2019 年 67 巻 9 号 p. 935-939
2019 年 67 巻 9 号 p. 935-939
Chafuroside A and chafuroside B are flavone C-glycosides isolated from oolong tea leaves. They have a number of beneficial pharmacological activities related to antiinflammation at various concentrations. However, no crystallographic study of chafurosides has yet been reported. In the present study, the crystal structures of chafuroside A and chafuroside B were investigated using single-crystal X-ray diffraction. The asymmetric unit of the chafuroside A crystal consists of one chafuroside A and two water molecules, and that of chafuroside B contains one chafuroside B and one water molecule. The flavone moiety of chafuroside A is curved, i.e., the angle between the best-fit planes of the chromene and phenyl rings is 18.9°, whereas the chafuroside B flavone moiety is relatively flat. A comparison of the curvatures of the flavone moieties of various C-glycosides showed that the curvature of chafuroside A is significantly larger than those of the others. This structural feature might contribute to the differences between the strengths of the pharmacological activities of chafurosides A and B.
Tea leaves contain a wide variety of polyphenols that are beneficial to health and may have therapeutic applications.1–3) The polyphenolic compounds chafuroside A (II) and chafuroside B (IV) are flavone C-glycosides first isolated from oolong tea leaves4–6) (Fig. 1). A structural feature of chafurosides A and B is the unusual ether linkage between the C2″-hydroxyl group of the mannose and the aglycone, which is generated by the fermentation process of tea leaves through the intramolecular ring-closing reaction of isovitexin-2″-sulfate (I) and vitexin-2″-sulfate (III) (prechafurosides A and B), respectively5,6) (Fig. 1).

These flavonoids have a number of pharmacological activities. The oral administration of chafuroside A (1–10 µg/kg) and chafuroside B (100 µg/kg) reduced the skin reaction of sensitized mice after epicutaneous exposure to 2,4-dinitrofluorobenzene or 2,4,6-trinitro-1-chlorobenzene. This indicates that they have antiinflammatory effects.4,7) In addition, an oral diet containing 10 or 20 ppm chafuroside A reduced the number of polyps formed in adenomatous polyposis coli-deficient mice and hindered the development of azoxymethane-induced colon aberrant crypt foci in rats.8) Chafuroside B (0.3–1 µM) suppressed the production of UV-B-induced immunosuppressive mediators such as interleukin-10, tumor necrosis factor α, and prostaglandin E2, and the expression of the receptor activator of nuclear factor κB ligand mRNA. These effects ameliorate cell damage by blocking DNA damage and UV-B-induced apoptosis in normal human epidermal keratinocytes.9) The results indicate that these flavonoids are promising candidates for new active pharmaceutical ingredients.
Formulations of chafurosides have been designed to extend their therapeutic applications. Orally disintegrating tablets containing powdered tea leaves have been developed using a microwave treatment method,10) which increases the contents of chafurosides A and B by promoting their formation from precursor molecules while retaining immediate disintegration properties and tablet hardness.11) A self-assembled micellar formulation of chafuroside A with improved solubility and, as a result, enhanced antiinflammatory activity, was reported.12) The molecular structure significantly affects the chemical, physical, and biopharmaceutical properties of pharmaceutical preparations; therefore the crystal structures of chafurosides are needed to ensure therapeutic efficiency, minimize toxicity, and optimize the production costs of formulations. In particular, since the water solubility of chafurosides is markedly low, pharmaceutical techniques such as salt formation, co-crystallization, amorphization, and polymorphic transitions are required to overcome that intrinsic physicochemical property. However, no crystallographic study of chafurosides has yet been reported. Here, we report the single-crystal structures of chafuroside A dihydrate and chafuroside B monohydrate, which were extracted from oolong tea leaves and purified, and discuss the effects of their structures on their pharmacological activities.
Chafurosides A and B were extracted from oolong tea leaves, purified with several column chromatographies, and crystallized from aqueous methanol solution using the method reported by Ishida et al.5) Prior to extraction, the oolong tea leaves were heated at above 388 K to promote the conversion of prechafurosides A and B to chafurosides A and B, respectively.6) Purified and recrystallized samples were stored in a desiccator over dry silica gel at room temperature until use.
