2019 年 67 巻 8 号 p. 849-854
2019 年 67 巻 8 号 p. 849-854
Regenerative therapy with keratinocyte growth factor (KGF) is a novel therapeutic approach for treatment of chronic wounds. However, KGF cannot be used directly to the wound site due to its physicochemical instability. In previous study, sacran, a natural megamolecular polysaccharide, showed potential properties as a biomaterial for hydrogel film in wound healing. In this study, we fabricated sacran hydrogel film containing KGF (Sac/KGF-HF) and evaluated the effects of Sac/KGF-HF on fibroblasts migration and re-epithelialization process. We successfully prepared a homogenous and -amorphous Sac/KGF-HF by a casting method. In addition, Sac/KGF-HF had a high swelling ratio and flexibility. Sac/KGF-HF promoted a migration process of NIH3T3 cells and improved wound healing ability in mice with a percentage of wound closure reaching 90.4% at 9 d. Interestingly, the addition of KGF in Sac-HF considerably increased the number of epithelial cells compared to control, which is important in the re-epithelialization process. It could be concluded that KGF in Sac-HF has the potential for promoting Sac-HF abilities in wound healing process.
Human skin, the outer layer of body, is an important organ for body stimulation, protection against physical damage, radiation, and germs, as well as for regulating body temperature.1) The physiological imbalances can cause significant skin injuries.2) The healing of skin injuries is a complex process which is believed to consist of four stages.3) A homeostatic stage which starts immediately after wound injuries, through platelet aggregation, degranulation, and fibrin formation. The next stage begins with inflammation by releasing cytokines and growth factors. While inflammation, neutrophils infiltrate to the base of the wound followed by monocytes, which turn into macrophages. In the proliferation stage, fibroblasts migrate to the wound and deposit a new extracellular matrix to initiate re-epithelialization and followed by angiogenesis, collagen synthesis, and extracellular matrix formation. The final stage is remodeling, which is characterized by collagen restructuring, vascular regression, maturation and scaring.4,5)
Growth factors, a natural polypeptides, activate intracellular signaling pathways that regulate various aspects of subcellular physiology and cellular function.6,7) Several studies have shown that a deficiency of growth factor is responsible for chronic wounds compared to acute wounds, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor alpha (TGF-α), insulin-like growth factor (IGF), basic fibroblast growth factor (bFGF), and transforming growth factor beta 1 (TGF-β1).8–17)
Nowadays, growth factor delivery systems are a promising treatment as regenerative therapy in wound healing.18) Keratinocyte growth factor (KGF), a secreted growth factor, is involved in the repair of skin injury, which stimulates cell proliferation and migration in the re-epithelialization process. Re-epithelialization process is very important in wound healing as a parameter in the success of wound healing.19–21) Growth factor delivery systems are necessary to increase stability and control a release of growth factors in chronic wound therapy.22) To deliver KGF to the wound area as wound dressing, a topical dosage form is needed such as ointments, creams, and hydrogels. In human studies, the hydrogel was well tolerated and improved a healing process in patients with chronic diabetic ulcers and decubitus lesions.10)
Nowadays, a hydrogel film for wound dressing application has gained a lot of attention due to their excellent properties like impermeability to bacteria, film transparency, and less painless.23) Many hydrogel films are constructed by synthetic or semi-synthetic polymers, such as hydroxy propyl methyl cellulose, polyurethanes and/or polyvinyl alcohol.24–26) Natural polysaccharides have been known as biopolymers for wound dressing materials which have biomedical activities and a lower toxicity than synthetic or semi-synthetic polymers.27) Sacran is a natural megamolecular polysaccharide extracted from cyanobacterium of Aphanothece sacrum (Suizenji-nori) found in Kumamoto and Fukuoka prefectures in Japan.28) In addition, sacran also has an anti-inflammatory effect for alleviating hemorrhoid syndrome, atopic dermatitis and contact eczema.28–30) Previous studies showed that sacran as a premising biomaterial in hydrogel film (Sac-HF) could accelerate a wound healing process by maintaining skin moisture in wound site.31) Furthermore, Sac-HF can be used as an effective wound dressing biomaterial to deliver water soluble drugs.32) In the present study, we investigated the potential of Sac-HF in protein delivery as wound healing therapy, we fabricated a sacran hydrogel film containing KGF (Sac/KGF-HF) and evaluated its physicochemical properties, and wound healing abilities in vitro and in vivo.
