2019 年 67 巻 7 号 p. 666-674
2019 年 67 巻 7 号 p. 666-674
Dimeric sesquiterpene thioalkaloids from the rhizomes of Nuphar pumilum exhibited immunosuppressive effects using a sheep erythrocyte plaque forming cell (PFC) assay, as well as an anti-metastasis effect, and rapid apoptosis-inducing effects in tumor cell lines. In particular, dimeric sesquiterpene thioalkaloids with a hydroxy group (6-hydroxythiobinupharidine, 6,6′-dihydroxythiobinupharidine, 6-hydroxythionuphlutine B) showed substantial effects, whereas dimeric sesquiterpene thioalkaloids lacking the hydroxy group (thiobinupharidine, thionuphlutine B, 6′-hydroxythionuphlutine B, neothiobinupharidine, thionuphlutine B β-sulfoxide, neothiobinupharidine β-sulfoxide) and monomeric sesquiterpene alkaloids (nupharidine, 7-epideoxynupharidine, nupharolutine) showed weak activity. In this review, we summarize our studies of the biofunctional effects of these alkaloids.
Nupharis Rhizoma [Japanese name “Senkotsu (川骨)], the dried rhizomes of Nuphar (N.) japonicum DC. (Nymphaceae), is listed in Japanese Pharmacopea XVII and has been prescribed in Japanese traditional prescriptions as an antipyretic, analgesic, and anti-inflammatory. In contrast, Chinese Nupharis Rhizoma [Chinese name “Ping peng cao gen (萍蓬草根)], the dried rhizomes of N. pumilum (TIMM.) DC., has been prescribed as a tonic, a haemostatic, and for diuretic purposes in traditional Chinese medicine.
In the course of our studies of natural medicines originating from aquatic plants, we isolated thiohemiaminal-type dimeric sesquiterpene thioalkaloids such as 6-hydroxythiobinupharidine (2), 6,6′-dihydroxythiobinupharidine (3), 6-hydroxythionuphlutine B (5), and 6′-hydroxythionuphlutine B (6) from the rhizomes of N. pumilum collected in China or Russia, and found a new rearrangement reaction of the thiaspirane ring in thiohemiaminal-type alkaloids with the 6-hydroxy group.1) In addition, we reported thiaspirane sulfoxide-type dimeric sesquiterpene alkaloids, named nupharpumilamines A–D (11, 13, 15, 16).2) From the rhizome of N. japonicus, nupharidine (17) and deoxynupharidine (18) were characterized more than half a century ago.3–5) As pharmacological studies of nuphar alkaloids, the central paralysis effect of deoxynupharidine (18),6) the anti-fungal, anti-methicillin-resistant Staphylococcus aureus (MRSA), and anti-vancomycin-resistant enterococci (VRE) activities of 3,7–9) and the immunosuppressive,10,11) anti-invasion and/or anti-metastatic,12) and rapid apoptosis-inducing effects of 2, 3 and 5 in several tumor cell lines,13) as well as cytotoxic effects on several multidrug resistant cell lines14) of 2, 3 and 5 have been reported to date. In this review, we summarize our previous studies of dimeric sesquiterpene thioalkaloids with a thiaspirane structure, including recent developments by other groups for the use of these as anti-cancer agents.
With regard to dimeric thiaspirane-type sesquiterpene alkaloids, during the 1970s, LaLonde et al. originally reported the isolation and chemical determination of 6-hydroxythiobinupharidine (2), 6,6′-dihydroxythiobinupharidine (3), thionuphlutine B (4), 6-hydroxythionuphlutine B (5), 6′-hydroxythionuphlutine B (6), 6,6′-dihydroxythionuphlutine B (7), and neothiobinupharidine (8) from the rhizome of Nuphar plants such as N. luteum.15–18) With regard to sulfoxide-type dimeric sesquiterpene alkaloids, Wróbel et al. previously reported on thionuphrutin B β-sulfoxide (12) and neothiobinupharidine B β-sulfoxide (14).19–22) Iwanow et al. also reported on syn-thiobinupharidine sulfoxide (9) and anti-thiobinupharidine sulfoxide (10),23) as well as thiobinupharidine (1) derived from 2 by reduction with sodium borohydride.24) Several monomeric sesquiterpene alkaloids, nupharidine (17), deoxynupharidine (18), 7-epideoxynupharidine (19), nupharolutine (20), (−)-anhydronupharamine (21), were isolated from the rhizomes of N. japonicum.3–5,25–29) The chemical structures of these alkaloids 1–21 are shown in Fig. 1.

The production of antigen-specific antibodies represents a major defense mechanism in humoral immune responses, but their overproduction is one cause of allergies. Several assays have been developed to assess T-cell-dependent antibody responses. Of these assays, the antibody forming cell (AFC) assay or PFC assay and enzyme-linked immunosorbent assay (ELISA) have been used to assess immunosuppression.
