2020 年 68 巻 10 号 p. 946-953
2020 年 68 巻 10 号 p. 946-953
In the present study, a novel cocrystal of felodipine (FEL) and β-resorcylic acid (βRA) was developed. We specially focused on the change of binding pattern with bovine serum albumin (BSA) induced by cocrystallization of FEL with βRA. The solid characterizations and density functional theory (DFT) simulation verified that FEL–βRA cocrystal formed in equimolar ratio (1 : 1 M ratio) through C=O…H–O hydrogen bond between C=O group in FEL and O–H group in βRA. The binding interactions between FEL–βRA system and BSA were studied using fluorescence spectral and molecular docking methods. Two guest molecule systems, including a physical mixture of FEL and βRA and FEL–βRA cocrystal were performed binding to BSA in molecular docking. According to the Kb and binding energy, the supramolecular form of FEL–βRA system was retained during binding to BSA. Molecular docking simulation suggested that FEL and its cocrystal inserted into the subdomain IIIA (site II′) of BSA. The interactions between FEL and BSA including hydrogen bonding with ASN390 residue and intermolecular hydrophobic interactions with LEU429 and LEU452 residues. However, the size of supramolecular FEL–βRA better matched that of active cavity of BSA; the cocrystal is closely bound to BSA through hydrogen bonding with ASN390 residue and intermolecular hydrophobic interactions with LEU429, VAL432, LEU452 and ILE387 residues. This change on binding affinity of FEL to BSA induced by cocrystallization with βRA provided theoretical basis to evaluate the transportation, distribution and metabolism of cocrystal drug.
Most new chemical entities in the pharmaceutical development pipeline are poorly water-soluble, thereby posing a challenge for oral formulation development.1) In recent decades, the design of cocrystals has received considerable attention due to its unique advantages on improving the solubility, dissolution rate, stability, and bioavailability.2–5) Cocrystals are constructed using active pharmaceutical ingredients (APIs) and cocrystal formers (CCFs) through various intermolecular interactions, such as aromatic π–π stacking, hydrogen bonds, or van der Waals forces.6) In most cases, the hydrogen bond is one of the most important interaction force occurring between API and CCF molecules in cocrystal.3,7)
Most studies on cocrystals focused on their screening, structural characterization, evaluations on physicochemical properties in vitro.8,9) Sun et al. investigated the solubility behavior of the (indomethacin + nicotinamide) cocrystal in different solvent, suggesting the existence of 1 : 1 indomethacin–nicotinamide solution complex in methanol or methanol/ethyl acetate mixture solvent.10) However, few investigations considering in vivo behavior have been reported. It is required by the authorities to demonstrate that the pure drug should be released from the cocrystal structure in vivo. So, it is currently considered that the cocrystallization of API with CCF should not change the pharmacological profile of the API. After entering the body, API binds to the biological macromolecules such as proteins by weak forces including hydrogen bond, intermolecular force and hydrophobic interaction, and then the API/protein complex is transported to the targeting site and API is released. In the cocrystal, API and CCF are also connected with weak intermolecular forces. If the weak intermolecular forces in the cocrystal are still maintained during transport, it is an interesting question whether the weak forces between API and CCF affect the binding between API and proteins? Generally, a short lifetime or poor distribution of API are induced by the weak binding affinity between API and serum albumin, in case the strong binding leads to declining the concentration of free API component in plasma. The investigations on change in binding affinity of API to bovine serum albumin (BSA) induced by cocrystallization with CCF would provide a theoretical basis to evaluate the transportation, distribution, and metabolism of the cocrystal.
