2019 Volume 67 Issue 8 Pages 888-895
2019 Volume 67 Issue 8 Pages 888-895
New sugar hydrazones incorporating furan and/or 1,3,4-thiadiazole ring systems were synthesized by reaction of the corresponding hydrazide with different aldose sugars. Heterocyclization of the formed hydrazones afforded the derived acyclic nucleoside analogues possessing the 1,3,4-oxadiazoline as modified nucleobase via acetylation followed by the heterocyclization process. The anticancer activity of the synthesized compounds was studied against human liver carcinoma cell (HepG-2) and at human normal retina pigmented epithelium cells (RPE-1). High activities were revealed by compounds 3, 12 and 14 with IC50 values near to that of the reference drug doxorubicin.
Substituted furans can be employed as building blocks for formation of naturally occurring metabolites and various substituted furans used as pharmaceutical agents.1) Furan containing compounds gained wide attention in the field of medicinal chemistry research due to their activities associated with chemotherapeutic behavior.2) Substituents at the C-2 position afforded derivatives which are greatly distributed in nature; for example, ailanthoidol, a neolignan derivative, has revealed antiviral, antioxidant, and antifungal activities.3) Moreover, a number of furan based compounds exhibited antimicrobial, anticancer, antitumor, anti-inflammatory, and antitubulin activities and are also used for treatment of cardiac arrhythmias.4–8) Furthermore, it has been explored that the tetrahydrofuryl moiety is a basic structural motif in many important C-glycosides9–12) of significant biological and chemical interest. Being a bioisostere of pyrimidine and oxadiazole, thiadiazole compounds have, extensively, found outstanding pharmacological applications. The thiadiazole sulfur atom revealed better liposolubility, and these compounds exhibited the ability to cross cellular membranes and interact with biological targets with prominent affinities due to the mesoionic nature of 1,3,4-thiadiazoles. 1,3,4-Thiadiazoles have been characterized by important biological activities, such as anticancer, antiepileptic, antiviral, antibacterial, antifungal, antidiabetic, analgesic, and anti-inflammatory activities.13–17) Interesting research investigations have indicated that 1,3,4-thiadiazole is a promising motif of great significance in drug discovery research including divers cancer cell lines by inhibition of diversified molecular targets for cancer medication.13–23) As another remarkable hetrocyclic scaffold, 1,3,4-oxadiazole, has extensive important applications in designing promising agrochemicals in addition to the recently reported various bioactivities of related derivatives.24–27) On the other hand, structurally modified nucleosides as acyclic and C-nucleoside analogues revealed a spectrum of medicinal properties, including antibiotic, antiviral, and antitumor activities.28–33) The efficacy of the strategy of combinations of different pharmacophoric motifs in novel hybrid structures for constructing new drugs and our interest in synthesizing potent sugar based heterocycles28,34,35) promoted us to synthesize new compounds incorporating furan, thiadiazole and oxadiazole sugar derivatives as modified acyclic C-nucleoside analogs with investigation of their anticancer activity against human liver carcinoma cell.
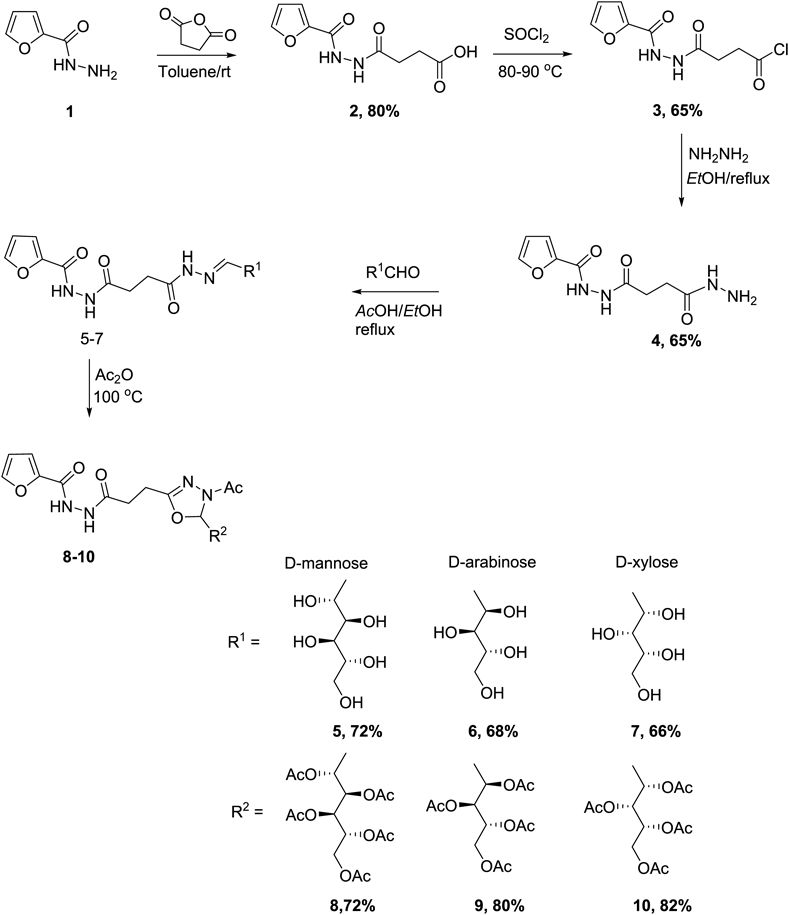
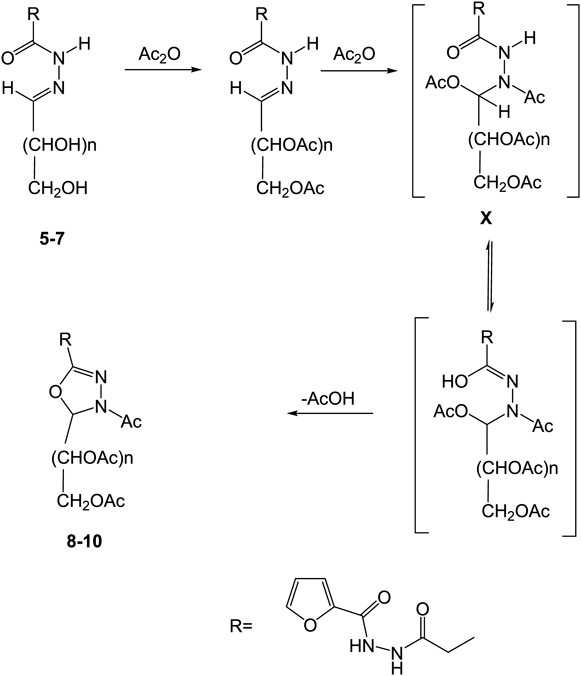
In the current investigation, two types of target hybrid compounds involving furan based 1,3,4-oxadiazole linked to sugar moieties and furan based 1,3,4-thiadiazolyl-1,3,4-oxadiazole attached to sugar units. The synthesis of the first was started with furan-2-carbohydrazide which was converted to the corresponding 4-(2-(furan-2-carbonyl)hydrazinyl)-4-oxobutanoic acid 2 via reaction with succinic anhydride. The succinohydrazide derivative 4 of the resulting hydrazinyl-4-oxobutanoic acid derivative was obtained by reaction of 2 with thionyl chloride to afford the corresponding acid chloride 3 which was then allowed to undergo hydrazinolysis reaction by means of hydrazine hydrate in absolute ethanol. The IR spectra of the afforded compounds showed the characteristic carbonyl functional group absorption band in addition to the NH bands. The 1H-NMR spectra exhibited the signals of the two methylene protons in addition to furyl and NH signals confirming the assigned structures. The integration of the additional heterocycle with the furan ring was based on heterocyclization of the proposed sugar hydrazones of the key acyl hydrazide 4. Thus, reaction of the latter hydrazide 4 with monosacharide aldoses; namely D-mannose, D-arabinose and D-xylose in catalytic acetic acid led to the formation of the derived sugar hydrazones 5–7, respectively in 66–72% yields. The 1H-NMR spectra of the afforded sugar hydrazones demonstrated the absence of the two proton signals of the NH2 group and existence of the attributed signals to the hydroxyl protons as well as the alditol chain protons. The resulting chemical shift of the methine proton (i.e., H-1) and existence of a signal assigned to the NH confirmed the acyclic form feature of the sugar part in the resulting sugar hydrazone as the anomeric hydrogen H-1 in glycosides (possessing cyclic form of the sugar) would be shown at lower shift values. Acetylation of the hydrazones 5–7 by using of acetic anhydride at 100°C, led to the formation of products which have been revealed as 1,3,4-oxadiazoline linked acyclic O-acetylated-sugar derivatives 8–10 via a heterocylization process in addition to acetylation of the reacted sugar hydrazones (cf. Chart 1). This is also in accordance with the mode of their preparation and agrees with our previously reported Heterocyclization of sugar hydrazones by means of acetic anhydride.28,36) The mechanism of this acetylating Heterocyclization leading to the oxadiazoline substituted sugars 8–10 began with the addition of acetic anhydride molecule to the C=N group in the respective hydrazone. Accordingly, the partially positive carbon atom has been attacked by the acetate anion while the acetyl ion was provided to the nitrogen atom to form an intermediate which then readily loosed a molecule of acetic acid to afford the substituted oxadiazoline linked sugar products37) (cf. Chart 2). The bands relating to the carbonyl functionalities have been revealed in the IR spectra of 8–10 in addition to the absence of the characteristic hydroxyl group signals. The 1H-NMR spectra of the later products indicated the existence of signals of the acetyl-methyl and the sugar chain protons as well as the appearance of the signal of H-5 in the oxadiazoline [cf. Exp. Part].
Molecular hybridization, including incorporation of more than heterocyclic system in one hybrid molecule, represents one of the pivotal strategies followed recently for designing new bioactive leads. In the current investigation, another five membered heterocyclic core, 1,3,4-thiadiazole, was incorporated into the final heterocyclic sugar derivatives. Thus, reaction of the acid chloride 3 with ethyl 2-((5-amino-1,3,4-thiadiazol-2-yl)thio)acetate (11)38) led to the formation of the ester functionalized furyl linked 1,3,4-thiadiazolyl derivative 12 in 70% yield. The latter produced ester derivative was converted to the derived acid hydrazide 13 via hydrazinolysis by means of hydrazine hydrate. The 1H-NMR spectrum of the ester 12 showed the characteristic ethyl signals as triplet and quartet for the CH3and CH2 protons, respectively which have been disappeared in the corresponding 1H-NMR spectrum for the hydrazide 13 and instead the NH2 protons signals have been revealed. The target 1,3,4-oxadiazoline sugar derivatives 16 and 17 attached to furyl linked 1,3,4-thiadiazole system were prepared following the synthetic strategy described for preparation of compounds 8–10 via formation of the derived sugar hydrazones 14 and 15 possessing D-mannose and D-arabinose sugars, respectively followed by heterocyclization using acetic anhydride at 100°C (Chart 3). The IR spectra of the hydrazones 14 and 15 exhibited the absorption bands of the hydroxyl groups in the sugar chain at 3381–3438 cm−1 in addition to the amide carbonyl frequency at their characteristic region. The 1H-NMR spectra of the sugar hydrazones showed in addition to all protons according to the assigned structure, the H-1 proton at δ 7.40–7.55 ppm indicating its sp2 nature and accounting for the acyclic structure of the sugar moiety in the produced hydrazones. The IR spectra of the oxadiazole sugar derivatives 16 and 17 showed the carbonyl absorption bands in their characteristic area for the acetyl-carbonyl functionality. Their 1H-NMR spectra revealed the signal at δ 5.88–5.90 ppm attributed to the H-5 in the oxadiazoline ring (originally H-1 in the sugar moiety) indicating that heterocyclization had been taken place in addition to acetylation step, in addition to the acetyl-methyl and NH signals.
In this study, the newly synthesized compounds were examined in vitro for their antitumor activities against HepG 2 and RPE-1 cell lines by applying lactate dehydrogenase (LDH) assay, to investigate the permeabilization (rupture) of the cellular membrane and severe irreversible cell damage. The activity of all new compounds was studied but compounds with very little values (at more than 100 micro-mole) or that had no activity were not involved in the table. In case of HepG-2 liver cancer type, the results showed that, three compounds; 3, 12 and 14 exhibited significantly high anticancer activities compared to Doxorubicin® (positive control) with IC50 values 5.5 ± 1.2, 7.29 ± 1.5 and 4.2 ± 1.2, respectively. A number of synthesized derivatives (5, 7, 9, 13 and 15) showed moderate activities against the latter cancer cell line while, the remaining of the prepared compounds showed weak activities (Fig. 1, Table 1). The observed activity results for the human normal cells revealed that seven synthesized compounds (1, 2, 3, 4, 8, 7 and 12) showed moderate cytotoxicity compared to the positive control whereas the rest of the compounds showed weak cytotoxic effect against the normal cells.

