) | 51) | ||
| Stems | Nigakilactone F (60) | 51) | ||
| Picrolemma | P. sprucei | Leaves, roots, stems | Isobrucein B (135) | 8) |
| Leaves, roots, stems | Neosergeolide (182) | 52) | ||
| Quassia | Q. amara | Leaves | Picrasin K (34) | 53) |
| Leaves | Picrasin H (41) | 54) | ||
| Leaves | Picrasin I (42) | 54) | ||
| Leaves | Picrasin J (43) | 54) | ||
| Leaves | Simalikalactone E (138) | 55) | ||
| Q. indica | Barks, stems | Samaderine B (20) | 56) | |
| Barks, stems | Samaderine E (130) | 56) | ||
| Q. undulata | Roots, stems | 15-Desacethylundulatone (100) | 57) | |
| Simaba | S. cedron | Seeds | 1,4-Dehirocedro-nolactone A (17) | 58) |
| S. orinocensis | Barks, roots | Orinocinolide (126) | 59) | |
| Barks, roots | Simalikalactone D (129) | 59) | ||
| S. madagascariensis | Leaves | 5β,6-Dihydrosa-maderine A (1) | 60) | |
| Leaves | Samaderine A (2) | 60) | ||
| Leaves | 2-Chlorosamaderine A (3) | 60) | ||
| Leaves | 3,4β-Dihydrosa-maderine C (18) | 60) | ||
| Leaves | Cedronin (19) | 60) | ||
| Leaves | Samaderolactone A (21) | 60) | ||
| Simarouba | S. berteroana | Barks, stems | Glaucarubinone (87) | 44) |
| Barks, stems | Ailanqyassin (128) | 61) |
The 190 quassinoids are divided into five types: C18 (1–3), C19 (4–30), C20 (31–181), C22 (182), and C25 (183–190) types according to the carbon number of their aglycones (Figs. 1–4). Interestingly, all the C18-type quassinoids (1–3) were isolated from Simaba madagascariensis. C19-type quassinoids were found in 6 species: Ailanthus excelsa (27, 28), Brucea javanica (26), E. longifolia (4–16, 22–25, 29, 30), Quassia indica (20), S. cedron (17), and S. madagascariensis (18, 19, 21). Only one compound named neosergeolide (182) with C22 aglycone has been found in natural products to date. Meanwhile, C25-type quassinoids were obtained from C. grevei (183), Harrisonia perforata (189, 190), Odyendyea gabonensis (184–186), and Picrasma javanica (187, 188). C20-type quassinoids have been found in all plants of Simaroubaceae family except S. cedron.




Most of the 190 quassinoids were with C19 or C20 aglycone, and more than half of them were obtained from B. javanica (26, 44–48, 51, 52, 54, 76, 112–125, 127, 131–133, 137, 139–181) and E. longifolia (4–16, 22–25, 29, 30, 50, 53, 81, 93–97, 101–103, 105–108, 110). To provide chemical evidence for plant identification, the structural characteristics of these two types of quassinoids obtained from B. javanica and E. longifolia were discussed.
