2020 Volume 68 Issue 1 Pages 46-57
2020 Volume 68 Issue 1 Pages 46-57
Over the past decade, a number of new 1,4-naphthoquinones have been isolated from natural sources and new 1,4-naphthoquinones with diverse structural features have been synthesized. Cardioprotective, anti-ischemic, hepatoprotective, neuroprotective and some other new properties were found for these compounds; their role in protecting against neurodegenerative diseases has been established. Their anti-inflammatory, antimicrobial and antitumor activities have been studied in more detail; new, previously unknown intracellular molecular targets and mechanisms of action have been discovered. Some compounds of this class are already being used as a medicinal drugs and some substances can be used as biochemical tools and probes for non-invasive detection of pathological areas in cells and tissues in myocardial infarction and neurodegenerative diseases using modern molecular imaging techniques.
1,4-Naphthoquinone (1) or para-naphthoquinone is a natural organic compound derived from naphthalene. Oxidation of two atoms of the benzene ring in the α-position of the naphthalene nucleus leads to 1,4-naphthoquinone, a compound in which the 1,4-quinoid nucleus is annulated with an aromatic (benzene) ring. The quinone ring contains a system of double bonds conjugated with carbonyl groups; it is easily susceptible to reduction, oxidation and addition of O-, N-, and S-nucleophiles. 1,4-naphthoquinone is easily reduced by various agents and converted to 1,4-dihydroxynaphthalene. Natural hydroxyderivatives of 1,4-naphthoquinone lawsone (2) and juglone (3) with hydroxyl groups in the α- and β-positions of the naphthalene core form salts and complexes with cations of various metals and are used as dyes. 5,8-Dihydroxy derivative of 1,4-naphthoquinone (naphthazarin) (4) exists in various tautomeric forms which react with the formation of different reaction products (Fig. 1). The tautomeric equilibrium in the naphthazarin core depends on the nature of the substituents on the core. The high reactivity of naphthoquinones and the well-developed methods of chemical modification of naphthoquinones make this group of compounds attractive for the profound development of new types of substances with high biological activity.

Although there exist several reviews, books and surveys related to the isolation of natural quinones, determination of their structure and to studies on their biological activity, complete synthesis and numerous modifications of their structure, these works do not cover all the available data in this area.1–5) The present report discusses modern aspects of the problem and reviews relevant publications up to 2019.
1,4-Naphthoquinones are common metabolites of plants, animals, fungi and bacteria.1,6) The range of biological effects of natural and synthetic 1,4-naphthoquinones is diverse and includes antimicrobial, antifungal, antiviral, antiprotozoal, wound healing, cytotoxic, antitumor and some other properties.7–15) Historically, natural naphthoquinones such as lawsone (2), juglone (3) and lapachol (5) were among the first to be investigated.6)
Lawsone (2), one of the best-known naphthoquinones, was isolated from a tropical shrub Lawsonia inermis (henna). It was well-recognized since ancient times for its ability to dye wool and silk in orange color. The paste made from ground leaves of henna is used by women to dye their hair in a distinctive red color.16)
The 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone lapachol (5) and its cyclic derivatives α- and β-lapachone (6–7) were isolated from tropical plants of the family Bignoniaceae. Lapachol (5) and their cyclic derivatives α- and β-lapachone (6–7) are used in traditional Brazilian medicine as anti-parasitic, antimalarial, anti-inflammatory, antiseptic and anticancer agents3,17) (Fig. 2).

Juglone (5-hydroxy-1,4-naphthoquinone, 3) was isolated from leaves and nuts of various plants (Juglandaceae)1,6) and has antibacterial and fungicidal properties. The related 7-methyljuglone (8) and its dimer diospyrin (9) (Fig. 3) were identified as active components of root extracts of Euclea natalensis with antitubercular properties.18) A series of synthetic and plant-based naphthoquinone derivates of the 7-methyljuglone scaffold have been isolated and evaluated for antibacterial activity against Mycobacterium tuberculosis.19)

Subsequent studies have shown that the introduction of hydrophobic substituents in positions 2 and 3 of the quinone core of juglone leads to increase in antimicrobial activity.20) It appears that the introduction of the hydrophobic tetra-O-acetyl-O-β-D-glucopyranosyl substituents into positions 2 and 3, leads to the formation of compounds (10) and (11) with a significant (2–4 times) increase in antifungal activity against Trichophyton mentagraphytes.21) The antitumor activity of juglone and its derivatives has also been the subject of numerous studies, but the high toxicity of juglone has prevented its use for the treatment of tumors. A promising approach to the modification of juglone was the attachment to the quinone core of the per-O-acetyl-O- and per-O-acetyl-S-glycoside substituents, which led to new O- and S-glycoside derivatives (10–13) (Fig. 4) and other related products active in vitro in human leukemia HL60 cells.22,23) The glycosides (10) and (11) were evaluated in vivo for their antitumor activity in mouse Ehrlich ascites carcinoma. The most effective glucoside (10) increased the average lifespan of mice by a factor of two or longer.24)