Sacran (lot OGS-00022) was obtained from Green Science Material (Kumamoto, Japan). KGF solution (50 ppm KGF suspended in ammonium sulfate solution) was purchased from Skin Actives Scientific (Gilbert, AZ, U.S.A.). Dulbecco’s Modified Eagle Medium/DMEM (containing 4.5 g/L D-glucose, L-glutamine and pyruvate) (Gibco, Waltham, MA, U.S.A.), fetal bovine serum/FBS (Gibco, N.Y., U.S.A.), and Pen Strep/Penicillin Streptomisin (Gibco, Waltham, MA, USA.) were purchased from PT. Elo Karsa Utama (Bandung, Indonesia).
Cell CultureNIH/3T3 cells, a murine fibroblast cell line, were provided by PT. Prodia Stem Cell Indonesia (Jakarta, Indonesia). NIH/3T3 cells were grown in DMEM culture medium containing penicillin (100 units/mL) and streptomycin (100 µg/mL) supplemented with 10% FBS, at 36°C in a humidified atmosphere containing 5% CO2 and 95% air.
Preparation of Sac-HFThe preparation method of Sac-HF was slightly modified from our previous studies.30) Briefly, sacran (250 mg) was dissolved in distilled water on a water bath (80°C), stirred for 24 h, and sonicated for 10 min. Then, the resulting sacran solution was transferred into a propylene box (5 × 5 × 4 cm3) and dried with oven (Memmert 854, Schwabach, Germany) at 60°C for 48 h. Finally, Sac-HF was obtained by heating at 110°C for 2 h.
Preparation of Sac/KGF-HFThe preparation method of Sac/KGF-HF using a casting method was previously mentioned in our studies.33) Briefly, Sac-HF (1 × 1 cm2) was casted by 10 µL of KGF (100 ng) and kept at 4°C for 24 h. Then, Sac/KGF-HF was dried at 37°C for 4 h.
Differential Scanning Calorimetry (DSC)DSC analysis was carried out using Auto Q20 (TA Instruments, Tokyo, Japan). Sac-HF and Sac/KGF-HF were heated in an aluminum pan with heating rate of 10°C/min, started from 50°C until 250°C under nitrogen atmosphere.30)
Swelling AbilitySac-HF and Sac/KGF-HF (1 × 1 cm2) were weighed (Wd) and immersed in phosphate buffered saline (PBS) (pH 7.4). The completely expanded-hydrogel film sheet was immediately weighed (Ws) after removing the excess PBS on the surface at 3 and 24 h. The swelling ratio was calculated using the formula Ws/Wd.34)
Mechanical StrengthSac-HF and Sac/KGF-HF (1 × 1 cm2) were clamped with 2 clips and pulled at a rate of 0.5 mm/s in Textechnofavigraph (Moenchengladbach, Germany). The result was calculated as Elongation at Break (%) = (L − Lo)/Lo × 100, where L and Lo were expressed as the length of the breakpoint and the initial length of the sample, respectively.35)
Proliferation StudyNIH/3T3 cells (1 × 105 cells/well plate) were seeded in 24 well plates for 24 h and treated with Sac-HF and Sac/KGF-HF (2.5 × 2.5 mm2) for 4 h. Afterward, it was rinsed 2 times with PBS (270 µL), then 30 µL of WST-8 reagent was added to each well and incubated at 37°C for 4 h. The absorbance of the solution was measured at 450 nm, with a reference wavelength at 665 nm, using microplate reader (NanoQuant Plate™, Tokyo, Japan).31)
Cell Migration StudyThe study was conducted on NIH/3T3 cells with the scratch method described by Li et al.36) Briefly, the 24 well plates containing NIH/3T3 cells (1 × 105 cells/well plate) were linearly scratched with a sterile tip (0.1–10 µL). The monolayer of NIH/3T3 cells was washed with PBS to remove the floating cells or other impurities. The results of the scratches were observed under a microscope and then the width of the scratches was recorded. Sac-HF and Sac/KGF-HF were added to their corresponding wells. The cell migration was monitored by a microscope (ZEISS axio, Jakarta, Indonesia) and photographed at 6, 12, and 18 h. The distance between cells that migrate from the opposite side of the scratch line was expressed as the percentage of the initial migration area.37)
Wound Healing StudyEighteen healthy mice (BALB/c mice aged 8 weeks with a body weight of 30–35 g) were given diazepam intramuscularly at a dose of 2.5 mg/kg.38) The full-thickness excisional wound was made on the back side of hairless mice using 8-mm sterile biopsy punch.30) Mice were divided into three test groups as HF-Sac, HF-Sac/KGF, and control (without treatment). HF-Sac and HF-Sac/KGF were applied precisely on the wound area. The wound area was photographed using Canon’s EOS 1200D Camera (Tokyo, Japan) and the wound size was analyzed by ImageJ software on days 0, 4, 6, and 9 d post treatment. All the animal research studies were approved by Ethical Committee of Medical Faculty Universitas Padjadjaran with ethical approval number 1296/UN.C10/PN/2017.