| Compound | HepG2 | RPE-1 |
|---|---|---|
| IC50 (µM) ± S.D. | ||
| 1 | 128.3 ± 7.2 | 55.3 ± 6.7 |
| 2 | 108.5 ± 9.4 | 43.7 ± 5.9 |
| 3 | 5.5 ± 1.2 | 55.4 ± 4.9 |
| 4 | 102.9 ± 10.3 | 68.0 ± 6.5 |
| 5 | 41.3 ± 6.9 | 128.8 ± 12.9 |
| 6 | 70.6 ± 9.2 | 124.1 ± 10.8 |
| 7 | 31.7 ± 4.9 | 64.9 ± 7.3 |
| 8 | 99.9 ± 10.2 | 62.3 ± 5.9 |
| 9 | 52.1 ± 5.5 | 139.8 ± 11.5 |
| 10 | 115.2 ± 14.2 | 99.5 ± 8.9 |
| 12 | 7.29 ± 1.5 | 63.3 ± 5.9 |
| 13 | 53.7 ± 5.8 | 74.9 ± 10.2 |
| 14 | 4.2 ± 1.2 | 172.0 ± 12.9 |
| 15 | 30.3 ± 6.1 | 96.8 ± 9.3 |
| 17 | 102.9 ± 11.5 | 90.9 ± 9.5 |
| Doxorubicin | 3.4 ± 1.9 | 8.9 ± 2.1 |
Interestingly, by comparing the observed results on both cell lines in this investigation, it is obvious that, compounds 3, 12 and 14 showed 10, 8.7 and 41 folds more toxicity on cancer cells than on normal cells, respectively. However, the positive control showed only 2.6 folds more toxicity on cancer cells than on normal cells. These results may reveal that the three compounds 3, 12 and 14 may be used as effective anticancer candidate drugs, as they possess much less toxic effect on the normal cells compared to the positive control.
In correlation of the afforded activity results with the structural features of the most active compounds, it was revealed that in addition to the acyl chloride derivative 3, two candidates comprising the furan and 1,3,4-thiadiazole ring systems, compounds 12 and 14, were found to be the best in activity. The results indicated that the integration of the two five membered heterocycles afforded compounds of higher activities than those possessing only furan and 1,3,4-oxadiazole systems which showed the importance of the 1,3,4-thiadiazole ring system. The importance of 1,3,4-thiadiazole core incorporation has been revealed clearly by comparing the activity of the two hydrazides 4 and 13 in addition to comparing the results obtained for the sugar hydrazone 5 versus its analogue 13. It has been revealed that incorporation of 1,3,4-thiadiazole ring into these structural skeletons (compounds 13 and 14) resulted in enhanced activities. Similarly, the difference in activities of the hydrazones 5, 7 versus the derived oxadiazolyl sugars 8, 10, respectively accounts clearly for the importance of the 1,3,4-oxadiazole ring. Obviously, compounds possessing such ring system showed increased activities against the HepG2 cancer cell line. However, this was not the case for the structural skeleton exhibiting both thiadiazole and oxadiazole rings as the activity was lost in compound 17 when compared with its hydrazone precursor 15. On the other hand, it has been found interestingly that, the attachment of acyclic sugar moiety, via hydrazone linkage in the sugar hydrazones 5–7, resulted in markedly increased cytotoxic activity as their IC50 values were markedly decreased when compared to their hydrazide precursor 4. These results are in the same trending of those obtained for compounds 13–15 possessing similar structural characteristics since the sugar hydrazones 14 and 15 are highly active than their acid hydrazide precursor 13. Moreover, it was well observed that, the attachment of six-carbon acyclic sugar chain via a hydrazinyl acetylthio-linkage to the 1,3,4-thiadiazole system in compound 14 afforded the highly active compound. Furthermore, the 1,3,4-thiadiazole sugar hydrazone incorporating the free hydroxyl mannose moiety showed higher inhibition activity than its analog possessing the arabinose sugar moiety. In addition, the (hydrazinyl)oxobutanoyl chloride 3 showed markedly increased cytotoxic activity than its substituted propionic acid precursor and also its derived acetohydrazide product 4. On the other hand, the thioacetate ester 12 in which the 1,3,4-thiadiazole derivative linked to propanamido furic hydrazide exhibited highly increased activity than its derived hydrazide showing the difference in activity between the alkyloxy and hydrazinyl chains.
New 1,3,4-oxadiazole sugar derivatives based furyl ring system and furyl linked 1,3,4-thiadiazole core were synthesized. The anticancer activity against human liver carcinoma cell was studied and compounds possessing the furan and 1,3,4-thiadiazole rings showed the highest activities. The results showed the importance of the integration of the latter ring systems in the produced hybrid structure in addition to the high activity exhibited by molecules incorporating six carbon sugar moieties.
TLC was performed using aluminum plates pre-coated with silica gel 60 or 60 F254 (Merck, Germany) and visualized by iodine or UV light (254 nm). Melting points were determined on a Böetius PHMK (Veb Analytik Dresden, Germany) apparatus. The NMR spectra were recorded on a Varian Gemini 300 and Bruker DRX 400 spectrometer at 25°C, unless otherwise stated. 1H-NMR and 13C-NMR signals were referenced to tetramethylsilane and the solvent shift ((CD3)2SO δ H 2.50 and δC 39.5). Coupling constants are given in Hz and without sign. The IR-spectra were recorded (KBr) on a Jasco FT/IR-410 instrument. Mass spectrometry was carried out on a Varian FINNIGAN MAT 212 instrument and the elemental analysis on the PerkinElmer, Inc. 240 instrument. The Anti-cancer evaluation was performed at National Research Center (NRC), Dokki, Cairo, Egypt.