Another representative compound of the naphthazarine series used in medicine is shikonin (14)—a quinone secondary metabolite produced by the plant Lithospermum erythrorhizon (red root). Shikonin has antitumor, antiviral, wound healing, anti-inflammatory and antimicrobial properties.25–27) In addition to shikonin, the plant has been found to contain its derivatives (15–17)—side-chain hydroxyl esters, mainly acetyl (15) and isobutyryl shikonin (17) (Fig. 5).
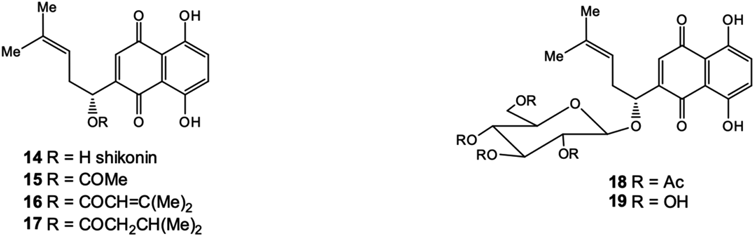
Glucosidation of hydroxyl group in side chain of shikonin28) leads to the formation of new glycosides (18, 19) (Fig. 5) that induce 70 kDa heat shock proteins (HSP70) biosynthesis in cells.29) Yehua et al. also synthesized the glucoside (16) and related shikonin glycosides with various mono- and disaccharides moietes and tested these derivatives in comparison to shikonin against both drug-sensitive cell lines (K562, MCF-7 and HL60) and their drug-resistant cell sublines (K562/ADR, MCF-7/ADR and HL60/ADR).30) Most of the shikonin derivatives exhibited good cytotoxicity against all three cancer cell lines evaluated. The results suggest that some of the synthesized glycosides, such as D-xyloside and lactoside, are the potential compounds for treatment of certain drug-resistant cancers. Currently, shikonin (14) is produced by biotechnology and this quinone is a good platform for the versatile structural modifications directed to the synthesis of new antitumor and antiviral substances capable to suppress the replication of human immunodeficiency virus type 1 (HIV-1) drug-resistant strains.26,27)
In the last decade, along with the search for new cytotoxic and antimicrobial 1,4-naphthoquinones, investigations are being focused on naphthoquinones in relation with their protective functions in cells. The group includes substances with cardioprotective, neuroprotective, hepatoprotective and anti-inflammatory properties. 1,4-Naphthoquinones with anti-bacterial and antitumor activity have been studied in more detail. An expert review31) of works on natural and synthetic naphthoquinones for the period 2001–2012 have demonstrated a significant increase in the number of papers and patents describing the synthesis, modification and study of the biological activity of natural substances based on the 1,4-naphthoquinone core.
2.2. Cardioprotective and Anti-ischemic PropertiesThe polyhydroxynaphthoquinones of the naphthazarin group (5,8-dihydroxy-1,4-naphthoquinone) occupy a special place among naphthoquinones. A distinctive feature of these compounds is their ability to effectively intercept free radicals and bind the Fe2+ ions responsible for the formation of reactive oxygen forms, the excessive content of which in the body leads to the development of various pathological conditions. It is believed that the cardioprotective properties of naphthoquinones are due to their antioxidant effect.32,33)
The best-known and most accessible compound of this class is echinochrome (2,3,5,6,8-pentahydroxy-7-ethyl-1,4-naphthoquinone, 20) (Fig. 6)—a natural pigment of marine origin found in the shells and needles of many species of sea urchins but mainly isolated from sea urchin “sand dollar” Scaphechinus mirabilis. Echinochrome was used to develop the medicinal drug histochrome (Histochrome™). Histochrome has found application in cardiology, ophthalmology and in the treatment of chronic lung diseases.34,35) The therapeutic effect is based on the ability of histochrome to capture free radicals and stabilize cell membranes. As also known, ferrous cations accumulate in myocardium tissue damaged by ischemia and initiate non-enzymatic oxidation of membrane lipids. In contrast to basic endogenic cardioantioxidants (vitamin A and ubiquinon), histochrome neutralizes these cations and prevents myocardium from toxic peroxides.