HistopathologyThe histopathology study was carried out by taking the middle part of the wound site from wound healing studies. The wounds of HF-Sac and HF-Sac/KGF groups were immersed in 10% formalin (BNF) to prevent decay. Then, the wounds were blocked in paraffin and cut in 3–5 µm using a microtome (Medimeas MRM-1120 A, Haryana, India). The slice of clan on the slide was processed for immunohistochemistry by Malory Azan staining. The scoring number of fibroblasts, collagen, and epithelium on the skin surface was assessed on a scale of 0–3, where 0 = weak staining; 1 = moderate coloring; 2 = strong coloring; and 3 = very strong coloring.39)
Data AnalysisThe quantitative data were expressed as the mean ± standard error of the mean (S.E.M.). The statistical comparisons were carried out by the statistically used variance of one-way ANOVA Scheffe method.
Homogenous and amorphous hydrogel films are needed for effective wound-dressing application.40–42) Sac-HF was successfully fabricated using the physically-crosslinked method (Fig. 1A). The sacran solution was dried in oven at 60°C and heated at 110°C to obtain the Sac-HF. The purpose of drying at 60°C was to vaporize water solvents, while upon heating at 110°C, crosslinks between sacran chains were formed.30) To obtain a homogenous Sac/KGF-HF, KGF was casted on the surface of Sac-HF. DSC analysis confirmed that Sac/KGF-HF and Sac-HF were in amorphous form (Fig. 1B). Herein, the melting point of KGF appeared at 113.31°C and disappeared in the thermogram of Sac/KGF-HF. In our previous study, X-ray diffractometer (XRD) studies verified that Sac/KGF-HF showed a hallow pattern, indicating an amorphous form.33) In addition, the endothermic peaks of KGF, Sac-HF, and HF-Sac/KGF at around 100°C at DSC represented a heat absorption of water. These results suggest that a homogenous and amorphous Sac/KGF-HF was successfully prepared by a casting method.
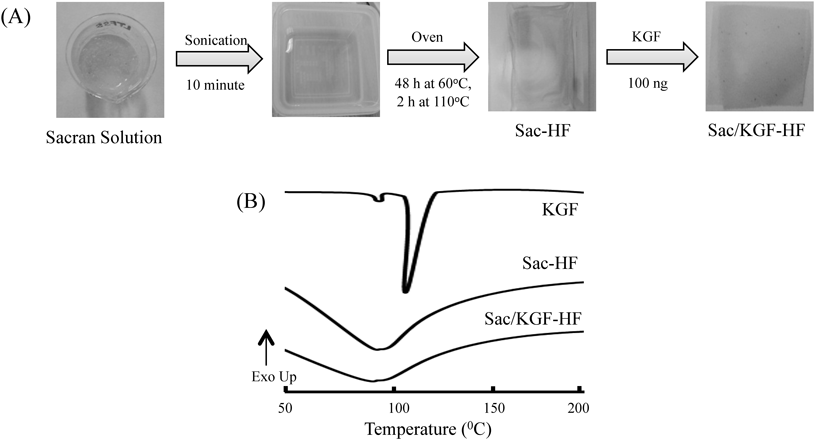
(A) Preparation of Sac/KGF-HF (B) DSC thermograms of Sac/KGF-HF. The experiments were performed three times, and representative data are shown.