To find new biofunctionalities of rhizomes of N. pumilum, we investigated the effects of a methanolic (MeOH) extract and alkaloid fraction from the dried rhizomes on the immune system via PFC assay using SRBC. As a result, both the extract and alkaloid fraction were found to inhibit antibody formation from SRBC-immunized splenocytes.11)
The alkaloid fraction significantly inhibited the primary response in PFC formation, from 0.1 to 10 µg/mL. Hydrocortisone and cyclosporin A potently inhibited the PFC formation, from 0.01 to 1 µM (Fig. 2). Monomeric sesquiterpene alkaloids (17–19) and dimeric sesquiterpene thioalkaloids (1, 4, 8) lacking the hydroxy group showed no significant effect. 7-Epideoxynupharidine (19, 0.01–1 µM) tended to increase the number of PFC, while nuphalorutine (20), a monomeric sesquiterpene possessing a hydroxy group at the 7-position, tended to suppress the number of PFC. However, the differences were not significant. On the other hand, dimeric sesquiterpene thioalkaloids [6-hydroxythiobinupharidine (2), 6,6′-dihydroxythiobinupharidine (3), 6-hydroxythionuphlutine B (5), 6′-hydroxythionuphlutine B (6), 6,6′-dihydroxythionuphlutine B (7)] having the hydroxy group in the quinolizidine ring, were found to suppress the PFC formation at 1 µM.11)

Mouse spleen was removed, and a splenocyte suspension (8 × 106 cells/mL) in RPMI-1640 medium was prepared and seeded in a 24-well multi-plate. Then, SRBC (8 × 106 cells/mL), 50 µM 2-mercaptoethanol and test samples dissolved in DMSO were added to the medium, and the whole was cultured at 37°C in a 5% CO2 atmosphere. The final concentration of DMSO was adjusted to less than 0.01%. Four days later, cells were resuspended in 160 µL of new medium, and 20 µL of diluted (×2) guinea pig complements and a similar volume of 30% (v/v) SRBC were added. The whole mixture was transferred into a Cunningham’s chamber and incubated at 37°C for 2 h. After incubation, the numbers of PFCs were counted under a microscope (×40). Each column represents the mean and standard error of the mean (S.E.M.) (n = 3–6), and the column pattern shows the concentration of each sample, □: 0.01, ■: 0.1, ■: 1 µM, respectively. Significantly different from the control: * p < 0.05, ** p < 0.01. a) Data were taken from ref. 11.
Next, the dimeric sesquiterpene thioalkaloids (2, 3, 5–7) that inhibited the primary PFC formation were examined for secondary PFC formation. As a result, all compounds completely suppressed the PFC formation at 1 µM.11) From this observation, the active compounds (2, 3, 5–7) appeared to directly suppress anti-SRBC antibody production in immunized B lymphocytes.
Finally, the trypan blue dye exclusion test and WST-1 assay were performed to clarify whether the immunosuppressive activities of dimeric sesquiterpene thioalkaloids depended on their cytotoxic effects. Splenocytes were cultured for 4 d with the test compounds. When splenocytes were cultured with 1 µM of each test compound for 4 d, compounds 3 and 7 and hydrocortisone slightly decreased the number of viable cells. However, the other compounds did not affect the rate of the viable cells.11) On the other hand, in an assay method for the evaluation of cell viability (WST-1 assay), two dimeric sesquiterpene thioalkaloids possessing two hydroxy groups (3, 7) at 1 µM significantly reduced the formazan formation following WST-1 uptake. The activities of these alkaloids were weaker than those of hydrocortisone and cyclosporine A.11) These findings suggested that the direct suppressive effects of the dimeric sesquiterpene thioalkaloids possessing two hydroxy groups on cell viability might be partly involved in their suppressive activities in antibody production.
In conclusion, the MeOH extract from the dried rhizome of N. pumilum suppressed antibody formation from mouse splenocytes, and four dimeric sesquiterpene thioalkaloids, 6-hydroxythiobinupharidine (2), 6,6′-dihydroxythiobinupharidine (3), 6-hydroxythionuphlutine B (5), and 6′-hydroxythionuphlutine B (6), and 6,6′-dihydrothionuphlutine B (7) were isolated as active principles. The hydroxy group in the quinolizidine ring appeared to be essential for this immunosuppressive activity, and an increase in the hydroxy groups may enhance their cytotoxic effects.
Metastatis of cancer, which is the major cause of death in cancer patients, occurs through a complex multistep process consisting of invasion into the circulation from the primary tumor, immigration to distant organs, adhesion to endothelial cells, and infiltration into the tissue. Therefore, its blockade has been considered essential to enhancing the survival of cancer patients.
4.1. Effects on the Invasion of B16 Melanoma CellsIn the continuing study of new biofunctional effects of nuphar alkaloids, we reported their effects on the invasion of B16 melanoma 4A5 cells across collagen-coated filters in vitro, and inhibitory effects of the alkaloid fraction and the principal dimeric sesquiterpene thioalkaloid 2 on lung metastasis of B16 melanoma cells in mice.12)
Effects of nuphar alkaloids (1–3, 5, 8, 9, 12, 14, 17–20) on the invasion of B16 melanoma 4A5 cells were examined. As a result, a reference compound, curcumin, strongly inhibited the invasion with an IC50 value of 20 µM. Those dimeric sesquiterpene thioalkaloids lacking the hydroxy group in the quinolizidine ring [neothiobinupharidine (8), syn-thiobinupharidine sulfoxide (9), thionuphlutine B β-sulfoxide (12), neothiobinupharidine β-sulfoxide (14)] and monomeric sesquiterpene alkaloids [nupharidine (17), deoxynupharidine (18), 7-epideoxynupharidine (19), and nupharolutine (20)] showed weak activity. On the other hand, dimeric sesquiterpene thioalkaloids with the hydroxy group at the 6-position [6-hydroxythiobinupharidine (2), 6,6′-dihydroxythiobinupharidine (3) and 6-hydroxythionuphlutine B (5)] were found to show strong activity, with IC50 values of 0.029, 0.087, and 0.36 µM, respectively. However, thiobinupharidine (1) derived from 2 lacked activity12) (Table 1). Similarly to the immunosuppressive effects of nuphar alkaloids, the thiohemiaminal structure with the hydroxy group at the 6-position is essential for strong activity.