Felodipine (FEL), a dihydropyridine calcium antagonist, can lower blood pressure by reducing peripheral vascular resistance through a highly selective action on smooth muscle in arteriolar resistance vessels.11) FEL is poorly water-soluble (BCS class II), as well as displays short biological half-life and extensive first-pass metabolism after oral administration, which leads to a low systemic bioavailability of approximately 20%. Hence, the efforts on improving solubility and bioavailability of FEL have received considerable attention.12,13)
BSA, a kind of preferred representative serum albumin in investigation on the binding interaction between protein and API, is well characterized, abundant, cost-effective, and highly structure homologous to human serum albumin (HSA).14–16) Herein, we used FEL as the API and β-resorcylic acid (2,4-dihydroxybenzoic acid, βRA) as the CCF (Fig. S1) to prepare FEL–βRA cocrystal, and the interaction between BSA and the cocrystal supramolecule was investigated using fluorescence spectra and molecular docking methods. The aim of this study was to elucidate the change in binding affinity between FEL and BSA induced by cocrystallization with βRA through obtaining information about fluorescence quenching mechanism, binding constant, thermodynamic parameters and binding site numbers. Importantly, molecular docking technology was used to reveal how FEL–βRA binds to BSA protein as a supramolecular entity or a physical mixture of FEL and βRA.
Powder X-ray diffraction (PXRD) patterns of the FEL, βRA, physical mixture of FEL and βRA, and FEL–βRA complex were shown in Fig. 1A. As seen in pattern of the FEL–βRA complex (Fig. 1A-d), new peaks were observed at 2θ values of 9.4°, 15.0°, 18.7° and 25.8° (◆: new peaks), which were apparently different from those of the parent materials FEL, βRA and their physical mixture. Generally, along with the appearance of new peaks, the disappearance of peaks corresponding to starting materials should be also observed if a new solid phase is formed. As expected, peaks at 10.2°, 16.2°, 25.4°, 32.7° belong to intact FEL and peaks at 13.6°, 15.8°, 25.3°, 26.8° belong to βRA disappeared in PXRD pattern of FEL–βRA complex. Suggesting the FEL–βRA complex prepared by EtOH solvent evaporation method was a new state of cocrystal in nature, not a simple mixture of FEL and βRA.17,18)
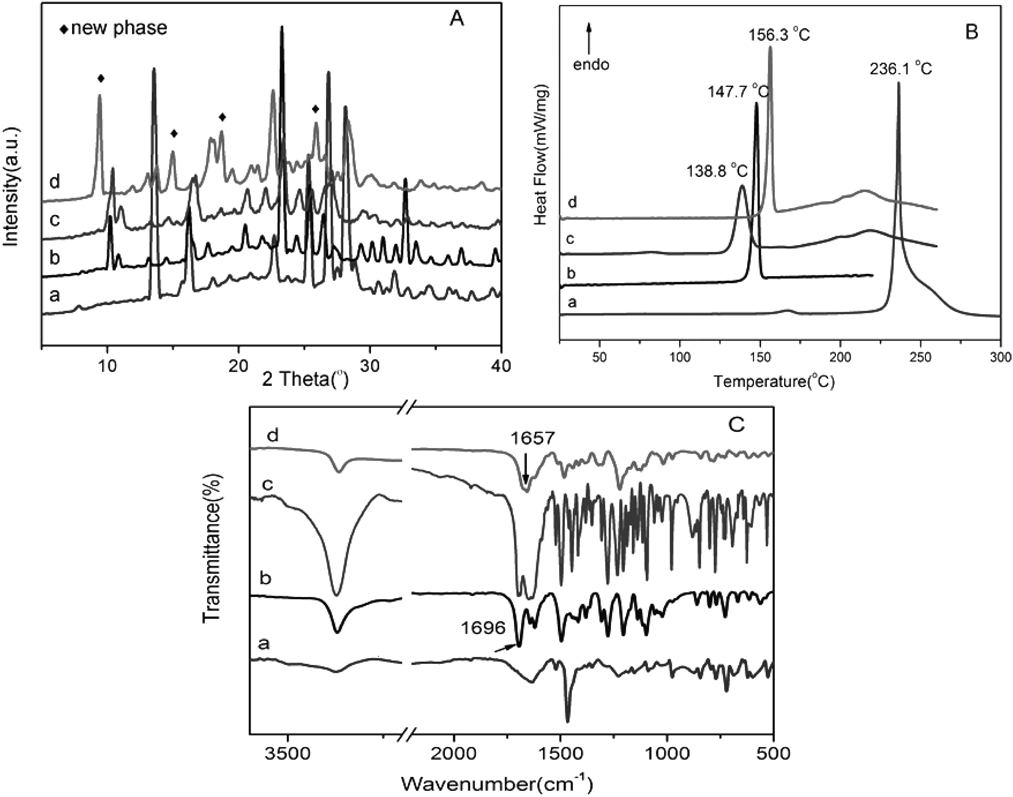
(a) βRA, (b) FEL, (c) physical mixture of FEL and βRA, (d) FEL–βRA cocrystal.