4-(2-(Furan-2-carbonyl)hydrazinyl)-4-oxobutanoic Acid (2)A solution of furan-2-carbohydrazide (1.29 g, 10 mmol) and succinic anhydride (1.02 g, 10 mmol) in toluene (20 mL) was stirred at room temperature for 24 h. The solvent was evaporated under reduced pressure at 50°C to give a residue, which was triturated with diethyl ether (25 mL) to afford a solid which was filtered, dried and crystallized from ethanol to afford compound 2 as a yellowish solid. Yield: 1.85 g, 80%; mp 148–149°C; 1H-NMR (DMSO-d6) δ/ppm: 3.04 (t, 2H, J = 6.2 Hz, CH2), 3.15 (t, 2H, J = 6.2 Hz, CH2), 6.70 (m, 1H, furanH-4), 7.58 (d, 1H, J = 7.4 Hz, furan H-3), 8.12 (d, 1H, J = 7.6 Hz, furanH-5), 9.24 (br s, 1H, NH exchangeable), 9.50 (br s, 1H, NH exchangeable), 11.84 (s, 1H, OH); 13C-NMR (DMSO-d6) δ: 30.5, 31.8 (2CH2), 111.5, 115.3, 143.0, 146.5 (furyl-C), 158.2, 168.1, 175 (3C = O); IR (KBr) cm−1, ν: 3344 (OH), 3335, 3243 (NH), 2925 (CH), 1740, 1686 (C=O); MS m/z: 226 (M+, 12%). Anal. Calcd for C9H10N2O5 (226.19): C, 47.79; H, 4.46; N, 12.39; Found: C, 48.0; H, 4.48; N, 12.50.
4-(2-(Furan-2-carbonyl)hydrazinyl)-4-oxobutanoyl Chloride (3)A solution of the carboxylic acid derivative 2 (1.98 g, 10 mmol) and a catalytic amount of N,N-dimethylformamide (DMF) in thionyl chloride (5 mL) was stirred at 80–90°C for 5 h. After removal of half of the solvent under reduced pressure, the formed precipitate was filtered, washed with cold ethanol, dried and recrystallized from methanol to give the acid chloride 3 as a brownish solid. Yield: 1.586 g, 65%; mp 178–179°C; 1H-NMR (DMSO-d6) δ/ppm: 3.05 (t, 2H, J = 6.2 Hz, CH2), 3.16 (t, 2H, J = 6.2 Hz, CH2), 6.62 (m, 1H, furanH-4), 7.57 (d, 1H, J = 7.4 Hz, furan H-3), 8.09 (d, 1H, J = 7.6 Hz, furan H-5), 9.25 (br s, 1H, NH exchangeable), 9.50 (br s, 1H, NH exchangeable). 13C-NMR (DMSO-d6) δ: 30.5, 42.8 (2CH2), 111.5, 115.3, 143.0, 146.5 (furyl-C), 158.2, 168.1, 176.0 (3C = O): IR (KBr) cm−1, ν: 3330, 3133 (NH), 2924 (CH), 1726 (C=O), 1650 (C=O); MS m/z: 244 (M+, 14); Anal. Calcd for C9H9ClN2O4 (244.63): C, 44.19; H, 3.71; N, 11.45; Found: C, 44.12; H, 3.82; N, 11.71.
N′-(Furan-2-carbonyl)succinohydrazide (4)Hydrazine hydrate (2 mL) was added blow (5°C) using ice bath to a solution of acid chloride derivative 3 (2.16 g, 10 mmol) in ethanol (50 mL) then stirred for 15 min at room temperature and then refluxed for 5 h.The reaction mixture was allowed to cool and stand at room temperature for about 5 h and the precipitated product was filtered and dried to give acid hydrazide 4 as yellowish solid. Yield: 1.56 g, 65%; mp 188–189°C; 1H-NMR (DMSO-d6) δ/ppm: 3.26 (t, 2H, J = 6.6 Hz, CH2), 3.53 (t, 2H, J = 6.6 Hz, CH2), 5.22 (br s, 2H, NH2 exchangeable), 6.71 (m, 1H, furanH-4), 7.55 (m, 1H, furanH-3), 8.14 (d, 1H, J = 7.6 Hz, furanH-5), 9.14 (br s, 1H, NH exchangeable), 9.50 (br s, 1H, NH exchangeable), 10.12 (br s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 33.5, 34.0 (2CH2), 111.5, 115.3, 143.0, 146.5 (furyl-C), 158.2, 168.1, 176.0 (3C = O); IR (KBr) cm−1, ν: 3431, 3330 (NH, NH2), 2926 (CH), 1640 (C=O), 1655 (C=O); MS m/z: 240 (M+, 88%). Anal. Calcd for C9H12N4O4 (240.22): C, 45.00; H, 5.04; N, 23.32; Found: C, 44.88; H, 5.09; N, 23.28.
Sugar N′-(3-Hydrazinyl-3-oxopropyl)furan-2-carbohydrazones (5–7)General Procedure: The monosaccharide namely; D-mannose, D-arabinose or D-xylose (6 mmol) dissolved in water (1 mL) was added to a solution of the hydrazide 4 (5 mmol) in ethanol (20 mL) containing glacial acetic acid (0.3 mL). The reaction mixture was heated at reflux temperature for 4 h then concentrated till removal of half of the amount of solvent and left to cool at room temperature. The formed precipitate was filtered off, washed with water and cold ethanol, then dried and recrystallized from ethanol to give compounds 5–7, respectively.
D-Mannose N′-(3-Hydrazinyl-3-oxopropyl)furan-2-succinohydrazide (5)Yield: 2.89 g, 72%; mp 289–290°C; 1H-NMR (DMSO-d6) δ/ppm: 3.23 (t, 2H, J = 6.6 Hz, CH2), 3.37–3.42 (m, 2H, H-6′, H-6″), 3.52 (t, 2H, J = 6.6 Hz, CH2), 3.62–3.65 (m, 1H, H-5′), 4.18–4.25 (m, 2H, H-4′, H-3′), 4.41–4.52 (m, 3H, H-2′, 2OH), 4.85–4.91 (m, 2H, 2OH), 5.39 (m, 1H, OH), 6.76 (m, 1H, furan H-4), 7.55 (m, 2H, furan H-3, H-1′), 8.15 (d, 1H, J = 7.6 Hz, furan H-5), 10.06 (br s, 1H, NH exchangeable), 10.25 (br s, 1H, NH exchangeable), 10.50 (br s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 35.8, 46.9 (2CH2), 63.7 (C-6) 66.4 (C-5), 69.8 (C-4), 71.3 (C-3), 73.4 (C-2), 111.5, 115.3, 143.0, 146.5, 154.3 (furyl-C, C-1), 163.2, 168.1, 175 (3C = O); IR (KBr) cm−1, ν: 3433–3405 (OH), 3250 (NH), 2923 (CH), 1652 (C=O), 1633 (C=O), 1618 (C=N); Anal. Calcd for C15H22N4O9 (402.36): C, 44.78; H, 5.51; N, 13.92. Found: C, 44.97; H, 5.88; N, 13.80.