| Inhibition (%) | |||||
|---|---|---|---|---|---|
| 0.01(µM) | 0.1 (µM) | 1 (µM) | 10 (µM) | 100 (µM) | |
| Thiobinupharidine (1) | — | 5.2 ± 17 | 9.7 ± 8.3 | 15.6 ± 5.3 | 22.7 ± 8.8† |
| 6-Hydroxythiobinupharidine (2) | 50.9 ± 5.5** | 57.5 ± 3.1** | 66.5 ± 1.8** | 84.8 ± 0.3**,† | 86.8 ± 2.4**,† |
| 6,6′-Dihydroxythiobinupharidine (3) | 40.3 ± 4.1** | 43.1 ± 6.9** | 68.5 ± 3.1** | 83.2 ± 2.3**,† | 86.6 ± 1.5**,† |
| 6-Hydroxythionuphlutine B (5) | 5.9 ± 14.6 | 31.6 ± 11.9 | 62.7 ± 2.2** | 61.4 ± 3.6**,† | 47.1 ± 4.6**,† |
| Neothiobinupharidine (8) | — | 11.9 ± 6.8 | 18.9 ± 4.1 | 33.2 ± 4.1 | 28.3 ± 2.1† |
| syn-Thiobinupharidine sulfoxide (9) | — | 16.9 ± 11 | 38.3 ± 14.1 | 42.2 ± 5.1 | 32.5 ± 5.3† |
| Thionuphlutine B β-sulfoxide (12) | — | −8.1 ± 4.5 | 10.2 ± 1.5 | 9.2 ± 10.5 | 0.0 ± 4.5 |
| Neothiobinupharidin β-sulfoxide (14) | — | 10.6 ± 5.0 | 18.5 ± 2.2 | 12.8 ± 3.5 | 15.7 ± 4.6 |
| Nupharidine (17) | — | 19.6 ± 3.1 | 34.7 ± 2.7** | 41.4 ± 2.5** | 47.2 ± 3.8** |
| Deoxynupharidine (18) | — | −5.1 ± 4.9 | 14.0 ± 4.7 | 28.5 ± 5.2 | 19.1 ± 2.6 |
| 7-Epideoxynupharidine (19) | — | 5.1 ± 3.7 | 25.3 ± 3.7 | 20.9 ± 1.3 | 23.0 ± 4.1 |
| Nupharolutine (20) | — | 25.5 ± 9.6 | 1.7 ± 4.8 | 16.8 ± 11.5 | −0.6 ± 10.3 |
The invasion assay of B16 melanoma 4A5 cells was performed using Cell Culture Insert™ and 24-well microplates. The upper side of each filter of Cell Culture Insert™ was pre-coated with collagen (type I). Cell Culture Insert™ with collagen-coated filters were inserted into the 24-well multi-plates with 700 µL/well of DMEM [FBS (+)], and these were pre-incubated for 20 min. A mixture of B16 melanoma 4A5 cells (5 × 105 cells) suspended in 150 µL DMEM [FBS (−)] and test sample solution in 150 µL DMEM [FBS (−)] were then added onto the filters and incubated for 24 h. After incubation, the cells crossing the filters were fixed with 25% glutaraldehyde solution and stained with crystal violet, and the numbers of cells were counted under a microscope. The test sample was dissolved in dimethylsulfoxide (DMSO), and the final concentration of DMSO in the medium was 0.1%. All experiments were performed in quadruplicate, and results are expressed as the percentage inhibition of invasion. Each value represents the mean ± S.E.M. (n = 4). Significantly different from the control, ** p < 0.01. † Cytotoxic effect was observed. a) Data were taken from ref. 12.
Next, we reported the effects of the alkaloid fraction and the principal dimeric sesquiterpene thioalkaloid, 2, on the metastasis of B16 melanoma in mice.12) B16 melanoma is a highly lung metastatic cell line derived from B16 melanoma 4A5 obtained by the in vivo selection method.
Ten days after injection of the melanoma cells, approx. 20 colonies of the cells were observed on the surfaces of both lungs in mice. As shown in Table 2, the alkaloid fraction (20 mg/kg/d, per os (p.o.)) and 2 (5 mg/kg/d, p.o.) strongly suppressed the lung metastasis of the cells, with inhibition of 93 and 94%, respectively.12)
| Dose (mg/kg, p.o.) | n | Numbers of colonies | Inhibition (%) | |
|---|---|---|---|---|
| Control | — | 6 | 19.5 ± 5.3 | — |
| Alkaloid fraction | 20 | 5 | 1.4 ± 0.5** | 93 |
| 6-Hydroxythiobinupharidine (2) | 5 | 6 | 1.2 ± 0.7** | 94 |
| Curcumin | 20 | 6 | 2.5 ± 0.6** | 87 |
Highly metastatic B16 melanoma cells were obtained from B16 melanoma 4A5 in vivo selection. The cells were resuspended to a concentration of 1 × 106 cells/mL in PBS. Mice were given an intravenous injection of the melanoma cells (5 × 105 cells/200 µL). Ten days later, the mice were killed, the lungs were excised, and tumor colonies were counted. Curcumin was used as a reference compound. Each test compound was given orally once a day. Each value represents the mean ± S.E.M. Significantly different from the control, ** p < 0.01. a) Data were taken from ref. 12.