The differential scanning calorimetry (DSC) curves of FEL, βRA, physical mixture of FEL and βRA, and FEL–βRA cocrystal were presented in Fig. 1B. The DSC curve of the cocrystal showed a sharp and narrow endothermic peak at 156.3°C (ΔHfusion = 70.3 J·g−1), which may be attributed to the melting of the cocrystal. The melting point of the cocrystal differed from those of parent materials FEL (Tpeak = 147.7°C, ΔHfusion = 83.2 J·g−1), βRA (Tpeak = 236.1°C, ΔHfusion = 634.1 J·g−1) and their physical mixture (Tpeak = 138.8°C, ΔHfusion = 69.6 J·g−1). Obviously, the melting point of FEL–βRA cocrystal was between those of FEL and βRA. For two-parent components, the disappearance of melting peaks and the occurrence of a new single melting peak proved the formation of a new homogeneous phase. Generally, the melting point of cocrystal lies between or below those of two-parent components.19,20) According to the survey of Schultheiss and Newman 51% cocrystals displayed melting points between those of the API and CCF.21) Obviously, the screened FEL–βRA cocrystal contributed to this group.
The hydrogen bond is an attractive interaction that presents in the cocrystal systems. IR spectroscopy was performed to gain insight into the possible molecular-level interactions, such as hydrogen bonding, between API and CCF in the complex.8) The IR spectra of the FEL, βRA, physical mixture, and FEL–βRA cocrystal were shown in Fig. 1C. A comparison of the absorption peaks illustrated that the spectrum of the physical mixture was superimposition by that of individual FEL and βRA, indicating there was no interaction between them in the physical mixture. However, the IR spectrum for the cocrystal system was markedly different from that of the physical mixture. It is accepted that the characteristic IR absorption peaks of functional groups will shift after hydrogen bonding. Among FEL complexes, NH group commonly acts as a hydrogen bond receptor or be protonated. For example, Chadha et al. reported the NH group of pyridyl ring in felodipine interacts with hetero nitrogen of imidazole molecule to form N–H…N hydrogen bond and carbonyl O of ethyl ester group in felodipine interacts with NH of imidazole to form O…H–N hydrogen bond.22) However, in the current study, the peak position and shape assigned to N–H stretching vibration (3371 cm−1) in FEL–βRA cocrystal are almost the same as that of intact FEL (Fig. 1C), which in support the conclusion that NH group in FEL was not involved in the formation of hydrogen bonds or ionic bond. However, the peak at 1696 cm−1 assigned to the C=O stretching vibrations of ethyl ester in FEL shifted to 1657 cm−1 in the cocrystal. Meanwhile, the peak at 3376 cm−1 resulted from stretching vibration of phenolic hydroxyl O–H in βRA disappeared in the cocrystal. Generally, the IR vibrations of the hydrogen bonding sites will red-shift 20–50 cm−1 after complexation.9) The shift of the C=O group in FEL was in accordance with this law. Considering the preference of forming intramolecular hydrogen bond between the O–H group and ortho-position C=O group in the βRA molecule, we deduced the hydrogen bonds constructed between 4–OH group in βRA and C=O group of ethyl ester in FEL. That is, in the cocrystal of FEL–βRA, one FEL molecule and one βRA molecule are bound together through C=O…H–O hydrogen bond.