D-Arabinose N′-(3-Hydrazinyl-3-oxopropyl)furan-2-succinohydrazide (6)Yield: 2.52 g, 68%; mp 280–281°C; 1H-NMR (DMSO-d6) δ/ppm: 3.23 (t, 2H, J = 6.6 Hz, CH2), 3.52 (t, 2H, J = 6.6 Hz, CH2), 3.62–3.67 (m, 2H, H-5′, H5″), 3.75–3.78 (m, 1H, H-4′), 4.31–4.34 (m, 1H, H-3′), 4.41 (dd, 1H, J = 6.8, J = 7.6 Hz, H-2′), 4.91–4.94 (m, 1H, OH), 4.99 (m, 1H, OH), 5.19–5.23 (m, 2H, 2OH), 6.78 (m, 1H, furan H-4), 8.30–8.35 (m, 3H, furan H-3, H-1′, H-5), 9.50 (br s, 1H, NH exchangeable), 10.06 (br s, 1H, NH exchangeable), 11.75 (s, 1H, NH exchangeable). 13C-NMR (DMSO-d6) δ: 35.4, 46.8 (2CH2), 64.1 (C-5) 67.1 (C-4), 69.7 (C-3), 73.4 (C-2), 111.5, 115.3, 143.0, 146.5, 153.6 (furyl-C, C-1), 159.8, 167.8, 175 (3C = O); IR (KBr) cm−1, ν: 3427–3404 (OH), 3333 (NH), 2924 (CH), 1657 (C=O), 1642 (C=O), 1618 (C=N); Anal. Calcd for C14H20N4O8 (372.33): C, 45.16; H, 5.41; N, 15.05; Found: C, 45.02; H, 5.50; N, 15.37.
D-Xylose N′-(3-Hydrazinyl-3-oxopropyl)furan-2-succinohydrazide (7)Yield: 2.45 g, 66%; mp 283–284°C; 1H-NMR (DMSO-d6) δ/ppm: 3.25 (t, 2H, J = 6.6 Hz, CH2), 3.54 (t, 2H, J = 6.6 Hz, CH2), 3.62–3.67 (m, 2H, H-5′, H-5″), 3.75–3.78 (m, 1H, H-4′), 4.35–4.41 (m, 2H, H-3′, 2′), 4.91–4.99 (m, 2H, 2OH), 5.19–5.25 (m, 2H, 2OH), 6.90 (m, 1H, furan H-4), 7.58–7.61 (m, 2H, furan H-3, H-1′), 8.17 (d, 1H, J = 7.6 Hz, furan H-5), 9.50 (br s, 1H, NH exchangeable), 10.25 (br s, 1H, NH exchangeable), 11.06 (br s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 35.4, 46.8 (2CH2), 64.1 (C-5) 67.1 (C-4), 69.7 (C-3), 73.4 (C-2), 111.5, 115.3, 143.0, 146.5, 153.6 (furyl-C, C-1), 159.8, 167.8, 175 (3C = O); IR (KBr) cm−1, ν: 3438–3398 (OH), 3246 (NH), 2923 (CH), 1638–1642 (C=O), 1618 (C=N); Anal. Calcd for C14H20N4O8 (372.33): C, 45.16; H, 5.41; N, 15.05; Found: C, 45.05; H, 5.48; N, 15.19.
5-(O-Acetyl Sugar)-1-(3-acetyl-5-(2-(furan-2-carbonyl)hydrazinyl)ethyl)-2,3-dihydro-1,3,4-oxadiazole (8–10)General Procedure: A solution of the sugar hydrazones 5–7 (5 mmol) in acetic anhydride (10 mL) was heated at 100°C while connected with reflux condenser for 3 h. The resulting solution was cooled then ice-cold water (50 mL) was added with continuous shaking. The product was extracted with chloroform (40 mL) followed by addition of sodium hydrogen carbonate and the mixture was stirred for 45 min then filtered. The solvent was dried with calcium chloride and evaporated till dryness to afford the corresponding oxadiazoline substituted acetyl sugar derivatives 8–10.
N′-(2-(4-Acetyl-5-(penta-O-acetyl-D-mannopentitolyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)ethyl)furan-2-succinohydrazide (8)Yield: 4.70 g, 72%; brownish foam; mp 127–129°C;1 H-NMR (DMSO-d6) δ/ppm: 1.98, 1.99, 2.01, 2.02, 2.05, 2.18 (6s, 18H, 6CH3), 3.53 (t, 2H, J = 6.6 Hz, CH2), 3.64 (t, 2H, J = 6.6 Hz, CH2), 3.97–4.05 (m, 2H, H-5′, H-5″), 4.86 (m, 1H, H-4′), 4.94 (dd, 1H, J = 6.5, J = 7.4 Hz, H-3′), 5.25 (t, 1H, J = 7.4 Hz, H-2′), 5.41 (dd, 1H,J = 7.4, J = 8.8 Hz, H-1′), 5.78 (d, 1H, J = 8.8 Hz, oxadiazoline H-5), 6.61 (m, 1H, furan H-4), 7.18 (d, 1H, J = 7.8 Hz, furanH-3), 7.88 (d, 1H, J = 7.6 Hz, furan H-5), 9.25 (s, 1H, NH exchangeable), 9.5 (s, 1H, NH exchangeable). 13C-NMR (DMSO-d6) δ: 19.7, 20.1, 20.3, 20.7, 21.1, 23.4 (6CH3) 35.7, 44.3 (2CH2), 61.8 (C-5), 62.9 (C-4), 67.5 (C-3), 69.5 (C-2), 74.7 (C-1), 88.7 (oxadiazoline C-2), 111.5, 115.3, 143.0, 146.5, 156.2 (furyl-C, oxadiazoline C-5), 159.1, 165.5, 169.1, 169.4, 169.7, 169.9, 170.3, 175 (8C = O); IR (KBr) cm−1, ν: 3259 (NH), 2923 (CH), 1740 (C=O), 1637 (C=O), 1617 (CN); Anal. Calcd for C27H34N4O15 (654.58): C, 49.54; H, 5.24; N, 8.56; Found: C, 49.21; H, 5.03; N, 8.39.