In our previous study, we reported the cytotoxic effects of a methanolic extract and alkaloid fraction from the rhizomes of N. pumilum on a human leukemia cell (U937), mouse melanoma cell (B16F10), and human fibrosarcoma (HT1080).13)
The cytotoxic effects of nuphar alkaloids (1–12, 14), and a reference compound, camptothecin, on the tumor cell lines are summarized in Table 3. Camptothecin showed strong cytotoxic effects in a concentration and time-dependent manner, but did not produce complete cell death (inhibition of cell viability: 60.6–75.0%) after incubation for 24 h, even at a high concentration (10 µM). The dimeric sesquiterpene thioalkaloids lacking the hydroxyl group at the 6-position [6′-hydroxythionuphlutine B (6), neothiobinupharidine (8), thionuphlutine B β-sulfoxide (12), neothiobinupharidine β-sulfoxide (14)], and monomeric sesquiterpene alkaloids [nupharidine (17), 7-epideoxynupharidine (19), and nupharolutine (20)] showed weak activity at 10 µM after incubation for 72 h. On the other hand, dimeric sesquiterpene thioalkaloids with the 6-hydroxy group in the quinolizidine ring [6-hydroxythiobinupharidine (2), 6,6′-dihydroxythiobinupharidine (3), and 6-hydroxythionuphlutine B (5)] were found to show substantial cytotoxic effects at 10 µM, and their cytotoxic effects at 24 h were almost equivalent to that of camptothecin. Furthermore, thiobinupharidine (1) and thionuphlutine B (4) derived from 2 and 5 also lacked the activity. These findings suggest that the thiohemiaminal structure with the hydroxy group at the 6-position is essential for strong activity.
| Cell line | Incubation time (h) | Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| 0 (µM) | 0.1 (µM) | 1.0 (µM) | 10 (µM) | |||
| Thiobinupharidine (1) | ||||||
| U937 | 72 | 0.0 ± 2.1 | — | 6.9 ± 2.2 | 10.9 ± 3.1** | |
| B16F10 | 72 | 0.0 ± 1.7 | 6.8 ± 1.2 | 7.9 ± 0.7 | 4.4 ± 1.5 | |
| HT1080 | 72 | 0.0 ± 1.1 | 9.9 ± 1.4 | 6.3 ± 1.1 | −11.7 ± 1.9** | |
| 6-Hydroxythiobinupharidine (2) | ||||||
| U937 | 24 | 0.0 ± 0.3 | — | 3.6 ± 2.4 | 83.2 ± 0.2** | |
| 48 | 0.0 ± 0.6 | — | 1.3 ± 1.0 | 92.5 ± 0.0** | ||
| 72 | 0.0 ± 0.4 | — | −1.0 ± 0.8 | 94.3 ± 0.1** | ||
| B16F10 | 24 | 0.0 ± 2.8 | — | — | 88.6 ± 0.3** | |
| 48 | 0.0 ± 5.4 | 10.3 ± 2.4 | 27.3 ± 3.9** | 97.3 ± 0.4** | ||
| 72 | 0.0 ± 1.2 | 11.1 ± 1.7** | 20.8 ± 3.5** | 98.7 ± 0.2** | ||
| HT1080 | 24 | 0.0 ± 2.3 | 1.8 ± 2.3 | 14.0 ± 2.3** | 96.1 ± 0.1** | |
| 48 | 0.0 ± 2.7 | 1.6 ± 2.7 | 8.3 ± 2.7 | 97.5 ± 0.3** | ||
| 72 | 0.0 ± 1.3 | 5.4 ± 1.6 | 15.7 ± 2.3** | 99.2 ± 0.6** | ||
| 6,6′-Hydroxythiobinupharidine (3) | ||||||
| U937 | 24 | 0.0 ± 2.2 | — | 5.3 ± 1.1 | 54.7 ± 0.6** | |
| 48 | 0.0 ± 0.7 | — | 2.3 ± 1.7 | 80.0 ± 0.6** | ||
| 72 | 0.0 ± 1.1 | — | 1.3 ± 1.2 | 81.6 ± 3.1** | ||
| B16F10 | 24 | 0.0 ± 2.0 | — | — | 16.6 ± 4.1** | |
| 48 | 0.0 ± 2.2 | −8.0 ± 1.3 | −15.0 ± 1.8 | 51.8 ± 3.1** | ||
| 72 | 0.0 ± 1.4 | −3.5 ± 2.0 | −6.0 ± 2.6 | 54.8 ± 2.3** | ||
| HT1080 | 24 | 0.0 ± 2.7 | — | — | 19.3 ± 2.7** | |
| 48 | 0.0 ± 2.7 | 9.0 ± 3.3 | 0.5 ± 0.6 | 75.0 ± 1.2** | ||
| 72 | 0.0 ± 1.4 | 0.1 ± 1.6 | −0.9 ± 1.6 | 75.3 ± 2.1** | ||
| Thionuphlutine B (4) | ||||||
| U937 | 72 | 0.0 ± 2.1 | — | 9.1 ± 0.9 | 7.8 ± 2.4 | |
| B16F10 | 72 | 0.0 ± 0.9 | 1.7 ± 1.0 | −2.2 ± 1.4 | 2.2 ± 1.7 | |
| HT1080 | 72 | 0.0 ± 1.1 | −10.8 ± 1.3** | 0.9 ± 2.3 | −12.0 ± 2.8** | |
| 6-Hydroxythionuphlutine B (5) | ||||||
| U937 | 24 | 0.0 ± 2.2 | — | 74.6 ± 0.6** | 80.0 ± 0.3** | |