Single-Crystal Structure DeterminationDetails of the crystallographic data, data collection, and structure refinement are summarized in Table 1. The diffraction data for chafuroside A and B crystals were obtained at the SPring-8 BL02B1 and Aichi Synchrotron Center BL2S1, respectively. The initial structures were determined using SHELXT,13) and the structures were crystallographically refined using SHELXL14) and shelXle.15) Hydrogen atoms were located in difference Fourier maps and were treated as riding on their parent atom. The absolute structure of chafuroside A could not be reliably determined from the Flack parameter16) value (0.35(25)), and the absolute structure was set at that reported by Ishida et al.5) In the chafuroside B refinement, nine bond lengths and two angle distances of the disordered glucopyranoside ring were restrained to each of the average values calculated using Mogul in the CSD system. Although the hydrogen atoms of chafuroside B and a water molecule at the major site, with occupancies of 0.853(4), were located in difference Fourier maps, those at the minor site, with occupancies of 0.147(4), were not, and the hydrogen atoms were restrained so that reasonable hydrogen bonds were formed. Hydrogen atoms were treated as riding and the Uiso(H) values were treated as in the refinement of chafuroside A dihydrate. The Flack parameter of the chafuroside B monohydrate crystal, −0.11(15), suggested the correct assignment of the absolute structure, which is consistent with that reported by Ishida et al.5)
| Crystal data | Chafuroside A dihydrate | Chafuroside B monohydrate |
|---|---|---|
| Chemical formula | C21H18O9·2 (H2O) | C21H18O9·H2O |
| Space group | P212121 | P21 |
| Crystal color | Yellow | Yellow |
| Cell parameters (Å) | ||
| a | 6.8007 (7) | 4.880 (4) |
| b | 10.7162 (11) | 19.650 (4) |
| c | 26.047 (3) | 9.720 (2) |
| Z | 4 | 2 |
| Data collection | ||
| Crystal size (mm) | 0.2 × 0.01 × 0.01 | 0.4 × 0.02 × 0.01 |
| Temperature (K) | 100 | 100 |
| Wavelength (Å) | 0.7006 | 0.75 |
| (sin θ/λ)max (Å−1) | 0.649 | 0.666 |
| No. of reflections | ||
| Measured | 11792 | 30375 |
| Independent | 4288 | 4550 |
| Observed [I > 2σ] | 4157 | 4402 |
| Rint | 0.0314 | 0.098 |
| Refinement | ||
| No. of reflections | 4288 | 4550 |
| R[F2 > 2σ (F2)] | 0.0306 | 0.0434 |
| wR(F2) | 0.1002 | 0.124 |
| Δρmax, Δρmin (e Å−3) | 0.33, −0.23 | 0.35, −0.43 |
The atomic coordinates and diffraction data were deposited in the Cambridge Structural Database (CCDC numbers 1897680 for chafuroside A dihydrate and 1897681 for chafuroside B monohydrate).
The asymmetric unit of the crystal consists of one chafuroside A and two hydration water molecules (Fig. 2a). The torsion angle between the phenyl and chromene rings, O1–C2–C11–C12, is −9.5(2)°. In the crystal, the phenyl and chromene rings of the flavone moieties are stacked alternately along the a-axis. The stacking distance, represented by C10 of the chromene ring at (x, y, z) and C11 of the phenyl ring at (x − 1/2, −y + 3/2, 1 − z) is 3.400(3) Å (Fig. 2c). One intramolecular hydrogen bond is formed at the chromene ring between O5 as the hydrogen donor and O4 as the hydrogen acceptor (Table 2). The phenolic hydroxyl oxygen atom O6 acts as a hydrogen donor and forms an intermolecular hydrogen bond with the hydroxyl oxygen of the glucopyranoside moiety, O9, at (x − 1/2, −y + 3/2, −z + 1), and the O9 atom in turn is hydrogen bonded as a hydrogen donor to O7 at (−x + 1/2, −y + 2, z + 1/2) (Fig. 2b). The oxygen atom OW1 of a hydration water molecule acts as the hydrogen bond donor in formation of a hydrogen bond with the other hydration water oxygen atom OW2 at (x + 1, y, z) (Fig. 2c). This pair of hydration water molecules is surrounded by the hydroxyl and carbonyl oxygen atoms of chafuroside A to form a total of five hydrogen bonds.