Recent studies have shown that treating cardiomyoblast cells with echinochrome causes a significant increase in mitochondrial mass and oxidative phosphorylation functions, which enhances mitochondrial energy efficiency by modulating major mitochondria biogenesis regulatory genes, including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), estrogen-related receptor alpha (ERR-α), peroxisome proliferator-activated receptor gamma (PPAR-γ) and NRF-1, and some such mitochondrial transcription regulatory genes as mitochondrial transcription factor A (TFAM), dimethyladenosine transferase 2, mitochondrial (TFB2M), DNA-directed RNA polymerase, mitochondrial (POLMRT), single-stranded DNA-binding protein, mitochondrial (SSBP) and elongation factor Tu, mitochondrial (TUFM).36) Also, a novel mechanism of action for echinochrome that regulates phosphorylation of phospholamban resulting in a delay in Ca2+ uptake by sarcoplasmic/endoplasmic reticulum calcium ATPase 2a (SERCA2A) and consequently induces a negative inotropic effect, was discovered. Moreover, echinochrome was found to be a potential drug for enhancing cardiomyocyte differentiation in embryonic stem cells through direct binding to protein kinase C iota type (PKC-iota) and inhibition of its activity.37,38)
The protective effect of histochrome (echinochrome) pretreatment of human cardiac progenitor cells (hCPCs) against hydrogen peroxide (H2O2)-induced oxidative stress was investigated. It was found that histochrome-treated hCPCs showed significant protective effects against H2O2-induced cell death. The anti-apoptotic proteins B-cell lymphoma 2 regulator protein (Bcl-2) and Bcl-xL were significantly up-regulated, whereas the pro-apoptotic proteins apoptosis regulator BAX (Bax), H2O2-induced cleaved caspase-3, the DNA damage marker and phosphorylated histone (γH2A.X) foci were significantly down-regulated upon treatment of hCPCs in vitro. Furthermore, prolonged incubation with histochrome alleviated the replicative cellular senescence of hCPCs. Thus, the authors reported the protective effect of histochrome against oxidative stress and suggested the use of a potent and bio-safe cell priming agent as a potential therapeutic strategy in patient-derived hCPCs to treat heart disease.39)
The cardioprotective effects of spinochrome D (2,3,5,6,8-pentahydroxy-1,4-naphthoquinone, 21) (Fig. 6), a structural analogue of echinochrome isolated also from sea urchins, against doxorubicin were investigated using human cardiomyocyte cell line (AC16) and human breast cancer cell line (MCF-7). Spinochrome D was found to protect AC16 cells from doxorubicin cytotoxicity, but it did not have the antitumor properties. With spinochrome D (21) treatment, the mitochondrial membrane potential and mitochondrial calcium localization were significantly different between cardiomyocytes and cancer cell lines. These findings suggest that spinochrome D (21) could be cardioprotective against the cytotoxicity of doxorubicin.40)
It was shown that histochrome (echinochrome, 20) enhances the vasodilatory effect of selective beta1 blocker, nebivalol, in cerebral and renal arteries, using a combined therapy in the renovascular model of arterial hypertension, induced in Wistar and OXYS rat strains. Comparative magnetic resonance imaging (MRI) angiography and MRI morphometry of the cerebral and renal arteries in hypertensive rats showed that histochrome expands the therapeutic potential of nebivalol due to angioprotective effects in the vascular region of the target organs.41)
The diglutathionyl functional analogue of echinochrome (7-ethyl-2,3-diglutathionyl-6-hydroxynaphthazarin, 22) (Fig. 6) with additional two glutathione tripeptide residues was synthesized; it has a number of advantages over the initial echinochrome—improved solubility and stability in aqueous solutions, and reduced toxicity. Its effect (22) was evaluated in a model of focal ischemia (ischemic stroke) by occlusion of middle cerebral artery. The behavioral reactions of rats after occlusion showed an increase of cognitive frustration. Similarly to echinochrome, the diglutathionyl analogue (22) reduced the region of cytotoxic edema in rat brain after treatment and increased the survival rate of rats by more than 30%. The recovering effect of (22) begins within 48 h, while (20)—within 72 h. It was found that the compound (22) can be an ischemic rat-brain protector.42)
Also, the effects of therapeutic or preventive-therapeutic administration of other water-soluble echinochrome analog U-441 (5,7,8-trihydroxy-2,3-diglutathionyl-6-methyl-1,4-naphthoquinone, 23) (Fig. 6) on arrhythmia severity assessed by a set of myocardial spatio-temporal depolarization and repolarization parameters were examined in a model of acute myocardial ischemia in cats. Paradoxically, U-441 (23) produced no effect on arrhythmia severity.43)
2.3. Hepatoprotective PropertiesRecently, it was discovered that echinochrome (20) administration significantly improved liver function, as indicated by decreased activities of liver enzymes such as alanine transaminase (ALT), gamma-glutamyltransferase (GGT), lactate dehydrogenase (LDH), aspartate transaminase (AST) and alkaline phosphatase (ALP), as well as by a higher albumin content. Moreover, echinochrome could counteract the hepatic oxidative stress of sepsis-induced liver damage via a marked increment in the glutathione content and intensity of antioxidant enzyme activities, as well as down-regulation of malondialdehyde, nitric oxide and hydrogen peroxide formation. Additionally, echinochrome treatment repaired the abnormal architecture of hepatic tissues induced by polymicrobial infection. Therefore, echinochrome (20) could be used as a potential alternative antiseptic remedy via oxidative damage attenuation.44)
In other experiments it was demonstrated that echinochrome (20) exerted hepatoprotective and anticholestatic effects as assessed by a significant decrease in the activities of serum AST, ALT and ALP, the levels of total protein and bilirubin, and liver MDA and NO levels in the animal model of intrahepatic cholestasis in rats induced by hepatotoxin such as alpha-naphthylisothiocyanate.45) Additionally, this compound showed the ability to improve the functioning of the muscular system and lipid and protein metabolism in first- and second-type diabetic patients.46)
2.4. Anti-inflammatory and Analgesic PropertiesAnti-inflammatory and analgesic properties of a number of naphthoquinones have been discovered. According to Zhang,47) chimaphilin (24) (Fig. 7), by inhibiting the release of histamine, has an anti-inflammatory and analgesic effect, while the anti-inflammatory effect of shikonin (14) is realized by inhibiting cyclooxygenase-2 at doses of 5–20 mg/kg and the production of tumor necrosis factor (TNF)-α at doses of 2.5–10 mg/kg.48,49) Naphthoquinone (25) and its dihydroderivative (26) (Fig. 6) were isolated from the culture of the fungus Dendryphion nanum, which is present in the leaves of Ficus religiosa. Compound (25) has significant anti-inflammatory properties that inhibit LPS-induced cytokine production in human mononuclear cell lines THP-1 and block the production of TNF-α and interleukin (IL)-6 with an IC50 of 0.60 and 0.06 µM respectively, while (26) was inactive.50)