| 48 | 0.0 ± 0.7 | — | 89.0 ± 0.7** | 91.3 ± 0.1** | ||
| 72 | 0.0 ± 1.1 | — | 85.3 ± 3.6** | 94.1 ± 0.1** | ||
| B16F10 | 24 | 0.0 ± 3.9 | — | −22.9 ± 9.8 | 91.5 ± 0.6** | |
| 48 | 0.0 ± 2.8 | 0.3 ± 2.0 | 65.0 ± 0.4** | 98.2 ± 0.1** | ||
| 72 | 0.0 ± 1.3 | 0.9 ± 3.4 | 69.3 ± 2.0** | 99.1 ± 0.2** | ||
| HT1080 | 24 | 0.0 ± 0.6 | 6.3 ± 3.1 | 42.0 ± 3.1** | 95.9 ± 0.6** | |
| 48 | 0.0 ± 1.4 | 8.6 ± 1.4 | 53.3 ± 2.8** | 98.0 ± 0.1** | ||
| 72 | 0.0 ± 1.1 | 1.0 ± 1.6 | 63.5 ± 2.2** | 98.7 ± 0.1** | ||
| 6′-Hydroxythionuphlutine B (6) | ||||||
| U937 | 72 | 0.0 ± 1.1 | — | 0.1 ± 1.1 | 0.5 ± 0.3 | |
| B16F10 | 72 | 0.0 ± 1.4 | 1.7 ± 1.2 | −1.6 ± 1.2 | −4.8 ± 1.2 | |
| HT1080 | 72 | 0.0 ± 2.1 | −9.8 ± 3.5 | −7.3 ± 3.1 | −9.1 ± 2.8 | |
| Cell line | Incubation time (h) | 0 (µM) | 0.03 (µM) | 0.1 (µM) | 1.0 (µM) | 10 (µM) |
| Camptothecin | ||||||
| U937 | 24 | 0.0 ± 1.2 | 7.1 ± 1.8 | 46.0 ± 0.7** | 75.8 ± 0.7** | 75.0 ± 0.8** |
| 48 | 0.0 ± 1.1 | 61.9 ± 0.9** | 90.6 ± 0.4** | 94.6 ± 0.4** | 98.2 ± 0.1** | |
| 72 | 0.0 ± 2.9 | 78.5 ± 0.8** | 95.3 ± 0.3** | 98.2 ± 0.1** | 98.0 ± 0.1** | |
| B16F10 | 24 | 0.0 ± 4.8 | −1.9 ± 18.4 | 16.2 ± 9.3 | 47.8 ± 2.0** | 60.6 ± 1.7** |
| 48 | 0.0 ± 5.8 | 7.5 ± 7.7 | 39.1 ± 1.5** | 83.2 ± 1.6** | 94.7 ± 0.3** | |
| 72 | 0.0 ± 1.9 | 33.4 ± 1.3** | 76.9 ± 0.8** | 93.5 ± 0.1** | 98.1 ± 0.1** | |
| HT1080 | 24 | 0.0 ± 1.5 | −3.8 ± 1.0 | −0.4 ± 2.5 | 49.5 ± 1.9** | 62.0 ± 1.8** |
| 48 | 0.0 ± 1.9 | −7.1 ± 11.7 | −11.7 ± 4.6 | 77.0 ± 0.8** | 93.1 ± 0.6** | |
| 72 | 0.0 ± 5.5 | −3.2 ± 3.7 | 5.4 ± 1.8 | 65.8 ± 2.1** | 93.8 ± 0.6** | |
U937 cells (5 × 103 cells/well) were seeded onto 96-well microplates in 100 µL of RPMI medium containing 10% FBS, penicillin (100 unit/mL), and streptomycin (100 µg/mL). After incubation at 37°C for 20, 44, and 68 h, with or without the test compound, the viability of the cells was determined using Cell Counting Kit-8 (CCK-8, Dojindo Co., Ltd.) according to the manufacturer’s instruction. Similarly, B16F10 (2.5 × 103 cells/100 µL of RPMI/well) and HT1080 (5 × 103 cells/100 µL of MEM/well) were seeded onto 96-well microplates in 100 µL of each medium containing 10% FBS, penicillin (100 unit/mL), and streptomycin (100 µg/mL). After incubation at 37°C for 20, 44, and 68 h with the test compound, 10 µL of MTT (5 mg/mL in PBS) was added to each well. After a further 4 h in culture, the medium was removed, and isopropanol containing 0.04 M HCl was then added to dissolve the formazan produced by the cells. The optical density of the formazan solution was measured with a microplate reader at 570 nm (reference: 655 nm). The test compound was dissolved in dimethylsulfoxide (DMSO), and the final concentration of DMSO in the medium was 0.1%. Results are expressed as the percentage inhibition of cell proliferation. Each value represents the mean ± S.E.M. (n = 4–6). Significantly different from the control: ** p < 0.01. a) Data were taken from ref.13.
Apoptosis plays an important role in the maintenance of tissue homeostasis by the selective elimination of excessive cells. In addition, the induction of apoptosis of cancer cells is also recognized as useful for cancer treatment, since cytotoxic drugs (e.g. etoposide, cisplatin, and paclitaxel) used in the chemotherapy of leukemias and solid tumors are known to cause apoptosis in the target cells. Therefore, the apoptosis-inducing effect of 6-hydroxythiobinupharidine (2) in U937 cells was examined.