Fluorescence Quenching Mechanism between FEL–βRA Supramolecule and BSABSA could emit strong intrinsic fluorescence excited by a certain wavelength of UV light. Its fluorescence signal mainly came from tryptophan and tyrosine residues.23) Fluorescence quenching induced by quencher, here small API molecule, can sensitively provide adequate information of binding mechanism and binding parameters between API molecule and BSA protein. It was noted that free FEL and βRA showed no fluorescence intensity at the maximum wavelength of BSA emission at 337 nm (Fig. S2), so the interference of internal filtration was not considered in the present study. After being excited at 289 nm, fluorescence spectra of BSA were recorded over the emission wavelength range from 200 to 450 nm in the presence of various concentrations of FEL (Fig. 2), the fluorescence quenching of βRA to BSA at higher concentration (32 × 10−6 mol·L−1) proved in Fig. S3, the quenching degree of BSA by free βRA can be negligibly small. As can be seen in Fig. 2, both FEL and FEL–βRA system displayed ability of linear quenching the BSA fluorescence, and the emission wavelength or peak shape of the BSA did not change after FEL–BSA binding. The endogenous fluorescence quenching of BSA induced by FEL or FEL–βRA was suggestive of the interaction between BSA and FEL.23) Furthermore, comparing the fluorescence emission spectra of FEL and FEL–βRA system at the same concentration, the quenching degree of BSA by FEL–βRA system is greater than that of free FEL.

The quenching of fluorescence is classified into two mechanisms as static quenching (ground state complex formation) and dynamic quenching (collisional). The quenching rate constants are expected to decrease with increasing temperature in static quenching, however, the reverse effect is observed in dynamic quenching.24) The fluorescence quenching data are analyzed by the Stern–Volmer equation
 | (1) |
where F and F0 are the steady-state fluorescence intensities in presence and absence of quencher (here FEL and FEL–βRA system), respectively. Ksv is Stern–Volmer quenching constant and Kq is the value of the quenching rate constant. [Q] is the concentration of quencher; τ0 is the average lifetime of the protein without the quencher taking average 10−8 s.25) In order to reveal the quenching mechanism of FEL (or FEL–βRA)–BSA interaction, the fluorescence quenching data at three different temperatures (i.e., 298, 304 and 310 K) were recorded and analyzed using the Stern–Volmer Eq. (1). The Ksv values obtained from Stern–Volmer plots (provided in Fig. S4) of F0/F against FEL concentration for FEL and FEL–βRA system were summarized in Table 1. The value of KSV decreased with increasing temperature in FEL–BSA system. Furthermore, the values of the Kq had fallen in the order of 1012 at three experimental temperatures, which exceeded the value of the maximum dynamic quenching constant (2 × 1010 L·mol−1·s−1).26) It was suggested that the fluorescence quenching of BSA induced by FEL was static quenching resulted from the formation of FEL–BSA complex, but not initiated by dynamic quenching. Moreover, the same as FEL, the values of KSV of the FEL–βRA–BSA complex decreased with increasing temperature, and the values of Kq also exceeded the maximum dynamic quenching constant, suggesting FEL–βRA supramolecule followed static quenching mechanism to BSA. However, KSV values for FEL–βRA system were higher than those of FEL, indicating that the hydrogen bonding between FEL and βRA in supramolecule promoted the interaction between FEL and BSA to a certain degree.
| Systems | T/K | Ksv/L·mol−1 | Kq/L·mol−1·s−1 |
|---|---|---|---|
| FEL–BSA | 298 | 28938 | 2.89 × 1012 |
| 304 | 23811 | 2.38 × 1012 | |
| 310 | 20990 | 2.10 × 1012 | |
| FEL–βRA–BSA | 298 | 57758 | 5.78 × 1012 |
| 304 | 34622 | 3.46 × 1012 | |
| 310 | 28383 | 2.84 × 1012 |
The number of the binding sites n and binding constant Kb between quencher and protein are calculated according to the double log Stern–Volmer equation27):
 | (2) |
From a plot of log[(F0 − F)/F] versus log[Q] (Fig. 3), the y-axis intercept and slope of these plots yielded the values of Kb and n, respectively. As shown in Fig. 3, the values of the regression coefficient (r) nearly equaled to 1, indicating the validity of Eq. (2). As listed in Table 2, the values of Kb decreased with the rising temperature, which indicated that the binding affinity decreased with the increasing of temperature. This relationship between Kb and temperature also suggested the quenching mechanism to BSA for FEL and FEL–βRA system was mainly determined by static quenching.28) The value of binding constant Kb is closely related to the distribution of the drug in plasma since a weak binding affinity would lead to a short lifetime or poor distribution, while strong binding would lessen the concentration of free drug in plasma.29) The Kb value larger than 104 L·mol−1 indicated rather high binding affinity of fluorophore and quencher.30) In Table 2, the values of Kb for FEL and FEL–βRA supramolecule were found to be 104–105 order of magnitude, which proposed the presence of strong binding between FEL and BSA. Moreover, it was found that the numbers of binding site n as calculated for both FEL and FEL–βRA system were approximately equal to 1 (Table 2), indicating that there was one class of binding site for FEL towards BSA, meaning one FEL molecule was bound to one BSA molecule.