To date, a number of new 3-halogen and 3-aryl-2-hydroxy-1,4-naphthoquinones have been synthesized, among which a new class of effective inhibitors of P2X7 receptors has been established.51) In fact, currently this is the first published work demonstrating the effective anti-inflammatory and analgesic activity of 1,4-naphthoquinones associated with the blocking of P2X7 receptors. The inhibitory activity of 1,4-naphthoquinone derivatives (AN-03, 27) and (AN-04, 28) (Fig. 8) was higher than that of the well-known P2X7 receptor blockers like BBG and A740003 in experiments on blocking Ca2+ and ethidium bromide influx in mouse peritoneal macrophages (IC50 = 0.05 and 0.02 µM correspondingly), inhibiting pro-inflammatory cytokine interleukin-1β (IL-1β) production in THP-1 cells (IC50 = 0.04 and 0.01 µM correspondingly) and reducing edema in the carrageenan model of inflammation at doses of 0.01–1.0 mg/kg in vivo. The results of electrophysiological experiments and computer simulation in silico suggest that 1,4-naphthoquinones is promising in the development of new effective P2X7 receptor blockers and anti-inflammatory drugs.

A theoretical study of the relationship between the electronic structure and the activity of the P2X7R receptor from a series of 2-hydroxy-1,4-naphthoquinone derivatives, using the quantitative structure–activity relationship (QSAR) method was done.52) The results showed that there are specific zones of these naphthoquinone derivatives potentially involved in the biological process. The presence of the different substituents seems not to have direct participation in the interaction; their importance may be associated with the modification of the electronic structure of the common skeleton. Statistically significant relationships have been obtained between the electronic structure and the functional activity (IC50) for the P2X7R receptor. Based on the analysis of the results, a corresponding two-dimensional pharmacophore was proposed.51,52)
2.5. Antimicrobial ActivityThere are on-going studies on the antimicrobial activity of 1,4-naphthoquinones in relation to various microbial pathogens that are dangerous as sources of fatal diseases, epidemics and nosocomial infections. In some cases, not only the direct effect of new compounds on microbial cells was investigated, but also their effect on the viability of biofilms formed by reproducing microorganisms. Thus, several closely composed new 2-hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinones (29) were synthesized and evaluated against Gram-negative and Gram-positive bacterial strains and their biofilms to probe for potential lead structures. The structure modification applied in the series resulted in 12 new naphthoquinones with pronounced antimicrobial activity against Escherichia coli and Pseudomonas aeruginosa. Four molecules showed antibiofilm properties, among which (29) (Fig. 9) improved the inhibition of biofilm formation by more than 60% with a better profile than the standard antibacterial drug, ciprofloxacin.53,54)

1,4-Naphthoquinone derivatives were found to significantly affect some fungal strains and suppress various bacteria growth, hyphal formation and cell aggregation.55) A series of novel, substituted 2-chloro-3-[(thiazol-2-yl)amino]-1,4-naphthoquinones (30–36) (Fig. 9) have been shown to exhibit promising antimicrobial activity against Plasmodium falciparum, Mycobacterium tuberculosis and P. aeruginosa.56)
A new class of 1,4-naphthoquinone hybrids (37) (Fig. 10) which contain phenylamino-phenylthio moieties as significant counterparts demonstrated major antibacterial activity against bacterial strains Staphylococcus aureus, Listeria monocytogenes, E. coli, P. aeruginosa, Salmonella bongori and Klebsiella pneumoniae.57–59) Two naphthoquinone derivatives (38) and (39) (Fig. 10) had potential antimycobacterial activity against three Mycobacterium tuberculosis strains and did not exhibit cross-resistance with isoniazid and rifampicin; they could be promising scaffolds for the development of new therapeutic strategies against tuberculosis.60)

Two novel heterocyclic ligands, 2-[(5-fluoro-1,3-benzothiazol-2-yl)amino]naphthalene-1,4-dione (40) and 2-[(5-methyl-1,3-benzothiazol-2-yl)amino]naphthalene-1,4-dione (41) (Fig. 10), and their Pd(II), Ni(II) and Co(II) complexes were in vitro effective against several clinically isolated bacteria strains Klebsiella oxytoca, P. aeruginosa, E. coli, Bacillus cereus and S. aureus. Complexes with Ni(II) showed the best antibacterial results.61) Currently, for the treatment of malaria and pneumonia caused by the simplest microorganisms Pneumocystis jirovecii, the drug Malarone (Malarone®) is used, which contains a synthetic derivative of 1,4-naphthoquinone lawsone—atovaquone (42)62) as an active ingredient (Fig. 11).