There are three types of nucleus of C19 quassinoids: eurycolactone (4–21), eurylactone (22–28), and longilactone (29, 30). Among them, 4–16, 22–25, 29, and 30 were found from E. longifolia, and their structural characteristics were observed: for eurycolactone-type C19 quassinoids (4–16), the C-8 was substituted by methyl; and the C-14 of eurylactone-type compounds (22–25) was substituted by β-OH; on the other hand, longilactone-type ones (29, 30) were found to exist only in E. longifolia. The above mentioned relationship between structural characteristics and plant sources was also found in the C19 quassinoids by Vieira and Braz-Filho.1)
The aglycone of C20-type quassinoids is usually substituted by oxygen groups such as –OH, C=O, or –OCH3, olefinic bond, α,β-unsaturated ketone, bridged ring, as well as ester groups (Fig. 3). The oxygen groups usually locate at C-1, 2, 11–15 of C20-type quassinoids. Combining our roundup with that of Vieira and Braz-Filho,1) we found C20-type quassinoids without oxygen substituted at C-1 to distribute in B. javanica (112–120, 139–146, 149–153, 155, 158–177), Ailanthus altissima, A. excels, and B. antidysenteria. Moreover, almost all the C20-type quassinoids obtained from E. longifolia were substituted by β-OH at C-14 and C-15 positions (49, 50, 53, 81, 93–97, 101–103, 105–108, 110). On the other hand, it was found that the olefinic bond could form between C-1 and C-2, C-2 and C-3, C-3 and C-4, C-4 and C-5, C-12 and C-13, C-13 and C-21 in the structure of C20-type quassinoids. Among them, the plant origin of Δ1,2 ones was limited in E. longifolia (143–146, 159–170), as well as A. altissima and B. javanica.1) Although the α,β-unsaturated ketone could locate at C-1, C-2, and C-3; C-2, C-3 and C-4; C-3, C-4 and C-5; along with C-11, C-12 and C-13 of C20 type quassinoids’ aglycone, from E. longifolia, only C-2, C-3, and C-4 α,β-unsaturated ketone type (50, 53, 93–97, 102, 103, 105–108) has been found to date. Meanwhile, the bridge group always located at C-8 and C-11 or C-8 and C-13 position, the former ones (81, 93–97, 101–103 105–108, and 110) were mainly found from E. longifolia, while B. javanica was the main plant source of the latter ones (112–125, 127, 131–133, 137, 139–180) (Table 1). Moreover, esterification was another substitution type of C20 quassinoids; it could form at C-15 and C-21, and most of those esterified at C-15 were obtained from B. javanica (112–120, 122–124, 139–146, 149–153, 156–180).
The structural characteristics and plant sources of quassinoids and their relationships summarized above may play an important role in the identification of related plants.
Quassinoids exhibit extensive bioactivities such as anti-cancer, anti-parasitic, anti-viral, anti-inflammation, anti-microbial, anti-diabetes, fertility improvement,7,62,63) gastro-protection, and hepato-protection.64) Among them, the anti-cancer, anti-parasitic, anti-viral, and anti-inflammatory activities have attracted much attention from researchers. On the basis of our above generalized rule, the substitutes of quassinoids’ aglycones include oxygen groups, olefinic bond, α,β-unsaturated ketone, bridged ring, ester groups, methyl or –CH2OH, and so on. And different kinds of substituted quassinoids possess different bioactivities. Thus the SARs between the four kinds of activities and substitute forms of quassinoids were summarized and discussed in this section.
3.1. Anti-cancer ActivityThe anti-cancer activity of quassinoids is exerted mainly by their anti-proliferative effects on various cancer cell lines. During this process, the substitution of oxygen groups, methyl or –CH2OH, glycosyl, α,β-unsaturated ketone group, and olefenic bond are considered key roles influencing the anti-cancer activities of quassinoids (Fig. 5).