To examine whether Sac/KGF-HF possesses ideal properties for use as wound dressing materials, we studied their swelling ability and mechanical properties. Swelling ability is an important property to understand the hydrogel film ability for absorbing exudates in the wound site, which can accelerate the wound healing process.43,44) To obtain the swelling ratio of Sac/KGF-HF, the gravimetric method was used through comparing the weight of hydrogel film before and after immersion in PBS at 3 h and 24 h (Fig. 2A). Figure 2B shows that the swelling ratio of Sac-HF and Sac/KGF-HF increased approximately 37 and 44 times at 3 and 24 h, respectively, compared to initial weight of their dried hydrogel films, indicating that both Sac-HF and Sac/KGF-HF have-high swelling ratios. In addition, the addition of KGF in Sac-HF did not affect the potential swelling ability of Sac-HF. Hence, these results suggest that Sac/KGF-HF may maintain moist environment in the wound and improve its wound healing ability as reported previously regarding Sac-HF.31)
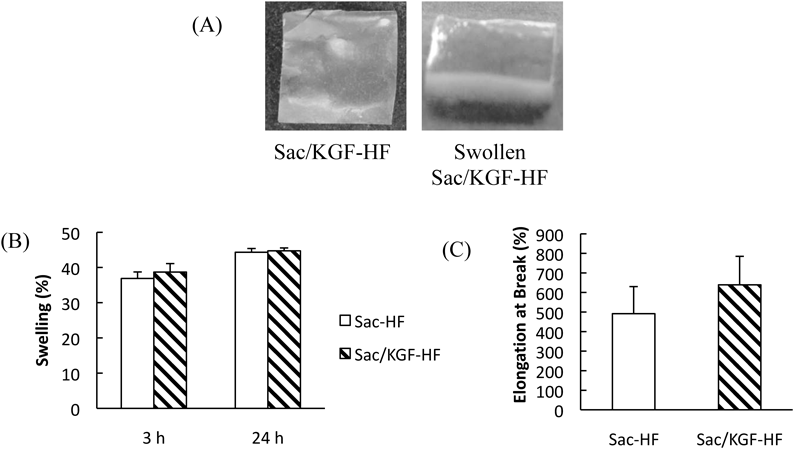
(A) Appearances of Sac/KGF-HF (B) Swelling ratio of Sac/KGF-HF. Sac/KGF-HF (1 × 1 cm2) was weighed (Wd), immersed in PBS at 3 and 24 h (B) Elongation at Break of Sac/KGF-HF. Sac/KGF-HF (1 × 1 cm2) was clamped with 2 clips and pulled by at a rate of 0.5 mm/s. Calculated as Elongation at Break (%) = (L − Lo)/Lo × 100 formula. Each value represents the mean ± S.E.M. of 3 experiments.
To investigate mechanical properties of Sac/KGF-HF, we next analyzed the elongation at break of Sac/KGF-HF using Textechnofavigraph. A high elongation of break of hydrogel film can be attributed to its better moisture maintenance ability.45) The results showed that the elongation at break of Sac/KGF-HF (638%) was higher than that of Sac-HF (491%) (Fig. 2C). It is likely that Sac/KGF-HF was more flexible than Sac-HF, probably due to a physical interaction between KGF structure and sacran molecular chains. However, it is still unclear. Thereby, further investigation is needed to clarify these finding.
Proliferation StudyTo determine the effect of Sac/KGF-HF on NIH/3T3 cells proliferation, the proliferation assay was carried out during 4 h incubation of Sac/KGF-HF on NIH/3T3 cells using WST-8 method. NIH/3T3 fibroblast cells were chosen as model cells because fibroblast cells are directly involved in the skin tissue regeneration and re-capitalization process.46) As seen in Fig. 3, Sac/KGF-HF increased cell proliferation by about 1.8 times compared to control. This result suggests that Sac/KGF-HF treated cells showed a high cell proliferation and biocompatibility.
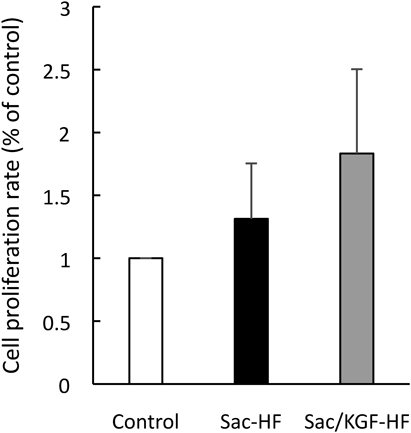
Proliferation assay was carried out during 4 h incubation of Sac/KGF-HF on NIH3T3 cells using WST-8 method. Each value represents the mean ± S.E.M. of 3 experiments.