A new group of hydroxynaphthoquinones was isolated from the callus culture of the tropical liana Triphyllum peltatum, three of which—dioncoquinone B (43), its 6-O β-glucoside (44) and dioncoquinone C (45) (Fig. 11), showed a significant activity against the parasitic protists of Leishmania major. In subsequent studies, the biologically active nucleus of natural quinones was modified by organic synthesis63,64) and the newly obtained biologically active derivatives of these 1,4-naphthoquinones were patented.65)
The efficacy of a series of 2-hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinones against different Trypanosoma cruzi discrete type units of relevant clinical forms of Chagas disease were analyzed. Compound (46) (Fig. 11) showed higher susceptibility to bloodstream trypomastigotes and exhibited a greater efficacy against trypomastigotes from different T. cruzi of Brazil and CL strains through the induction of intracellular autophagy and elevation of ROS level, leading to parasite death.66)
2.6. Cytotoxic and Anticancer PropertiesMany medicinal anticancer drugs based on natural compounds, such as menadione and anthracycline glycosides (doxorubicin, daunorubicin, aclacinomycin A), have a 1,4-naphthoquinoid fragment in their structure. And the search for new effective and selective antitumor naphthoquinones continues.
Synthetic 5-hydroxy-1,4-naphthoquinone derivatives were tested for the activity against three cancer cell lines: colon adenocarcinoma, breast ductal carcinoma and chronic myelogenous leukemia. Among them, 2-(5-hydroxy-1,4-dioxo-1,4-dihydronaphthalen-2-yloxy) acetamide and 2-(5-hydroxy-1,4-dioxo-1,4-dihydronaphthalen-2-yloxy)-N,N-dimethylacetamide (47–49) (Fig. 12) showed moderate-to-excellent activity and can be leads for more potent 5-hydroxy-2-substituted naphthoquinones as novel anticancer agents.67) In the course of investigation of biological properties of quinone derivatives, the N(H)-, S- and S,S-substituted-1,4-naphthoquinones were synthesized. Although all derivatives inhibited human cervical cancer (HeLa) cell growth, the most active compound was 2-(tert-butylthio)-3-chloronaphthalene-1,4-dione (50).68) A new group of 1,4-naphthoquinones bearing halogen and alkyl substituents in positions 2 and 3 was synthesized; the compounds active against acute myeloid leukemia were found among them. The 2-chloro-3-ethyl-5,6,7-trimethoxy-1,4-naphthoquinone (51) (Fig. 12) was the most potent against HL60 leukemia cells.69)

It is reported that some cytotoxic glycosides of 1,4-naphthoquinones exhibit less anticancer potential in vitro than the corresponding aglycones. For example, two shikonin glucosides—shikonin-1′,8-di-O-β-D-glucopyranoside and shikonin-1′-O-β-D-glucopyranoside, were recently biosynthesized through in vitro enzymatic glycosylation, with high water-solubility and stability than those of the parent compound. Unfortunately, both these shikonin glucosides exhibited weak cytotoxicity against MCF-7, MDA-MB-231, H1975, HNE1, SG7901 and I-10 cancer cell lines with IC50 values around 50–60 µM compared to the parent compound, shikonin (14), with IC50 values ranging from 1 to 3 µM.70)
There exists an opinion that the cytotoxic effects of quinones are mainly due to the induction of the formation of semiquinone radical that can transfer an electron to oxygen to produce super oxide, which is catalyzed by flavoenzymes such as NADPH-CYP reductase. Both semiquinone and super oxide of quinones can generate the hydroxyl radical, which is the cause of DNA strand breaks.71) A structure–activity relationship study confirmed that cytotoxic activity of 1,4-naphthoquinones is closely associated with their electron accepting capability, which gives rise to reactive oxygen species (ROS) production leading to DNA damage and cell death.72–74) Besides the mechanism of ROS production, a cytotoxic effect of clinically used quinone-based anticancer drugs is closely related to inhibition of DNA topoisomerase II enzyme, which is an essential enzyme required for DNA replication, chromosome condensation and chromosome segregation.75,76) However, recently along with the study of the effect of these compounds on the viability and proliferation of tumor cells of various natures, there has been a series of investigations regarding the antitumor activity of 1,4-naphthoquinones, which studied their ability to affect key membrane and intracellular molecular targets of tumor cells involved in oncogenesis.
Thus, synthetic compound (4-(((3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)methyl)-N-(pyridin-3-yl)benzamide, 52) (Fig. 13) was found to significantly affect the proliferation of multiple myeloma cells due to strong inhibition of Itch (astrophin-1 interacting protein-4), a HECT domain-E3 ligase.77)
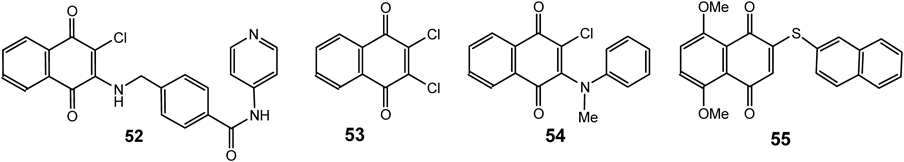
Several 2-amino(chloro)-3-chloro-1,4-naphthoquinone derivatives were investigated for their aromatase inhibitory activities. 2,3-dichloro 1,4-naphthoquinones (53) and 2-methylaminophenyl-3-chloro derivatives (54) were found to be the most potent compounds affording IC50 values 5.2 times lower than that of the reference drug, ketoconazole. The prediction of aromatase inhibitory activity in silico suggested that the 2,3-disubstitution of 1,4-naphthoquinone ring with halogen atoms (i.e., Br, I and F) is the most effective modification for potent activity for these compounds.78)
A novel synthetic naphthoquinone derivative, 2-(naphthalene-2-thio)-5,8-dimethoxy-1,4-naphthoquinone (55) (Fig. 13) exhibited the cytotoxic effects on gastric cancer cells and induced cell apoptosis via ROS-mediated regulation of the mitogen-activated protein kinase (MAPK), protein kinase B (Akt) and signal transducer and activator of transcription 3 (STAT3) signaling pathways.79–80)
Recently, the cytotoxic activity of the synthesized new sulfanyl-phenylamino-1,4-naphthoquinone derivatives (56–59) (Fig. 14) against three human cancer cell lines, A549, HeLa and MCF-7, was evaluated. Some of the compounds showed remarkable cytotoxic activity against cancer cells and induced apoptosis and arrest of the cell cycle at the G1 phase in MCF-7 cells via up-regulation of caspase-3 and caspase-7 protein and gene expression levels.58)