Picrajavanicins B (35), I (37), H (64), L (70), M (71), K (72), and javanicin F (66) isolated from P. javanica were revealed to possess anti-proliferative activities on PANC-1 cell line.49) Comparing their activities, it was found that their anti-proliferative potency could be increased by the substitution of (1) oxygen groups at C-4 (IC50 values: 3.25, 7.37, 8.48, >100 µM for 72, 71, 70, and 66, respectively); (2) –CH2OH group at C-13 (64 vs. 66, 37 vs. 35; IC50 values: 4.33, >100 µM, 17.41, >100 µM for 64, 66, 37, and 35, respectively). In addition, the methylation of 2-OH could also obviously increase the cytotoxicity on MCF-7 [picrasin H (41) vs picrasin B (38); IC50 values at 0.8, 8.9 µM for 41 and 38, respectively].54)
It was also found that glycosylation of 3-OH decreased the anti-tumor activities both on MCF-7 cells and MDA-MB-231 cells [brucein A (114) vs. yadanzioside B (172), brusatol (115) vs. bruceoside B (173), bruceantinol (119) vs. yadanzioside K (176); IC50 values on MCF-7 cells: 0.182 ± 0.048, 0.083 ± 0.038, 0.063 ± 0.016 µM for 114, 115, 119, respectively and 172, 173, 176 all >50 µM; IC50 values on MDA-MB-231 cells: 0.228 ± 0.020, 0.088 ± 0.012, 0.081 ± 0. 017 for 114, 115, 119, respectively; but 172, 173, 176 all >50 µM].20) The speculation could be confirmed further by comparison of the cytotoxicity against five human cancer cell lines of yadanziolides T (51) (IC50 values at 3.36, 4.40, 3.00, >10, and >10 µM on HCT-8, Bel-7402, BGC-823, A549, and A2780 cell lines, respectively) and U (76) (not active).31)
Furthermore, α,β-unsaturated ketone group was suggested to play a significant role on the anti-cancer activities of quassinoids, which was confirmed by comparing the activities between ailanthinone (86) and excelsin (191), 2′-(R)-O-acetylglaucarubinone (89) and 2′-(R)-O-acetylglaucarubin (78), as well as eurycomanone (93) and eurycomanol (81) using the same cell lines [ailanthinone (86) (IC50 values at 0.09, 0.13, 0.18, 0.37 µM towards DU145, A549, KB, KB-VIN cells, respectively) vs. excelsin (191) (IC50 values at 4.7, 16, 4.3, NA µM towards DU145, A549, KB, KB-VIN cells, respectively); 2′-(R)-O-acetylglaucarubinone (89) (IC50 values at 0.04, 0.06, 0.05, 0.42 µM towards DU145, A549, KB, KB-VIN cells, respectively) vs. 2′-(R)-O-acetylglaucarubin (78) (IC50 values at 0.83, 3.6, 0.67, NA µM towards DU145, A549, KB, KB-VIN cells, respectively)45); eurycomanone (93) (IC50 values at 44.1 and 14.1 µM towards MCF-7 and MGC-803 cell lines, respectively) vs. eurycomanol (81) (inactive)].41)
The assay on the cytotoxicities against multiple cancer cell lines of compounds from E. longifolia showed that eurycomanone (93) exhibited the IC50 values of 44.1 and 14.1 µM against MCF-7 and MGC-803 cell lines, respectively, while 13β,21-dihydroxyeurycomanone (102) was inactive. It was indicated that the olefinic bond between C-13 and C-21 might be an active unit for their anti-cancer effects.41)
3.2. Anti-parasitic ActivityThe anti-parasitic activity of quassinoids is one of the most widely studied biological effects. It was reported that quassinoids exhibit pronounced inhibitory potency on Plasmodium falciparum (the most common cause of malaria), Leishmania donovani, canine babesia, and trypanosome. Here, we generalize the different influences of the substituent types including oxygen groups, bridge ring, ester groups, α,β-unsaturated ketone, as well as dehydration of quassinoids’ aglycones on their parasitic inhibitory activities (Fig. 6).

The anti-malarial activity against P. falciparum assay indicated that the hydroxyl substitution exerted a positive influence on the inhibitory effects of quassinoids: brujavanol C (47) vs. brujavanol D (46) (IC50 values: 25.35 and 30.49 µM for 47 and 46, respectively).32)
Through comparison of the activities of 13,21-dihydroeurycomanone (97) and 14,15β-dihydroxyklaineanone (49) (97 vs. 49, IC50 values: 0.71 and 19.47 µM for 97 and 49, respectively),35) it was found that a methylenoxy bridge between C-8 and C-11 induced higher anti-malarial activity on P. falciparum. At the same time, the different activities of 14,15β-dihydroxyklaineanone (49) and bruceine D (131) (131 vs. 49, IC50 values: 0.58 and 5.02 µg/mL for 131 and 49, respectively)26) suggested that a methylenoxy bridge between C-8 and C-13 also increased the anti-malarial activity on P. falciparum.