To determine the apoptosis-inducing effect of a principal dimeric sesquiterpene thioalkaloid, 2, we examined the morphological changes in U937 after treatment with 2. We found that the characteristic breakdown of the cells into smaller units (apoptotic bodies) was observed in U937 at 5 and 10 µM at 1 h after treatment of 2. On the other hand, the reference compound camptothecin, even at a high concentration (10 µM), exhibited the characteristic changes 4 h after the treatment.13)
Furthermore, the apoptosis-inducing effect of 2 in U937 cells was examined using the terminal deoxynucleotidyl transferase biotin-deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assay (APO-BRDU™, BD Biosciences). As shown in Fig. 3, the TUNEL-positive cells (number of cells in M2 area >57%) were observed 1 h after treatment with 2 at 2.5–10 µM, but the TUNEL-positive cells were not observed at 1 µM after 24 h (cells in M2 area <1.2%). These results indicated that DNA fragmentation, which is characteristic of apoptotic cells, was observed after treatment of 2. On the other hand, camptothecin at 5 µM induced apoptosis of the cells by approx. 70% after 24 h, similar to the result of the cytotoxic assay.13)

TUNEL assay (APO-BRDU™, BD Biosciences) was performed according to the manufacturer’s instruction. Briefly, U937 cells (5 × 105 cells/5 mL/culture flask) in RPMI1640 containing 10% FBS with or without test compound were incubated at 37°C for 1 to 24 h in 5% CO2 atmosphere. After incubation, the U937 cells were fixed and stained with fluorescein-labelled BrdU and propidium iodide (PI). After incubation for 30 min at room temperature, the fluorescence intensity of each cell was analyzed by flow cytometry (FACSCalibur, Becton Dickinson). M1 and M2 indicate TUNEL-negative and positive cells, respectively. a) Data were taken from ref. 13.
In conclusion, we first reported that dimeric sesquiterpene thioalkaloids (2, 3, 5) exhibited rapid apoptosis-inducing activity within 1 h,13) and now suggest these active dimeric sesquiterpene thioalkaloids as new candidates to act as chemopreventive agents for cancers.
Recently, synthetic studies of nuphar alkaloids, including unnatural-type alkaloids, have been reported,30–36) and several of these studies are aimed at developing new anti-cancer agents. Despite the unique stereostructures of dimeric sesquiterpene thioalkaloids have been determined in the 1970s, their total synthesis was not completed until 2013 by Jansen and Shenvi30) and Korotkov et al. in 2015.31) Korotkov et al. first reported the total synthesis of (+)-6-hydroxythiobinupharidine (2), (+)-6,6′-dihydroxythiobinupharidine (3), and (−)-6,6′-dihydroxythionuphlutine, and they also reported the rapid apoptosis-inducing effects of them.31) In addition, they reported the discovery of the apoptically active monomeric nuphar alkaloid analogues, including the more potent than natural (+)-6-hydroxythiobinupharidine (2).32)
As a mechanism of action, we estimated the involvement of caspases 3 and 8 in preliminary experiments using several caspase inhibitors.13) However, recently, Mallick et al. clearly demonstrated that the mechanism of apoptosis by 2 involves the activation of caspase 9 and caspase 3, but not caspase 8. The release of cytochrome c from mitochondria occurred even in the absence of pro-apoptotic proteins BAX/BAK, and in cells that retained mitochondrial membrane potential.37) Furthermore, Ozer et al. reported that a nuphar extract inhibited nuclear factor-kappaB (NF-κB),38) but molecular targets of the nuphar alkaloids remain unknown.
In our previous study, we found a rearrangement of the thiospirane ring specific to thiohemiaminal type nuphar alkaloids with a 6-hydroxy group, and presumed that the thio-ether group of 2 and 3 might initially abstract a hydrogen atom from the neighboring 6-hydroxy group to form an epoxide intermediate with a thiol group, which would give the rearranged products 5 and 7, although some other pathway could be considered.2) We also considered that the intermediate might be the active form in the apoptosis-inducing effects. In contrast, Tada et al. have proposed that the sulfur atom of thiospirane iminium pharmacore reacts as an electrophile with nucleophilic thiols, and that sulfur-triggered retrodimerization can occur in the cells, which is then responsible for apoptosis.33)
Li et al. recently reported on the stereoselective synthesis and biological evaluation of C1-epimeric and desmethyl monomeric nuphar alkaloid analogs. They revealed that the desmethyl and C1-epimeric monomeric nuphar analogs are able to induce rapid apoptosis.35)
In the past, several anti-cancer drugs derived from plants have been used, such as taxanes, Vinca alkaloids, camptothecin and epi-podophyllotoxin. However, multi-drug resistance (MDR) has been a fatal problem in the development of anti-cancer drugs.39,40) Mechanisms of MDR include the overexpression of P-glycoprotein (P-gp) and specific gene mutations. Especially, P-gp is a well-known ATP-binding cassette (ABC) drug transporter, mediating resistance to a plethora of xenobiotic compounds.41)
To compare cytotoxic activity, we screened the cytotoxicity of nuphar alkaloids (1–4, 8–10, 17, 18, 21) toward drug-sensitive CCRF-CEM cells using the resazurin reduction assay. Dimeric sesquiterpene thioalkaloids (1–4, 8–10) isolated from N. pumilium showed stronger inhibition than monomeric sesquiterpenes (17, 18, 21) isolated from N. japonicum (Table 4). IC50 values and resistance degrees for seven active compounds (1–4, 8–10) were calculated using drug-sensitive CCRF-CEM cells and drug-resistant CEM/ADR5000 cells. 6,6′-Dihydroxythiobinupharidine (3) revealed weaker cytotoxic effects than doxorubicin toward CCRF-CEM cells. On the other hand, dimeric sesquiterpene alkaloids (1–4, 8–10) showed stronger cytotoxic effects than doxorubicin toward CEM/ADR5000 cells. In addition, dose-response curves showed that compounds 1–4 were more effective toward CCRF-CEM cells than CEM/ADR5000 cells. This finding suggested that the multidrug-resistant CEM/ADR5000 cells were cross-resistant to 3. We therefore hypothesized that these compounds are substrates for P-gp, and are extruded from the CEM/ADR5000 cells in a manner similar to doxorubicin.