A cell migration study was conducted to investigate the effect of Sac/KGF-HF on migration of NIH/3T3 cells using a scratch method. In this study, Sac-HF and Sac/KGF-HF were added to the plates on NIH/3T3 cells culture and observed at 6, 12, and 18 h after scratching a line, compared to the control group without addition of hydrogel films. Figure 4 showed that no significant difference in the migration was found among Sac/KGF-HF, Sac-HF and control groups at 6 h, although the migration of NIH/3T3 cells in Sac/KGF-HF group was faster than that of other groups. In addition, the migration speed of NIH/3T3 cells in the Sac-HF group was similar to that of the control group, suggesting the natural migration of NIH/3T3 cells. In general, when the monolayer cells were scratched, the cells around the scratch die and stimulate the cells to migrate towards the scratch.47) Meanwhile, the migration of NIH/3T3 cells in Sac/KGF-HF group at 12 h was significantly faster than its migration at 0 h, these results were not found in the other groups. Interestingly, only in the Sac/KGF-HF group, the scratch of NIH/3T3 cells was 100% closed at 18 h. Upon wounding, the growth factors promote cells to divide, produce new cells, and migrate under the influence of cytokines. KGF along the wound edge begins a histrionic reordering of their cytoplasmic and membrane structures. There is an equilibrium between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) with a net production of new tissue.47,48) Anyhow, these results corroborated the previous results of proliferation study and suggest that Sac/KGF-HF promoted a migration process of NIH/3T3 cells in vitro.
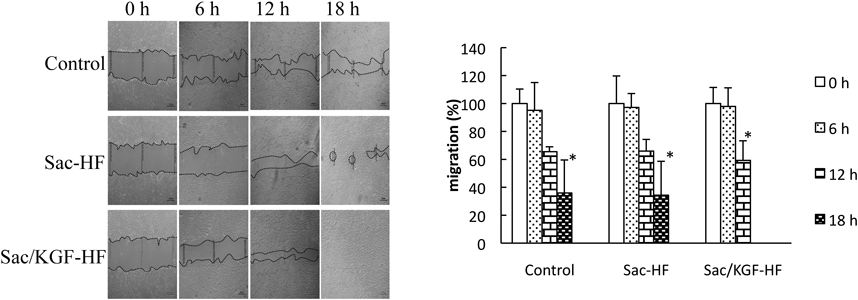
Cell migration tests were conducted on NIH/3T3 culture cells with “scratch” method. The 24 well plates containing NIH/3T3 cell culture was scratched with a sterile tip 0.1–10 µL. Each value represents the mean ± S.E.M. of 3 experiments. * p < 0.05 compared to 0 h.
To confirm the effect of KGF in Sac-HF on a wound healing process, we performed the in vivo wound closure study in full-thickness excisional wounds in BALB/c mice. Figure 5 shows that the Sac/KGF-HF group resulted in considerably faster wound closure than the other groups at 4, 6, and 9 d, suggesting that the addition of KGF in Sac-HF successfully accelerated wound healing ability of Sac-HF. On the last day of observation, the percentage of wound closure in Sac/KGF-HF, Sac-HF, and control groups were 90.4, 79, and 64.9%, respectively. In previous study, Sac-HF absorbed and maintained wound exudates, which promoted fibroblast proliferation and keratinocyte migration. According to a study conducted by Fukushima et al., it was found that sacran significantly improved skin barrier function in patients with atopic dermatitis and had anti-inflammatory effects in the wound area.29)
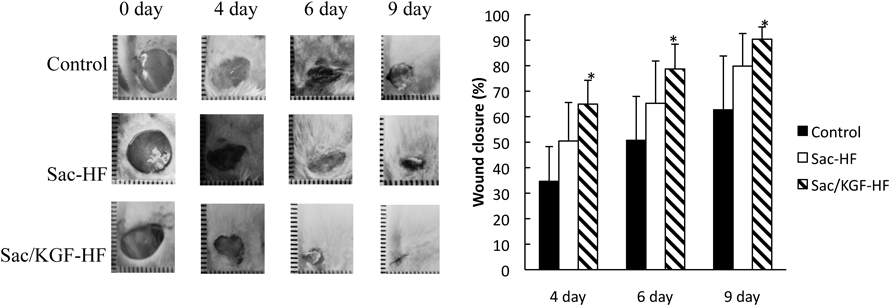
The in vivo wound closure study was performed in full-thickness excisional wounds of BALB-c mice at 4, 6, and 9 d. Each value represents the mean ± S.E.M. of 3 experiments. * p < 0.05 compared to control.