Esterification of quassinoids decreased their anti-malarial effects. For example, 14,15β-dihydroxyklaineanone (49) possessed excellent inhibition on P. falciparum with the IC50 value at 5.02 µg/mL, while 15β-O-acetyl-14-hydroxyklaineanone (51) showed moderate activities with the IC50 values at 13.71 µg/mL.26) The comparison of neosergeolide (182) and 12-acetylneosergeolide (192) (IC50 values: 0.004 and 0.118 µg/mL for 182 and 192, respectively) could further confirm the speculation.64)
α,β-Unsaturated ketone was reported to be an important influencing factor for the anti-parasitic activity of quassinoids on canine babesia. Brucein A (114), bruceantin (116), dehydrobruceine A (144), and dehydrobrusatol (145) were tested for their inhibitory activities against babesia.24) The results indicated that the α,β-unsaturated ketone of the C-15 side chain was critical for their anti-babesial activities (114 vs. 116, 145 vs. 144, IC50 values: 4.0, 13.4, 10.5, 31.0 ng/mL for 114, 116, 145, 144 respectively). While according to L. donovani inhibition assay, the α,β-unsaturated ketone at C-2, C-3, and C-4 was crucial as well (simalikalactone D (129) vs. orinocinolide (126), IC50 values: 0.035 and 25 µg/mL for 129 and 126, respectively).59)
Simultaneously, dehydration of ring A decreased the anti-trypanosomal activity as well (brucein A (114) vs. dehydrobruceine A (144), brusatol (115) vs. dehydrobrusatol (145), IC50 values: 2.9 ± 0.5 and 0.74 nM for 114 and 115, respectively, and 144, 145 both >1000 nM).19)
3.3. Anti-viral ActivityBesides anti-cancer and anti-parasitic activities, quassinoids exhibited strong inhibitory effects against plant viruses, especially tobacco mosaic virus (TMV). The SARs on anti-TMV were found to be related to the glycosylation, α,β-unsaturated ketone substitution and its substituted position (Fig. 7).

Bruceine B (113), brusatol (115), bruceoside A (162), yadanzioside I (171), and bruceoside B (173) isolated from B. javanica showed anti-TMV activity. Comparing the results, it was found that the glycosylation of 3-OH showed negative influence on the anti-TMV activity (113 vs. 171, 115 vs. 173, IC50 values: 3.47, 3.42, 4.22, and 4.64 ng/mL for 113, 115, 171, and 173, respectively). At the same time, bruceoside B (173), with a α,β-unsaturated ketone substituted at C-2, C-3, and C-4, showed strong anti-TMV activity, whereas bruceoside A (162) with a α,β-unsaturated ketone substituted at C-1, C-2, and C-3 was inactive. This indicated that the α,β-unsaturated ketone might be an active unit, and its substituted position played a significant role in their anti-TMV activity.23)
3.4. Anti-inflammatory ActivityThe anti-inflammatory effects of quassinoids were reported to be exhibited through their inhibition on the production of lipopolysaccharide (LPS)-induced nitric oxide (NO) and nuclear factor-kappaB (NF-κB) inhibitory abilities. According to the references, the substitution of α,β-unsaturated ketone, glycosyl, and Δ13,21 olefinic bond were considered as the main influencing factors on their anti-inflammatory activities (Fig. 8).
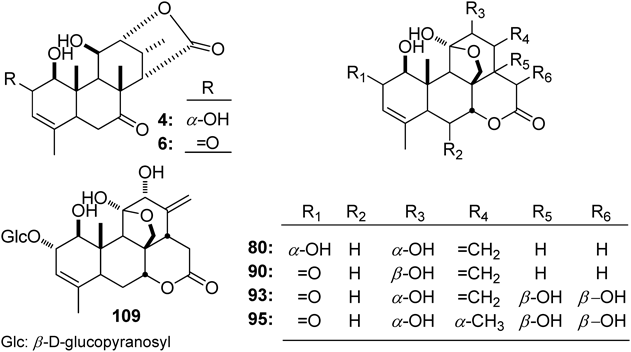
Shinjulactone A (80), ailanthone (90), and shinjuglycoside B (109) isolated from A. altissima were demonstrated to exhibit inhibitory effects on LPS-induced NO production in RAW264.7 cells at non-toxic concentrations. Comparing their activities, it was found that the α,β-unsaturated ketone substituted at C-2, C-3, and C-4 induced higher anti-NO production abilities (90 vs. 80, IC50 values: 5.18 and 56.4 µM for 90 and 80, respectively).5) This inference could be further confirmed by the activity comparison of eurycolactone E (4) and eurycomalactone (6) (6 vs. 4, IC50 values: 0.5 and 3.8 µM for 7 and 4, respectively).34) Meanwhile, the glycosylation of 2-OH was thought to decrease the activity (80 vs. 109, IC50 values: 56.4 and >100 µM for 80 and 109, respectively).5)
What is more, the different NF-κB inhibitory activities of eurycomanone (93) and 13α,21-dihydroeurycomanone (95) suggested that the methyl substituted at C-13 was crucial for the activity (95 vs. 93, IC50 values: 0.7 and 2.4 µM for 95 and 93, respectively).34)
On the basis of the above mentioned SARs, we found that the SARs of anti-cancer, anti-parasitic, anti-viral, and anti-inflammatory activities of quassinoids were closely related to the substituted groups type and position, which would provide a reference for quicker discovery of potent new drugs from natural products.