N′-(2-(4-Acetyl-5-(tetra-O-acetyl-D-arabinotetritolyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)ethyl)furan-2-succinohydrazide (9)Yield: 4.656 g, 80%; 153–154°C;1H-NMR (DMSO-d6) δ/ppm: 1.99, 2.00, 2.01, 2.02, 2.05 (5s, 15H, 5CH3), 3.67 (t, 2H, J = 6.6 Hz, CH2), 3.77–4.03 (m, 4H, CH2, H-4′, H-4″), 4.88–4.94 (m, 2H, H-3′, H-2′), 5.25 (t, 1H, J = 7.4 Hz, H-1′), 5.80 (d, 1H, J = 8.8 Hz, oxadiazoline H-5), 6.69 (m, 1H, furan H-4), 7.22 (d, 1H, J = 7.8 Hz, furanH-3), 8.68 (d, 1H, J = 7.6 Hz, furan H-5), 9.50 (s, 1H, NH exchangeable), 10.07 (s, 1H, NH exchangeable); 13C-NMR (DMSO-d6): δ 20.1, 20.4, 20.7, 21.2, 23.5 (5CH3), 35.7, 44.5 (2CH2), 62.9 (C-4), 63.7 (C-3), 72.3 (C-2), 76.7 (C-1), 88.2 (oxadiazoline C-2), 111.5, 115.3, 143.0, 146.5, 156.8 (furyl-C, oxadiazoline C-5), 159.2, 165.5, 169.3, 169.6, 169.0, 170.2, 175 (7 C=O); IR (KBr) cm−1, ν: 3278 (NH), 2923 (CH), 1742 (C=O), 1644 (C=O), 1615 (C=N); Anal. Calcd for C24H30N4O13 (582.52): C, 49.49; H, 5.19; N, 9.62; Found: C, 49.24; H, 5.14; N, 9.76.
N′-(2-(4-Acetyl-5-(tetra-O-acetyl-D-xylotetritolyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)ethyl)-furan-2-succinohydrazide (10)Yield: 4.77 g, 82%; mp 136–137°C; 1H-NMR (DMSO-d6) δ/ppm: 1.98, 1.99, 2.01, 2.04, 2.19 (5s, 15H, 5CH3), 3.63 (t, 2H, J = 6.6 Hz, CH2), 3.75–4.03 (m, 4H, CH2, H-4′, H-4″), 4.86–4.93 (m, 2H, H-3′, H-2′), 5.25 (dd, 1H, J = 7.4, 5.6 Hz, H-1′), 5.80 (d, 1H, J = 8.8 Hz, oxadiazoline H-5), 6.63 (m, 1H, furanH-4), 7.19 (d, 1H, J = 7.8 Hz, furanH-3), 7.89 (d, 1H, J = 7.6 Hz, H-5 furan), 7.91 (s, 1H, NH exchangeable), 9.50 (s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 20.1, 20.4, 20.7, 21.2, 23.5 (5CH3), 35.7, 44.5 (2CH2), 62.9 (C-4), 63.7 (C-3), 72.3 (C-2), 76.7 (C-1), 88.2 (oxadiazoline C-2), 111.5, 115.3, 143.0, 146.5, 156.8 (furyl-C, oxadiazoline C-5), 159.2, 165.5, 169.3, 169.6, 169.0, 170.2, 177 (7C = O); IR (KBr) cm−1, ν: 3278 (NH), 2924 (CH), 1741 (C=O), 1633 (C=O), 1564 (C=N); Anal. Calcd for C24H30N4O13 (582.52): C, 49.49; H, 5.19; N, 9.62; Found: C, 49.21; H, 5.11; N, 9.80.
Ethyl 2-((5-(4-(2-(Furan-2-carbonyl)hydrazinyl)-4-oxobutanamido)-1,3,4-thiadiazol-2-yl)-thio)acetate (12)Triethyl amine (0.35 mL) was added to a well stirred solution of the acid chloride 3 (2.16 g, 10 mmol) and ethyl 2-((5-amino-1,3,4-thiadiazol-2-yl)thio)acetate 11 (2.19 g, 10 mmol) in tetrahydrofuran (THF) (30 mL), then the mixture was heated under reflux for 5 h at which TLC indicated full consumption of the starting compounds. Crushed ice was added and the precipitated product was filtered off and recrystallized from acetic acid to give yellow crystals of compound 12.
Yield: 2.989 g, 70%; mp 175–176°C; 1H-NMR (DMSO-d6) δ/ppm: 1.17 (t, 3H, J = 6.2 Hz, CH3), 3.64 (t, 2H, J = 6.6 Hz, CH2), 3.96 (t, 2H, J = 6.6 Hz, CH2), 4.13–4.20 (m, 4H, 2CH2), 6.65–6.74 (m, 2H, NH, furanH-4), 7.03 (d, 1H, J = 7.6 Hz, furanH-3), 7.29 (d, 1H, J = 7.8 Hz, furanH-5), 9.50 (s, 1H, NH exchangeable), 11.65 (s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 14.2 (CH3), 31.9, 32.4, 33.5, 60.1 (4 CH2), 111.5, 115.3, 143.0, 146.5 (furyl-C), 152, 164 (thiadiazole-C), 157, 167, 173, 177 (4CO); IR (KBr) cm−1, ν 3278 (NH), 2922 (CH), 1735 (C=O), 1630 (C=O), 1618 (CN); MS m/z: 426 (M+ - 1, 15%). Anal. Calcd for C15H17N5O6S2 (427.45): C, 42.15; H, 4.01; N, 16.38; Found: C, 42.25; H, 4.21; N, 16.29.