The inserted figure was Van’t Hoff plots for the quenching of BSA.
| Samples | T/K | Kb/L·mol−1 | n | ΔH/kJ·mol−1 | ΔS/J·mol−1·K−1 | ΔG/kJ·mol−1 |
|---|---|---|---|---|---|---|
| FEL–BSA | 298 | 9.89 × 104 | 1.12 | −70.67 | −145.35 | −27.36 |
| 304 | 3.37 × 104 | 1.03 | −26.48 | |||
| 310 | 1.80 × 104 | 0.98 | −25.61 | |||
| FEL–βRA–BSA | 298 | 2.63 × 105 | 1.15 | −62.77 | −109.23 | −30.22 |
| 304 | 1.42 × 105 | 1.13 | −29.56 | |||
| 310 | 5.89 × 104 | 1.07 | −28.91 |
However, the binding constant Kb of FEL–βRA system with BSA was more than 3-fold higher than that of free FEL at 310 K. The change in binding affinity to BSA would induce variation on lifetime or distribution of FEL. It is necessary to clarify the mechanism of the change in binding affinity of FEL to BSA after cocrystallization with βRA.
Thermodynamic Parameters and Binding Force between FEL–βRA System and BSAGenerally, the interactions between small API molecule and protein can be described by four kinds of weak forces: hydrogen bonding, van der Waals, electrostatic and hydrophobic interactions.31) In order to confirm the interaction forces of FEL and FEL–βRA with BSA, the equations of Van’t Hoff and Gibbs–Helmholtz were used to calculate the values of enthalpy change (ΔH), entropy change (ΔS) and free energy (ΔG).
 | (3) |
 | (4) |
The values of ΔH and ΔS can be obtained from the slope of linear and the intercept of the Van’t Hoff equation, respectively (the insert in Fig. 3). As can be seen in Table 2, the negative sign of ΔH indicated an exothermic binding reaction, and the negative values of ΔS suggested the enthalpy-driven binding reaction between FEL and BSA. Owing to negative values of both ΔH and ΔS for FEL–BSA and FEL–βRA–BSA system, the interaction forces of BSA with FEL and FEL–βRA were van der Waals forces and/or hydrogen bond.32) And the negative value of free energy (ΔG) suggested thermodynamics spontaneous of the binding interaction between FEL and BSA. Moreover, ΔG value for FEL–βRA system was more negative than that of FEL at the same temperature, so the FEL–βRA supramolecular showed thermodynamics superiority on binding to BSA.
Molecular DockingSpectroscopic methodology confirmed that the parameters of binding reaction between FEL and BSA changed after introduction of βRA. Based on the spectroscopic results, molecular docking technology was used to reveal how the FEL–βRA system binds to BSA protein. To clarify the possible binding mechanism of FEL and FEL–βRA system with BSA, four binding patterns were designed, including FEL, βRA, the physical mixture of FEL and βRA and FEL–βRA cocrystal with BSA. The crystal structure of FEL–βRA cocrystal was simulated obtained using density functional theory (DFT) method, which is widely used to construct and optimize the geometries of cocrystals.33,34) The simulation results showed that the hydrogen bonds presented between FEL and βRA in the optimized configuration, which is consistent with the results derived from the IR spectrum.