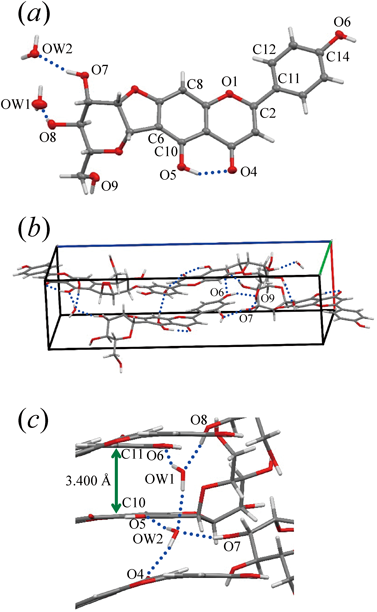
(a) Carbon, hydrogen, and oxygen atoms are shown in gray, white, and red, respectively. Hydrogen bonds are shown as blue dotted lines. Displacement ellipsoids are drawn at the 50% probability level. (b, c) The stacking distance is shown with a green arrow.
| Hydrogen bond | Distances (Å) | Angle (degree) | |||
|---|---|---|---|---|---|
| Donor (D) | Acceptor (A) | D…A | H…A | D–H…A | |
| Chafuroside A dihydrate | O5 | O4 | 2.559 | 1.80 | 149 |
| O7 | OW2 | 2.714 | 1.90 | 162 | |
| O8 | OW1 | 2.719 | 1.89 | 169 | |
| O6· | O9(i) | 2.752 | 1.98 | 153 | |
| O9 | O7(ii) | 2.842 | 2.04 | 159 | |
| OW1 | O6(iii) | 2.902 | 2.10 | 158 | |
| OW1 | OW2(iv) | 2.889 | 2.07 | 167 | |
| OW2 | O5(v) | 2.846 | 2.01 | 168 | |
| OW2 | O4(vi) | 2.802 | 1.96 | 172 | |
| Chafuroside B monohydrate | O5 | O4 | 2.598 | 1.85 | 148 |
| OWA | O7A | 2.802 | 2.00 | 162 | |
| OWB | O7B | 2.92 | 2.17 | 149 | |
| O7A | O4(vii) | 2.711 | 1.89 | 164 | |
| O7B | O4(vii) | 2.96 | 2.15 | 162 | |
| O8B | O5(viii) | 2.85 | 2.19 | 135 | |
| O9A | O4(ix) | 2.809 | 1.97 | 173 | |
Symmetry codes: (i) x − 1/2, −y + 3/2, −z + 1; (ii) − x + 1, y − 1/2, −z + 1/2; (iii) − x + 1/2, −y + 2, z − 1/2; (iv) x + 1, y, z; (v) − x, y + 1/2, −z + 1/2; (vi) − x + 1/2, −y + 1, z − 1/2; (vii) − x + 2, y + 1/2, −z + 2; (viii) − x + 3, y + 1/2, −z + 2; (ix) − x + 2, y + 1/2, −z + 1.
The asymmetric unit in the crystal contains one chafuroside B and one water molecule (Fig. 3a). The planes of the phenyl and chromene rings in the flavone moiety are twisted with a torsion angle (O1–C2–C11–C12) of −8.9(3)°. The glucopyranoside moiety of chafuroside B and a water molecule, which is hydrogen bonded to the hydroxyl oxygen O7 of the glucopyranoside moiety, are disordered and were modeled at two sets of sites (Table 2). The hydroxymethyl group (–C22A–O9A) of the glucopyranoside moiety in the major conformer adopts a gauche–trans conformation and is hydrogen bonded as a hydrogen donor to the symmetry-related carbonyl oxygen atom O4 of the flavone moiety (Fig. 3b). The hydroxymethyl group in the minor conformer (–C22B–O9B) adopts a gauche–gauche conformation and is hydrogen bonded as a hydrogen acceptor to the symmetry-related water molecule in the major conformation.