The lead compound, (NSC130362, 60) (Fig. 15), was selected from a small array of 1,4-naphthoquinines and quinoline-5,8-diones and effectively inhibited growth of pancreatic cancer cells via induction of oxidative stress or caspase 3/7 activity; it demonstrated antitumor activity in vivo. Its combination with different oxidative stress inducers, such as arsenic trioxide, myricetin and buthionine sulfoximine, was shown to cause cell death in a variety of breast, pancreatic, prostate and lung carcinoma cell lines as well as in human melanoma cells from patients.81)

The synthetic 1,4-naphthoquinone-2,3-bis-sulfides were most effective against melanoma UACC62 and prostate PC3 cancer cell lines. One compound (61) (Fig. 15) produces ROS which results in MCF-7 breast cancer cell death caused by apoptosis as evidenced by caspase 3/7 activation. Apoptosis was found to occur by the mitochondrial pathway and not by cell cycle arrest. Gene expression studies showed that p53, mouse double minute 2 homolog (Mdm-2) (a p53 regulator) and Bcl-2 (apoptosis inhibitor) were up-regulated during apoptosis induction by compound (61).82)
Two synthesized novel series of N-2,3-bis(6-substituted-4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)naphthalene-1,4-diones and substituted N-(methyl/ethyl)bisquinolinone triethylammonium salts were described as new candidates to extracellular signal-regulated kinases 1/2 (ERK1/2) with considerable antineoplastic activity. These compounds inhibited ETS-1 phosphorylation by ERK2 and showed highest potency for ERK2 inhibition with ATP-competitive inhibition mechanism. Two selected compounds (62–63) (Fig. 16) demonstrated broad spectrum antitumor activity against the nine tumor sub-panels tested.83)

A new series of 1,4-naphthoquinones, bearing various cyclic and aliphatic amines at 2-position, were designed and synthesized to identify antiproliferative agents for triple-negative breast cancer. Among them two compounds (64–65) (Fig. 16) significantly inhibited in vitro the proliferation of MDA-MB-231 cells. Using in silico molecular docking approach and pharmacological tests, the authors suggested that the active compounds may bind G protein-coupled receptor 55 (GPR55) and exert its pronounced antiproliferative activity against breast cancer cells.84)
Plumbagin (66) and menadione (67) (Fig. 16) were found to be toxic for C6 glioblastoma cells, increased the intracellular ROS amount, significantly uncoupled mitochondrial oxidation from phosphorylation impairing energy productions and caused cell death by necrosis.85)
2.7. Neuroprotective PropertiesAmong 1,4-naphthoquinones and their derivatives, compounds with neuroprotective activity were found. 5-hydroxy-1,4-naphthoquinone (juglone, 3) and 3-(p-hydroxyphenyl)-5-methoxy-1,4-naphthoquinone (68) (Fig. 17) inhibits the activity of β-secretase, which is directly involved in the formation of β-amyloid, reduces the aggregation of β-amyloid peptide and induces the disaggregation of pre-formed β-amyloid fibrils associated with Alzheimer’s disease.86)