| Cell lines | Compounds (µM) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| CCRF-CEM | 12.11 ± 0.85* | 1.86 ± 0.14* | 0.201 ± 0.030* | 18.26 ± 4.77* |
| CEM/ADR5000 | 19.99 ± 2.29* | 3.90 ± 0.39* | 1.14 ± 0.10* | 35.89 ± 2.39* |
| Degree of resistance | 1.65 | 2.10 | 5.33 | 1.97 |
| HCT116 (p53+/+) | 39.35 ± 7.15* | 6.36 ± 1.04* | 1.43 ± 0.52* | 55.42 ± 3.27* |
| HCT116 (p53−/−) | 47.21 ± 7.28* | 6.96 ± 1.56* | 1.72 ± 0.49* | 67.19 ± 13.40* |
| Degree of resistance | 1.20 | 1.10 | 1.20 | 1.21 |
| HEK293 | 43.44 ± 6.85* | 5.87 ± 1.05* | 1.33 ± 0.61* | 50.61 ± 6.23* |
| HEK293-ABCB5 | 41.32 ± 4.45* | 9.43 ± 1.76* | 2.62 ± 1.42* | 50.62 ± 1.28* |
| Degree of resistance | 0.95 | 1.61 | 1.97 | 1.00 |
| U87MG | 81.85 ± 5.89* | 8.34 ± 0.94* | 5.63 ± 4.08* | 45.21 ± 1.26* |
| U87MG.ΔEGFR | 49.73 ± 10.59* | 10.44 ± 1.64* | 4.44 ± 1.49* | 53.34 ± 9.66* |
| Degree of resistance | 0.61 | 1.25 | 0.79 | 1.18 |
| MDA-MB-231-pcDNA | 46.82 ± 6.17* | 7.26 ± 2.28* | 2.00 ± 0.66* | 45.49 ± 3.37* |
| MDA-MB-231-BCRP | 52.59 ± 6.87* | 8.98 ± 0.12* | 6.15 ± 1.79* | 53.79 ± 4.89* |
| Degree of resistance | 1.12 | 1.24 | 3.08 | 1.18 |
| Cell lines | Compounds (µM) | Dox (µM) | ||
| 8 | 9 | 10 | ||
| CCRF-CEM | 41.1 ± 3.83* | 17.89 ± 1.56* | 13.99 ± 0.60* | 0.028 ± 0.005* |
| CEM/ADR5000 | 38.4 ± 2.15* | 23.48 ± 0.76* | 19.87 ± 2.68* | 65.86 ± 4.35* |
| Degree of resistance | 0.92 | 1.31 | 1.42 | 2192 |
| HCT116 (p53+/+) | >100 | 34.89 ± 2.26* | 27.61 ± 2.70* | 0.125 ± 0.014* |
| HCT116 (p53−/−) | >100 | 36.36 ± 0.96* | 30.13 ± 3.96* | 0.520 ± 0.029* |
| Degree of resistance | — | 1.04 | 1.09 | 4.14 |
| HEK293 | >100 | 32.39 ± 7.96* | 33.73 ± 4.80* | 0.067 ± 0.036* |
| HEK293-ABCB5 | >100 | 32.02 ± 7.20* | 32.61 ± 3.50* | 0.045 ± 0.005* |
| Degree of resistance | — | 0.99 | 0.97 | 0.7 |
| U87MG | >100 | 42.81 ± 4.85* | 39.04 ± 2.91* | 0.044 ± 0.000* |
| U87MG.ΔEGFR | >100 | 36.77 ± 0.20* | 34.74 ± 1.78* | 0.081 ± 0.008* |
| Degree of resistance | — | 0.86 | 0.97 | 1.85 |
| MDA-MB-231-pcDNA | >100 | 38.06 ± 1.22* | 29.80 ± 7.49* | 0.032 ± 0.004* |
| MDA-MB-231-BCRP | >100 | 38.84 ± 1.91* | 22.27 ± 0.52* | 0.087 ± 0.017* |
| Degree of resistance | — | 1.02 | 0.75 | 2.71 |
Samples, IC50 values (µM) and degree of resistance. The degree of resistance was calculated by dividing the IC50 values of resistant cell lines with the IC50 values of sensitive cell lines. Each value represents mean ± S.D. of three independent experiments (* p < 0.05). a) Data were taken from ref. 14.
The dimeric sesquiterpene thioalkaloids (1–4, 8–10) exhibited stronger cytotoxic effects than the monomeric sesquiterpene alkaloids (17, 18, 21). Among the dimeric sesquiterpene thioalkaloids, compounds 2 and 3, having the hydroxy group at the 6-position, showed the strongest cytotoxic effects. There was not a considerable difference between 1 and 4 and between 9 and 10. Neothiobinupharidine (8) exhibited the weakest cytotoxic activity. These results also suggested that the hydroxy group at the 6-position was important to exhibiting cytotoxicity, and that the configurations of the sesquiterpene moiety were also important, similar to the results of our previous studies.10–13) In addition, 3, 1 and 10 were more effective than 2, 4 and 9, respectively. Namely, the hydroxy group at the 6′-position, the configuration of sesquiterpene moiety conjugated with the 2-position of tetrahydrothiophene, and the direction of sulfoxide (syn- or anti-) also affected the cytotoxic effects.
We also examined cytotoxic effects toward other sensitive and resistant cancer cell lines, i.e. HCT116 (p53+/+) colon cancer cells and its knockout clone HCT116 (p53−/−), HEK293 embryonic kidney cells and its transfectant subline HEK293-ABCB5, U87MG glioblastoma cells and its resistant subline U87MG.ΔEGFR, and MDA-MB-231-pcDNA breast cancer cells and its resistant subline MDA-MB-231-BCRP. The dimeric sesquiterpene thioalkaloids exhibited cytotoxic effects against all cell lines (Table 4). Especially, 2 and 3 were equal to or more effective towards sensitive and resistant cell lines than others.