4-(2-(Furan-2-carbonyl)hydrazinyl)-N-(5-((2-hydrazinyl-2-oxoethyl)thio)-1,3,4-thiadiazol-2-yl)-4-oxobutanamide (13)A solution of the thiadiazolyl ester 12 (3.83 g, 10 mmol) and hydrazine hydrate (98%) (2 mL) in absolute ethanol (30 mL) was heated under reflux for 6 h. The resulting mixture was cooled and left to stand at room temperature for 6 h. The formed precipitate was filtered, dried then recrystallized from ethanol–acetic acid mixture (1 : 1) to give compound the hydrazide product 13 as a yellow solid. Yield: 2.684 g, 65%; mp 250–251°C; 1H-NMR (DMSO-d6) δ/ppm: 3.27 (t, 2H, J = 6.6 Hz, CH2), 3.51 (t, 2H, J = 6.6 Hz, CH2), 4.12 (s, 2H, CH2), 5.29 (m, 2H, NH2 exchangeable), 6.61 (m, 1H, furanH-4), 7.56 (m, 1H, furanH-3), 8.10 (d, 1H, J = 7.6 Hz, furanH-5), 9.17–9.25 (br s, 2H, 2NH exchangeable), 9.50 (br s, 1H, NH exchangeable), 10.18 (br s, 1H, NH); 13C-NMR (DMSO-d6) δ: 31.9, 32.4, 33.5 (3CH2), 111.5, 115.3, 143.0, 146.5 (furyl-C), 152, 164 (thiadiazole-C), 157, 170, 173, 177 (4CO); IR (KBr) cm−1, ν 3430, 3305 (NH, NH2), 2920 (CH), 1658 (C=O), 1615 (CN); MS m/z: 413 (M+, 44%). Anal. Calcd for C13H15N7O5S2 (413.43): C, 37.77; H, 3.66; N, 23.72; Found: C, 37.79; H, 3.67; N, 23.59.
General Procedure for the Synthesis of Sugar Hydrazone Derivatives (14, 15)To a solution of the acid hydrazide 13 (10 mmol) in ethanol (20 mL) 3–5 drops of glacial acetic acid have been added. D-Mannose or D-arabinose (10 mmol) suspended in water (1 mL) was added and the mixture was refluxed for 6 h at which TLC indicated full consumption of the starting compounds. The resulting solution was concentrated and left to stand at room temperature overnight. The formed precipitate was filtered off, washed with water and cold ethanol, then dried and recrystallized from ethanol to give the sugar hydrazones 14 or 15, respectively.
3-(2-(Furan-2-carbonyl)hydrazinyl)-N-5-((2-oxo-2-(2-(D-mannopentitolylidenehydrazin-yl)-ethyl)thio)-1,3,4-thiadiazol-2-yl)butanamide (14)Yield: 3.737 g, 65%; mp 294–295°C; 1H-NMR (DMSO-d6) δ/ppm: 2.72 (t, 2H, J = 6.6 Hz, CH2), 2.88 (t, 2H, J = 6.6 Hz, CH2), 3.37–3.41 (m, 2H, H-6′, H-6″), 3.58–3.61 (m, 1H, H-5′), 4.16–4.23 (m, 4H, CH2, H-4′, H-3′), 4.43–4.55 (m, 3H, H-2′, 2OH), 4.84–4.92 (m, 2H, 2OH), 5.38 (m, 1H, OH), 6.65–7.93 (m, 3H, furan H-3,4,5), 7.98 (d, J = 8.4, 1H, H-1′), 9.50 (s, 1H, NH exchangeable), 10.06–10.25 (brs, 2H, 2NH), 11.75 (s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 35.6, 43.4, 46.7 (3CH2), 64.7 (C-6), 66.6 (C-5), 69.9 (C-4), 72.3 (C-3), 73.4 (C-2), 111.5, 115.3, 143.0, 146.5, 154.2, 155.6 (furyl-C, thiadiazole C-2, C-1), 157.3 (C-5 thiadiazole), 159.2, 164.5, 165.6, 173 (4C = O); IR (KBr) cm−1, ν: 3425–3381 (OH), 3245 (NH), 2924 (CH), 1655 (C=O), 1637 (C=O), 1617 (C=N); Anal. Calcd. For C19H25N7O10S2 (575.57): C, 39.65; H, 4.38; N, 17.04; Found: C, 39.55; H, 4.29; N, 17.17.
3-(2-(Furan-2-carbonyl)hydrazinyl)-N-5-((2-oxo-2-(2-(D-arabinotetritolylidenehydrazin-yl)-ethyl)thio)-1,3,4-thiadiazol-2-yl)butanamide (15)Yield: 3.433 g, 63%; mp 290–291°C; 1H-NMR (DMSO-d6) δ/ppm: 2.73 (t, 2H, J = 6.6 Hz, CH2), 2.89 (t, 2H, J = 6.6 Hz, CH2), 3.31–3.38 (m, 2H, H-5′, H-5″), 4.05–4.20 (m, 4H, CH2, H-4′, H-3′), 4.43–4.52 (m, 2H, H-2′, OH), 4.84–4.92 (m, 2H, 2OH), 5.35–5.38 (m, 1H, OH), 6.67–7.93 (m, 3H, H-3,4,5), 7.97 (d, J = 8.4, 1H, H-1′), 9.50 (s, 1H, NH exchangeable) 10.05–10.25 (br s, 2H, 2NH exchangeable), 11.71 (s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 35.6, 43.4, 46.7 (3CH2), 66.6 (C-5), 69.9 (C-4), 72.3 (C-3), 73.4 (C-2), 111.5, 115.3, 143.0, 146.5, 153.2, 155.6 (furyl-C, thiadiazole C-2, C-1), 157.3 (C-5 thiadiazole), 159.2, 164.5, 165.6, 173 (4C = O); IR (KBr) cm−1, ν: 3438–3400 (OH), 3243 (NH), 2924 (CH), 1654 (C=O), 1617 (C=N); Anal. Calcd for C18H23N7O9S2 (545.54): C, 39.63; H, 4.25; N, 17.97; Found: C, 39.47; H, 4.45; N, 17.68.
N-(5-(((4-Acetyl-5-(per-O-acetylsugar)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)thio)-1,3,4-thiadiazol-2-yl)-3-(2-(furan-2-carbonyl)hydrazinyl)propanamide (16, 17)A well stirred solution of the sugar hydrazones14 or 15 (10 mmol) in acetic anhydride (10 mL) was heated at 100°C 4 h at which TLC (methanol/chloroform; 0.4/9.6) showed completion of the reaction. The resulting solution was poured onto ice-cold water, and then extracted with chloroform (40 mL). Sodium hydrogen carbonate was added to the organic layer and mixture was stirred for 45 min and filtered. The chloroform layer was washed with water, dried with calcium chloride and evaporated till dryness to afford the corresponding acetyl sugar derivatives.