More recently, on a model of Parkinson’s disease in knockout rats, it was shown that the acetylated tris-O-glucoside of echinochrome (U-133, 69) (Fig. 18), activates the HSF1 transcription factor and increases the expression of inducible Hsp70, which leads to reversal of neurodegeneration processes.87) It was later found that echinochrome (20) is a non-competitive acetylcholinesterase inhibitor, which opens up prospects for the use of echinochrome for the treatment of neuromuscular disorders, as well as Alzheimer’s and Parkinson’s diseases.88)
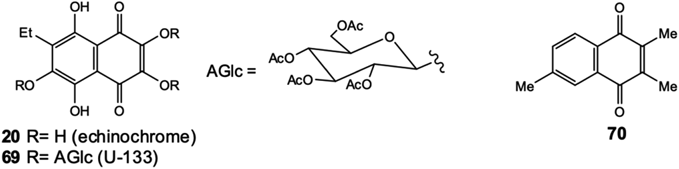
The monoamine oxidase (MAO) enzymes are of considerable pharmacological interest and inhibitors are used in the clinic for the treatment of major depressive disorder and neurodegenerative diseases. 2,3,6-trimethyl-1,4-naphthoquinone (70) (Fig. 18), contained in extracts of cured tobacco leaves, has MAO non-selective inhibition properties. Another compound, 5,8-dihydroxy-1,4-naphthoquinone (naphthazarin, 4), was found to be the most potent inhibitor for MAO-B. This study contributes to the discovery of novel MAO inhibitors, which may be useful in the treatment of disorders such as Parkinson’s disease, depressive illness, congestive heart failure and cancer.89)
2.8. 1,4-Naphthoquinones as Biomedical InstrumentsThe potential of some 1,4-naphthoquinones to rapidly visualize necrotic myocardium and the possible mechanisms of necrosis avidity was discovered. Not so long ago, six 1,4-naphthoquinones, 1,4-naphthoquinone (1), juglone (3), naphthazarin (4), plumbagin (66) and menadione (67) were radiolabeled with iodine-131; the necrotic myocardium imaging property and biodistribution of radio-labeled compounds were determined in rat models with re-perfused myocardial infarction. It was found that the necrotic myocardium could be clearly visualized by modern SPECT/CT tomography using this compound as early as 3 h post-injection. Among studied radiotracers, [131I]-naphthazarin (71) (Fig. 19) showed the highest necrotic-to-viable ratio due to its direct binding with exposed DNA in necrotic tissues.90)