P-gp-overexpressing CEM/ADR5000 cells showed cross-resistance to the dimeric sesquiterpene thioalkaloid 3. Therefore, we calculated the binding energies and simulated the interactions of these compounds to P-gp using the Lamarckian algorithm implemented in the Autodock 4 program. To compare the interactions of the dimeric sesquiterpene thioalkaloids (1–4, 8–10), doxorubicin (Dox) and 5-fluorouracil (5-FU) were used as positive and negative control compounds, respectively. Dox is a known P-gp substrate, and 5-FU is known not to be a P-gp substrate. The minimum predicted binding energy and predicted Ki values are shown in Table 5. The minimum predicted binding energies and predicted Ki values were −6.32 kcal/mol and 23.9 µM (for Dox) and −4.09 kcal/mol and 997.4 µM (for 5-FU). These results indicate that the dimeric sesquiterpene thioalkaloids, as well as Dox, bound to P-gp. The low binding energy of 5-FU indicates that this drug did not bind to P-gp. Dox interacted with the following amino acid residues of P-gp: Phe303, Ile306, Tyr307, Gln725, Phe728, Phe983, Met986, Ala987 and Gln990 (Table 5). Comparing the amino acid residues among the dimeric sesquiterpene thioalkaloids, the amino acid residues listed in Table 5 were involved. These alkaloids (2, 3, 4, 10) had common amino acid residues (Met949, Tyr953, Cys956, Val982 and Met986) and they interacted with the same binding site. In Fig. 4, a binding image of 6,6′-thiobinupharidine (3) and the specific amino acid residues are described as an example. Other natural products and synthetic drugs also bind to this pharmacophore of P-gp.42–44) However, the structure activity relationship was not clarified because each compound bound at a different angle against the binding site. The nuphar alkaloids, with lower values of predicted binding energy and Ki, and which showed lesser cytotoxic effects such as 8, could be useful for the development of P-gp inhibitors.
| Compounds | Binding energy (kcal/mol) | Ki value (nM) | Interacting residues |
|---|---|---|---|
| 1 | −9.2 ± 0.0 | 192.0 ± 7.3 | Leu225, Ser298, Ile299, Ala302, Ile306, Ser309, Ala342, Phe343 |
| 2 | −9.2 ± 0.2 | 177.5 ± 54.1 | Leu861, Ile864, Val865, Ile868, Ala869, Met949, Ser952, Tyr953, Cys956, Phe978, Val982, Ala985, Met986 |
| 3 | −8.8 ± 0.0 | 370 ± 3.1 | Ile864, Val865, Ile868, Met949, Ser952, Tyr953, Cys956, Phe978, Val982, Phe983, Met986 |
| 4 | −9.6 ± 0.1 | 87.5 ± 1.1 | Ile864, Val865, Ile868, Met949, Ser952, Tyr953, Cys956, Phe978, Val982, Phe983, Met986 |
| 8 | −10.3 ± 0.0 | 30.3 ± 0.4 | Ala292, Asn296, Ile299, Gln773, Gly774, Gly778, Lys826, Ser831, Ala834, Val835, Val991, Phe994, Pro996 |
| 9 | −9.4 ± 0.0 | 134.6 ± 2.8 | Met69, Tyr310, Leu339, Gln725, Met949, Tyr953, Phe983 |
| 10 | −9.9 ± 0.0 | 55.3 ± 0.2 | Leu861, Ile864, Val865, Ile868, Gly872, Met949, Ser952, Tyr953, Cys956, Val982, Ala985, Met986, Gly989 |
| Dox | −6.32 ± 0.2 | 23.9 ± 6.6 (µM) | Phe303, Ile306, Tyr307, Gln725, Phe728, Phe983, Met986, Ala987, Gln990 |
| 5-FU | −4.09 ± 0.0 | 997.4 ± 0.8 (µM) |
Lowest binding energies in kcal/mol and predicted inhibition constant (Ki) were determined as well as the interacting amino acids. a) Data were taken from ref.14.
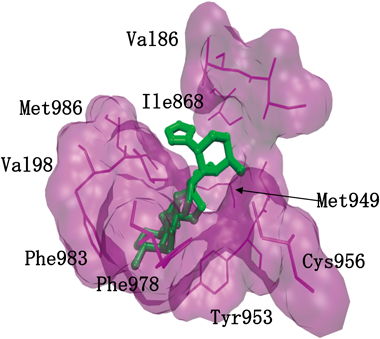
Specific amino acid residues of P-gp with which 3 interacted are presented. a) Data were taken from ref. 14.
Dimeric sesquiterpene thioalkaloids from Nuphar genus plants, having unique stereo structures, were isolated beginning in the 1970s. Recently, several biofunctional effects of these nuphar alkaloids have been reported. Especially, thiohemiaminal-type alkaloids such as 6-hydroxythiobinupharidine (2) and 6,6′-dihydroxythiobinupharidine (3), having a hydroxy group at the 6-position, have been shown to exhibit immunosuppression in PFC assays, an anti-metastasis effect, and rapid apoptosis-inducing effect in tumor cell lines such as U937 cells. Among their biofunctional effects, the rapid apoptosis-inducing effects and effectiveness on MDR cell lines are attractive features in the development of new anti-cancer agents, although the effective concentration range for apoptosis is relatively high (>1 µM). However, the mechanism of action is still unclear. Therefore, the target molecules of these active alkaloids should be clarified.
This research was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities 2015–2019. Tables 4, 5 and Fig. 4 were reproduced from ref. 14 with permission from the Royal Society of Chemistry.
The authors declare no conflict of interest.