N-(5-(((4-Acetyl-5-(penta-O-acetyl-D-mannopentitolyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)thio)-1,3,4-thiadiazol-2-yl)-3-(2-(furan-2-carbonyl)hydrazinyl) butanamide (16)Yield: 6.54 g, 79%; yellowish foam; 1H-NMR (DMSO-d6) δ/ppm: 1.83, 1.89, 1.93, 1.97, 2,01, 2.21 (6s, 18H, 6CH3), 2.68 (t, 2H, J = 6.6 Hz, CH2), 2.79–3.97 (m, 3H, CH2, H-5′), 4.03–4.15 (m, 3H, CH2, H-5″), 4.87–4.94 (m, 2H, H-4′, H-3′), 5.05–5.11 (dd, 1H, J = 7.4, J = 8.8 Hz, H-2′), 5.26 (t, 1H, J = 7.4 Hz, H-1′), 5.90 (d, 1H, J = 8.8 Hz, oxadiazoline H-5), 6.43 (m, 1H, furan H-4), 7.21 (d, 1H, J = 7.8 Hz, furan H-3), 7.89 (d, 1H, J = 7.6 Hz, furan H-5), 7.99–8.12 (br s, 2H, 2NH exchangeable), 9.50 (br s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 20.0, 20.3, 20.5, 21.8, 22.0, 23.2 (6CH3), 33.9, 34.3, 45.2 (3CH2), 61.5 (C-5), 62.9 (C-4), 64.9 (C-3), 67.8 (C-2), 76.8 (C-1), 83.9 (oxadiazoline C-5), 114.6, 115.3, 143.0, 146.5, 153.2, 153.1 (furyl-C, thiadiazole C-2, oxadiazole C-2), 157.1 (thiadiazole C-5), 158.3, 159.1, 169.5, 169.7, 170.0, 172.2, 174.2, 175, 177 (9 C=O); IR (KBr) cm−1, ν: 3238 (NH), 2911 (CH), 1740 (C=O), 1660 (C=O), 1615 (C=N); Anal. Calcd. for C31H37N7O16S2 (827.79): C, 44.98; H, 4.51; N, 11.84; Found: C, 44.76; H, 4.61; N, 12.02.
N-(5-(((4-Acetyl-5-(tetra-O-acetyl-D-arabinotetritolyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)thio)-1,3,4-thiadiazol-2-yl)-3-(2-(furan-2-carbonyl)hydrazinyl)propanamide (17)Yield: 5.517 g, 73%; yellowish foam; 1H-NMR (DMSO-d6) δ/ppm: 1.87, 1.89, 1.92, 1.96, 2.10 (5s, 15H, 5 CH3), 2.73 (t, 2H, J = 6.6 Hz, CH2), 2.83–3.98 (m, 3H, CH2, H-4′), 4.06–4.17 (m, 3H, CH2, H-4″), 4.89–4.93 (m, 1H, H-3′), 5.05–5.09 (dd, 1H, J = 7.2, J = 8.5 Hz, H-2′), 5.25 (t, 1H, J = 7.4 Hz, H-1′), 5.88 (d, 1H, J = 8.8 Hz, oxadiazoline H-5), 6.63 (m, 1H, furan H-4), 7.19 (d, 1H, J = 7.8 Hz, furan H-3), 7.89 (d, 1H, J = 7.6 Hz, furan H-5), 7.91–8.01 (br s, 2H, 2NH exchangeable), 9.5 (br s, 1H, NH exchangeable); 13C-NMR (DMSO-d6) δ: 20.0, 20.3, 20.5, 21.8, 23.2 (5CH3), 33.9, 34.3, 45.2 (3CH2), 62.9 (C-4), 64.9 (C-3), 67.8 (C-2), 76.8 (C-1), 83.9 (oxadiazoline C-5), 114.6, 115.3, 143.0, 146.5, 153.2, 153.1 (furyl-C, thiadiazole C-2, oxadiazole C-2), 157.1 (thiadiazole C-5), 158.3, 159.1, 169.5, 169.7, 170.0, 172.2, 175, 177 (8C = O); IR (KBr) cm−1, ν: 3243 (NH), 2926 (CH), 1738 (C=O), 1661 (C=O), 1617 (C=N); Anal. Calcd for C28H33N7O14S2 (755.73): C, 44.50; H, 4.40; N, 12.97; Found: C, 44.29; H, 4.62; N, 13.09.
In Vitro Anticancer ActivityDulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco, UK. Dimethyl sulfoxide (DMSO) was of HPLC grade and all other reagents and chemicals were of analytical reagent grade.
Cell CultureHepG-2 (Human liver carcinoma) and RPE-1 (human normal Retina pigmented epithelium) cell lines were purchased from the American Type Culture Collection (Rockville, MD, U.S.A.) and maintained in DMEM medium which was supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin and 100 U/mL streptomycin. The cells were grown at 37°C in a humidified atmosphere of 5% CO2. All experiments were conducted thrice in triplicate (n = 3). All the values were represented as means ± standard deviation (S.D.)
Lactate Dehydrogenase (LDH) AssayTo determine the effect of each synthesized compounds on membrane permeability in both HepG2 and RPE-1 cell lines, a LDH release assay was used.39) The cells were seeded in 24-well culture plates at a density of 2 × 105 cells/well in 500 µL volume and allowed to grow for 18h before treatment. After treatment with a series of different concentrations of each compound or Doxorubicin® (positive control), the plates were incubated for 48 h. Then, the supernatant (40 µL) was transferred to a new 96 well to determine LDH release and 6% triton X-100 (40 µL) was added to the original plate for determination of total LDH. An aliquot of 0.1 M potassium phosphate buffer (100 µL, pH 7.5) containing 4.6 mM pyruvic acid was mixed to the supernatant using repeated pipetting. Then, 0.1 M potassium phosphate buffer (100 µL, pH 7.5) containing 0.4 mg/mL reduced β-reduced nicotinamide adenine dinucleotide (NADH) was added to the wells. The kinetic changes were read for 1 min using enzyme-linked immunosorbent assay (ELISA) microplate reader in absorbance at wavelength 340 nm. This procedure was repeated with 40 µL of the total cell lysate to determine total LDH. The percentage of LDH release was determined by dividing the LDH released into the media by the total LDH following cell lysis in the same well.40) All experiments were conducted in triplicate (n = 3). All the values were represented as mean ± S.D. Significant differences between the means of parameters as well as IC50s were determined by probit analysis using SPSS software program (SPSS Inc., Chicago, IL, U.S.A.).
The authors declare no conflict of interest.