Recently, 41 novel 1,4-naphthoquinone derivatives were synthesized and 14 antiamyloidogenic compounds were initially discovered using in vitro β-amyloid aggregation assay. Follow-up docking and biophysical studies revealed that four of them (8-hydroxy-2-((3-methoxybenzyl)amino)naphthalene-1,4-dione (72), 2-(benzylamino)-5-hydroxynaphthalene-1,4-dione (73), 6-chloro-7-((2-iodophenyl)amino)isoquinoline-5,8-dione (74) and 1H-pyrrolo[3,2-g]isoquinoline-4,9-dione (75) (Fig. 19) penetrate the blood-brain barrier, bind directly to β-amyloid aggregates and enhance fluorescence properties upon interaction. These compounds specifically stain both diffuse and dense-core β-amyloid plaques in brain sections of APP/PS1 double transgenic Alzheimer’s mouse models and can be suggested as a new scaffold for β-amyloid imaging agents for early stage Alzheimer’s.91)
Over the past decade, a number of new 1,4-naphthoquinones have been isolated from natural sources and new 1,4-naphthoquinones with diverse structural features have been synthesized. Cardioprotective, anti-ischemic, hepatoprotective, neuroprotective and some other new properties were found for these compounds; their role in protecting against neurodegenerative diseases has been established. Their anti-inflammatory, antimicrobial and antitumor activity has been studied in more detail; new, previously unknown intracellular molecular targets and mechanisms of action have been discovered. Some compounds of this class are already being used as medicinal drugs and some substances can be used as biochemical tools and probes for non-invasive detection of pathological areas in cells and tissues in myocardial infarction and neurodegenerative diseases using modern molecular imaging techniques. The summarized data on biological properties of some 1,4-naphthoquinones are presented in Table 1. All of the above suggest that 1,4-naphthoquinones are a kind of unique molecules with a wide range of diverse biological activities and a very large potential for the use in medical practice.
| # | 1,4-Naphthoquinone | Activity/molecular target | Ref |
|---|---|---|---|
| Cardioprotective and anti-ischemic properties | |||
| (20) | 2,3,5,6,8-Pentahydroxy-7-ethyl-1,4-naphthoquinone (echinochrome) | Modulates PGC-1α, ERR-α, PPAR-γ and NRF-1 genes, TFAM, TFB2M, POLMRT, SSBP and TUFM genes, regulates phospholamban phosphorylation, inhibits SERCA2A; has protective effects against H2O2-induced cell death; inhibits PKC-iota; has angioprotective effects in the vascular region of the cerebral and renal arteries | 36) |
| 37) | |||
| 38) | |||
| 39) | |||
| 41) | |||
| (21) | 2,3,5,6,8-Pentahydroxy-1,4-naphthoquinone (spinochrome D) | Protects human cardiomyocyte cells from doxorubicin cytotoxicity | 40) |
| (22) | 7-Ethyl-2,3-diglutathionyl-6-hydroxynaphthazarin | Reduces the region of cytotoxic edema in rat brain after ischemic stroke | 42) |
| Hepatoprotective properties | |||
| (20) | 2,3,5,6,8-Pentahydroxy-7-ethyl-1,4-naphthoquinone (echinochrome) | Improves liver function by the decreased enzyme activities of ALT, GGT, LDH, AST, ALP; counteracts the hepatic oxidative stress; decreases the levels of serum total protein and bilirubin, liver MDA and NO level; improves the functioning of the muscular system, lipid and protein metabolism of diabetes patients of the first and second type | 44) |
| 45) | |||
| 46) | |||
| Anti-inflammatory and analgesic properties | |||
| (24) | Chimaphilin | Inhibits release of histamine | 47) |
| (14) | Shikonin | Inhibits cyclooxygenase-2 and normalizing the production of TNF-α | 48) |
| 49) | |||
| (27–28) | 3-Halogen and 3-aryl-2-hydroxy-1,4-naphthoquinones | Block P2X7 receptors | 51) |
| 52) | |||
| Anti-microbial activity | |||
| (29) | 2-Hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinones | Exhibit pronounced antimicrobial activity against E. coli and P. aeruginos in biofilms | 53) |
| 54) | |||
| (30–36) | 2-Chloro-3-[(thiazol-2-yl) amino]-1,4-naphthoquinones | Exhibit promising anti-microbial activity against P. falciparum, M. tuberculosis and P. aeruginosa | |
| (40) | 2-[(5-Fluoro-1,3-benzothiazol-2-yl)amino]naphthalene-1,4-dione; | Exhibit best antibacterial activity against K. oxytoca, P. aeruginosa, E. coli, B. cereus and S. aureus | 61) |
| (41) | 2-[(5-Methyl-1,3-benzothiazol-2-yl)amino]naphthalene-1,4-dione and their Ni(II) complexes | ||
| (46) | 2-Hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinones | Are effective against T. cruzi, induction of intracellular autophagy and elevation of ROS level | 66) |
| Cytotoxic and anticancer activity | |||
| (52) | (4-(((3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)methyl)-N-(pyridin-3-yl)benzamide | Inhibits cell proliferation; strongly inhibits Itch (astrophin-1 interacting protein-4), a HECT domain-E3 ligase | 76) |
| (53) | 2,3-Dichloro 1,4-naphthoquinone; 2-methylaminophenyl-3-chloro derivative | Inhibit aromatase | 77) |
| (54) | |||
| (55) | 2-(Naphthalene-2-thio)-5,8-dimethoxy-1,4-naphthoquinone | Inducts cell apoptosis via ROS-mediated regulation of MAPK, Akt and STAT3 signaling pathways | 78) |
| 79) | |||
| (56–59) | Sulfanyl-phenylamino-1,4-naphthoquinone derivatives | Induct apoptosis and arrest of cell cycle at the G1 phase via up-regulation of caspase-3 and caspase-7 | 58) |
| (60) | 1,4-Naphthoquinines and quinoline-5,8-dione | Induct oxidative stress and caspase 3/7 activity | 80) |
| (61) | 1,4-Naphthoquinone-2,3-bis-sulfide | Inducts apoptosis, caspase 3/7 activation, up-regulation of p53, Mdm-2 and Bcl-2, down-regulation of Bax gene expression | 81) |
| (62–63) | N-2,3-Bis(6-substituted-4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)naphthalene-1,4-diones | Inhibit ETS-1 phosphorylation by ERK2 | 82) |
| (64–65) | 1,4-Naphthoquinones, bearing various cyclic and aliphatic amines at 2-position | Bind G protein-coupled receptor 55 (GPR55) and exert its pronounced anti-proliferative activity | 83) |
| (66) | Plumbagin | Increase intracellular ROS amount, uncoupled mitochondrial oxidation from phosphorylation impairing energy production and cause cell death by necrosis | 84) |
| (67) | Menadione | ||
| Neuroprotective properties | |||
| (3) | 5-Hydroxy-1,4-naphthoquinone (juglone) | Inhibit β-secretase activity, reduce β-amyloid formation and aggregation | 85) |
| (68) | 3-(p-Hydroxyphenyl)-5-methoxy-1,4-naphthoquinone | ||
| (69) | Tris-O-glucoside of echinochrome; | Activates the HSF1 transcription factor and increases the expression of inducible Hsp70; | 87) |
| (18–19) | Shikonin glycosides | Induce HSP70 biosynthesis in cells | 29) |
| (70) | 2,3,6-Trimethyl-1,4-naphthoquinone | Inhibit MAO-B | 88) |
| (4) | 5,8-Dihydroxy-1,4-naphthoquinone | ||
This work was supported by the Russian Science Foundation (Grant No. 19-14-00047).
The authors declare no conflict of interest.