KGF increases the proliferation of keratinocytes which play an important role in the re-epithelization process.20) Research conducted by Staiano-Coico et al. showed that KGF given topically to partial wounds can stimulate the rate of re-epithelization and is associated with thickening of the epidermis. Wound care using KGF is also able to increase the quantity of jagged basal cells connected with improved deposition of collagen fibers in the superficial dermis adjacent to the epidermis. In addition, it shows normal orthokeratotic maturation and proliferation to basal cells.49) Based on the in vivo study, the synergistic effect between KGF and sacran in accelerating wound healing process is expected to occur.
Histopathology TestKGF converts fibroblasts into myofibroblasts and produces sequences of matrices such as type III collagen, glycosaminoglycans, and fibronectin which play a crucial role in wound healing.19) To examine the number fibroblasts and epithelial cells and collagen production in wound tissues, histopathology of wound specimens at 9 d with Malory Azan staining was conducted. From the observation (Fig. 6), both Sac/KGF-HF and Sac-HF were found to have a number of fibroblasts with significant differences in the control group. In addition, the addition of KGF in Sac-HF drastically increased the potential of Sac-HF in improving epithelial growth compared to control. However, no significant difference in collagen production between Sac/KGF-HF and control groups was observed. KGF is known to stimulate the release of keratinocytes in the wound area and transform fibroblasts-into myofibroblasts in order to begin re-epithelialization process which makes a significant contribution to the process of wound closure.19) From these results, it could be concluded that KGF in Sac-HF accelerated wound healing process by stimulating the number of fibroblasts and re-epithelialization process.
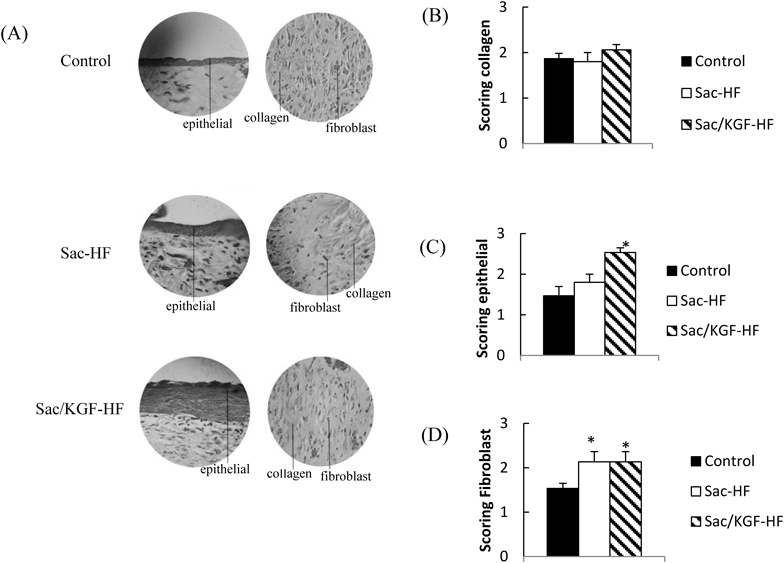
(A) Mallory Azan staining with 100 × zooming (B) Interpretation results of collage (C) Interpretation results of fibroblast (D) Interpretation results of epithelial. Each value represents the mean ± S.E.M. of 3 experiments. * p < 0.05 compared to control.
We successfully fabricated a homogenous and amorphous Sac/KGF-HF by a casting method. The physicochemical characterization results of Sac/KGF-HF showed that both Sac-HF and Sac/KGF-HF had a high swelling ratio and flexibility. In addition, Sac-KGF-HF accelerated a wound healing process in mice, probably by promoting the fibroblast cell migration, and stimulating fibroblasts and epithelial cells. It could be concluded that KGF in Sac-HF has the-potential for promoting Sac-HF abilities in wound healing process.
The authors thank Universitas Padjadjaran for providing research grant (No: 751t/UN6.O/PL/2018), Mr. Shinichiro Kaneko, a CEO of Green Science Material, for providing sacran, and Bayu Winata Putera, a manager in PT. Prodia Stem Cell Indonesia, for supplying NIH/3T3 cells.
The authors declare no conflict of interest.