抄録
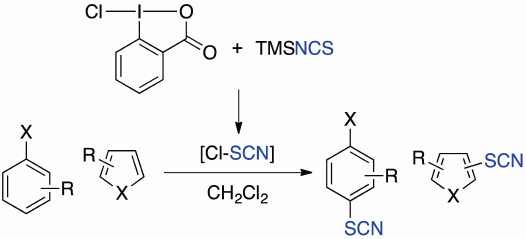
Thiocyanation of aromatic compounds has been investigated using the combination of 1-chloro-1,2-benziodoxol-3-(1H)-one (1) and (trimethylsilyl)isothiocyanate (TMSNCS). The reaction with electron rich aromatic compounds proceeded smoothly to provide the thiocyanated products in high yield, while electron deficient heteroaromatic compounds were not suitable for this reaction. In these reactions, the regioselectivity was generally high. Transformations of the products were also investigated to demonstrate the utility of the reaction. Based on NMR experiments, we propose that thiocyanogen chloride is generated in situ as an active species.