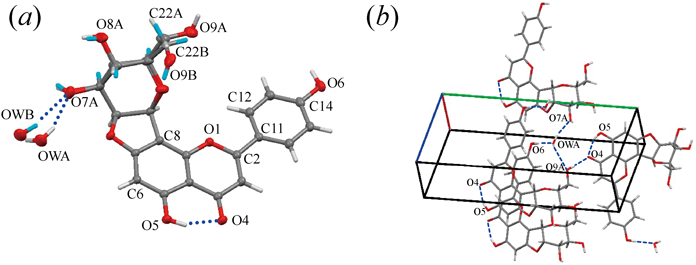
Atom coloring is the same as in Fig. 2, except that hydrogen atoms riding on the minor conformer are shown in sky blue.
Many hydrogen bonds are formed in the crystal structures of chafurosides and this might be involved in the stability of the structures, resulting in the poor water solubility of chafurosides. In addition, the flavone moiety of chafuroside A is curved, i.e., the angle between the best-fit planes of the chromene and phenyl rings is 18.9°, whereas the chafuroside B flavone moiety is relatively flat. The curvatures of the flavone moieties of flavone C-glycosides glycosidated at chromene C6 or C8, as in chafurosides A and B, were compared on the basis of dcurve, which is defined (Fig. 4a) as the distance between the best-fit plane of the chromene ring and the carbon atom of the phenyl group to which the hydroxyl group is bound (C14 in the cases of chafurosides A and B). Figure 4b shows a plot of the dcurve values of the flavone C-glycosides for which crystal structures have been reported. The average dcurve value ± the standard deviation is 0.37 ± 0.28 Å. The dcurve values of the flavone C-glycosides, except for chafuroside A, are evenly distributed around 0.0 Å, which indicates that the curvature of the flavone moiety of chafuroside A (dcurve = 1.01 Å) is significantly large (Fig. 4c). As mentioned in Introduction, although each chafuroside shows various pharmacological activities at different concentrations, the strengths of their pharmacological activities depend on the interactions between each chafuroside and receptors that have not yet been identified. Since the product structure generally affects the interactions, we strongly suggest that differences among the curvatures of the flavone moieties are also factors in the differences among the receptor affinities. In particular, the large curvature of the flavone moieties in chafuroside A can give high flexibility to the structure in solution, and this might be involved in the differences in the receptor affinities. Further studies of the structure–activity relationships are needed. However, this is the first evidence that curving of the flavone moiety may have important effects on the biological activities of the flavone C-glycosides chafurosides A and B.

(a, b) Flavonoids glycosidated at C6 or C8 are colored blue or red, respectively. CSDS IDs: MAJWIE,17) VEFHAP,18) LEKVIG,19) YULFIV,20) KOJXOW,21) OVEMUZ,22) and BAVTEX.23,24) (c) Chafurosides A and B and other flavonoids are shown in blue, red, and gray, respectively. Only the molecules with larger |dcurve| values are shown in the cases of YULFIV and KOJXOW. Hydrogen atoms are not shown for clarity.
The synchrotron radiation experiments on chafuroside A were conducted at SPring-8 BL02B1, with the approval of the Japan Synchrotron Radiation Research Institute (proposal no. 2015A1293). The synchrotron radiation experiments on chafuroside B were conducted at BL2S1 of the Aichi Synchrotron Radiation Center, Aichi Science & Technology Foundation, Aichi, Japan (proposal no. 2015N2011). This work was partly supported by the Japan Society for the Promotion of Science KAKENHI (Grant no. 17K08467).
The authors declare no conflict of interest.