Three typical specific guest molecule-binding sites, named as site I, site II and II′, are located at subdomain IIA and IIIA in active cavity of BSA, respectively. Molecular docking simulation provided the docking energy of molecular binding patterns in different possible binding sites of FEL and results were summarized in Table 3. As listed in Table 3, the lowest binding free energy (ΔGƟ = −26.55 kcal·mol−1) of FEL with BSA occurred at site II′ docking zone of BSA, indicating it is energetically favorable for FEL to bind to BSA in the hydrophobic cavity of site II′ of subdomain IIIA. Therefore, the binding configuration construction of FEL–BSA and FEL–βRA–BSA were performed at site II′ of subdomain IIIA and the dominating configurations with the lowest binding free energy were presented in Fig. 4.
| Binding site | ΔGƟ/kcal·mol−1 | ΔE1/kcal·mol−1 | ΔE2/kcal·mol−1 | ΔE3/kcal·mol−1 |
|---|---|---|---|---|
| Site I (subdomain IIA) | −9.47 | −29.64 | −18.78 | −10.86 |
| Site II (subdomain IIIA)a) | −21.89 | −32.17 | −26.99 | −5.18 |
| Site II′ (subdomain IIIA)a) | −26.55 | −45.53 | −39.15 | −6.38 |
a) Site II and site II′ are two different binding sites in subdomain IIIA of BSA. ΔGƟ is the binding free energy of FEL with BSA. ΔE1, ΔE2, and ΔE3 denote the total intermolecular interaction energy, the sum of van der Waals energy, hydrogen bond and desolvation energy, and the electrostatic energy, respectively.

Amino acid residues and hydrogen bonding interactions in active cavity of BSA for FEL–BSA (A), βRA–BSA (B), physical mixture of FEL and βRA (C), FEL–βRA cocrystal (D), and the docking conformations of FEL–BSA (A-1), FEL–βRA–BSA (D-1). The structure of FEL–βRA cocrystal was obtained from DFT simulation, which was conducted at the DFT B3lyp/6-31** level using a GAUSSIAN-03 program package.
As seen in Fig. 4A, FEL molecule inserted into the active cavity of BSA and was surrounded by the hydrophobic residues including LEU452, ARG484, SER488, LEU429, GLU449, VAL432, THR448, ILE387, ASN390, and ARG409. Among those residues, LEU429 and LEU452 residues displayed a binding affinity to FEL through intermolecular hydrophobic interactions. Moreover, a hydrogen bond force occurred between FEL and ASN390 residue (red line). As a result, FEL showed obvious binding affinity to BSA with the binding energy of −26.55 kcal·mol−1. In the fluorescence quenching experiment, CCF (βRA) displayed slight fluorescence quenching to BSA at higher concentration (Fig. S3). As a prospective result, the O–H group in βRA bound to ARG409 residue by N…H–O hydrogen bond with a binding energy of −12.37 kcal·mol−1 (Fig. 4B). Owing to the small size of βRA, it was not able to fill the hydrophobic active cavity of BSA, which resulted in lower binding affinity and slight fluorescence quenching to BSA. In order to reveal whether the FEL–βRA binds to BSA protein as a supramolecular entity or a physical mixture of FEL and βRA, two binding conformations between BSA and guest molecule were constructed, including the physical mixture of FEL and βRA and FEL–βRA supramolecule. The simulated binding pattern of the physical mixture of FEL and βRA with BSA was presented in Fig. 4C. It was much easier for βRA to enter the active cavity of βRA owing to its smaller size compared to FEL. If the βRA preferentially entered the cavity and bound to surrounding residue as a pattern illustrated in Fig. 4B, FEL molecule failed to enter the cavity normally and only collided with ARG409, ASN309, SER488 residues at the mouth of the cavity, which resulted in an unstable binding pattern.
However, if the FEL–βRA system entered the cavity of BSA in the form of supramolecule, in which the hydrogen bonds occurred between FEL and βRA (the right red line), the hydrogen bond between BSA and the FEL–βRA supramolecule was constructed with ASN390 residue (Fig. 4D). Moreover, methyl and ethyl groups at the end site and heterocyclic ring in FEL showed binding affinity to LEU429, VAL432, LEU452, ILE387 residues through intermolecular hydrophobic interactions. Most importantly, the size of the FEL–βRA supramolecule appropriately matched the size of the active cavity of BSA (Fig. 4D-1), leading to a stable binding conformation with a lower binding energy of −30.42 kcal·mol−1.