In the light of what we have summarized above, the anti-cancer effects were considered to be one of the major bioactivities of quassinoids. According to the references, anti-cancer mechanism of some representative quassinoids, eurycomanone (93), bruceine D (131), dehydrobruceine B (143), and brusatol (115) (Fig. 9) has been explored deeply.

Eurycomanone (93), the most abundant phytochemical quassinoid in E. longifolia roots, has been reported to exhibit pronounced inhibitory abilities against Jurkat, K562, A549, and human hepato carcinoma (HepG2) cells.36,37) Although there were different opinions on its anti-cancer mechanism, the importance of P53 pathway seems universally recognized. Zakaria et al.65) investigated the in vitro apoptosis-inducing effects towards HepG2, and found that 93 could promote expression of pro-apoptotic Bax and downregulate levels of anti-apoptotic Bcl-2 proteins, which were closely related to the P53 pathway. Moreover, study of 93 against lung cancer cell tumor markers suggested that P53 tumor suppressor protein and genes, heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1, prohibitin (PHB), annexin 1 (ANX1) and endoplasmic reticulum protein 28 (ERp28) as well as their related mRNA expressions were reduced obviously.66)
Bruceine D (131) is a major active quassinoid in B. javanica with excellent anti-proliferative activity on pancreatic cancer cells. The mitochondrial apoptotic and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways were considered to be significant routes in its cytotoxicity on pancreatic cancer cells. According to the results of cell cycle analysis, 131 was found to induce cellular apoptosis in Capan-2 cells through the mitochondrial pathway.67) Moreover, 131 was reported to induce apoptosis in PanCa cells by inhibiting activation of the PI3K/Akt signal pathway and reactive oxygen species (ROS) generation.68)
Dehydrobruceine B (143), also obtained from B. javanica, was found to possess cytotoxicity towards human lung cancer cell lines, A549 and NCI-H292.69,70) It could decrease cell viability and the level of mitochondrial membrane potential (MMP), at the same time, the concentration of cytochrome C in cytosol increased, and caspase-3, caspase-9, and poly(ADP-ribose) polymerase (PARP) were cleaved.70) On the other hand, it was found that as downregulation of the protein and its target genes of nuclear factor erythroid 2-related factor-2 (Nrf2) occurred, levels of intracellular ROS increased and mitochondrial apoptosis was induced.69) These findings suggested that the mitochondrial apoptotic pathway was a crucial in the anti-lung cancer activity for 143.
The other main quassinoid in B. javanica, brusatol, also displayed significant cytotoxicity against human PanCa cells in a dose- and time-dependent manner. The possible mechanism might be related to the ability of inducing apoptosis, which caused cell cycle arrest at G2/M phase, and inhibited NF-κB p65, Bcl-xL, and PCNA in PanCa cells.71)
In summary, the structures of 190 quassinoids from 2 families, 14 genus plants reported from 2004 to 2018, are summarized and classified according to their aglycones. In the process of summarizing the structures, the relevance between structural characteristics and plant sources was also found and summarized, which may play an important role in the identification of related plants. Meanwhile, the SARs of quassinoids were also discussed. The substituent groups and their positions were found to be the main influencing factors for the SARs. Moreover, because the most important pharmacological activity of quassinoids was anti-cancer, their possible anti-cancer mechanisms were also summarized. The above mentioned SARs and mechanisms will provide good references for the structural modification and transformation of bioactive compounds.
This research was supported by the National Natural Science Foundation of China (81673688; 81173524).
The authors declare no conflict of interest.