Furthermore, it can be observed that the conformations in binding state in BSA changed by comparing the molecular conformations of free guest molecules (Fig. 5), With regard to FEL molecule, methyl at the end site and chlorobenzene ring turned after binding to BSA (Figs. 5A and A-1), while, for FEL–βRA supramolecule, ethyl eater group and chlorobenzene ring in FEL and mother cycle of βRA turned in order to gain stronger affinity with surrounded residues in cavity of BSA (Figs. 5B and B-1).

It was suggested that both single FEL and FEL–βRA supramolecule possessed vigorous flexibility, which played an important role in the binding affinity to BSA.
Table 2 displayed that FEL–βRA cocrystal showed higher binding constant Kb than that of single FEL in the experiments test. Based on the combination of results on experiment and molecular docking, it was reasonable that the form of supramolecular FEL–βRA was more conducive to enhance its binding affinity to BSA protein. In recent years, molecular docking simulation provides an intuitive method to observe the interaction between API and binding protein, and gives a reasonable explanation on binding mechanism. Al-Otaibi et al. reported the binding reaction between pyrazinamide-based cocrystal and mycobacterium tuberculosis type II using molecular docking simulation.35) It indicated that the cocrystal showed certain inhibitory activity against mycobacterium tuberculosis and could be developed as a new anti-tuberculosis drug. Nadzri1 et al. performed molecular docking on four cocrystals containing 5-fluorouracil using human thymidylate synthase as the target protein.36) It was found that different cocrystals bound to human thymidylate synthase via the formation of hydrogen bonds with different amino acid residues, which revealed the potentially different anticancer activities of the cocrystals. However, no evidence from the experimental test on corresponding protein binding affinity, only one docking conformation from molecular docking is not enough to give a reasonable binding pattern of supramolecular cocrystal in protein. In the present study, based on the spectroscopic experimental result, two designed docking conformations, including physical mixture–BSA and supramolecular cocrystal–BSA, were used to clarify which binding manner was the reasonable one. In a conclusion, combined with the experimental binding constant Kb of FEL and FEL–βRA cocrystal to BSA, we deduced that the FEL–βRA cocrystal was bound to BSA in the form of “supramolecule.”
A new FEL–βRA cocrystal was successfully obtained by the solvent evaporation method and characterized using PXRD, DSC, FT-IR spectroscopy and DFT simulation verified that equimolar FEL and βRA molecules bound together through C=O…H–O hydrogen bond in the cocrystal. In the present study, we gave the possible binding conformation between API–CCF complex and BSA in vitro. The fluorescence experimental data gave an indication that free FEL and FEL–βRA complex showed a different binding affinity to BSA; the introduction of βRA remarkably increased the binding constant Kb. Molecular docking provided the binding mechanism; it was possible for the FEL–βRA system to bind to BSA retaining the form of supramolecule, not physical mixture and single FEL. The size of supramolecular FEL–βRA preferably matched the size of the active cavity of BSA, which led to a rather high binding affinity to BSA. This change on binding affinity to BSA induced by cocrystallization with βRA provided a theoretical basis to evaluate the transportation, distribution, and metabolism of cocrystal drug.
Materials The raw felodipine (>99.0%, commercially available, polymorph I) was purchased from Shanghai Tong Yuan Chemical Co., Ltd. (Shanghai, China). β-Resorcylic acid (>98.0%) was obtained from Tokyo Chemical Industry Co. (China). BSA (≥95%, molecular weight assumed to be 66430) was purchased from Beijing Aoboxing Biotechnology Co., Ltd. (Beijing, China). Trihydroxymethylaminomethane (Tris) was purchased from Beijing Yinuokai Technology Co., Ltd. (Beijing, China). All other reagents and solvents were of analytical grade. Doubly distilled water was used for the preparation of all solutions.
PXRD patterns were collected on a Bruker D8 advance X-ray diffractometer (Bruker, Germany) using CuKα radiation (λ = 1.5406 Å) and Lynx eye detector over the interval 5° to 40° (2θ) with step size of 0.02° (2θ) and time per step of 0.3 s. DSC was carried out on a NETZSCH DSC 214 instrument equipped with a refrigerated cooling accessory (IC40). Nitrogen was used as the purging gas with a flow rate of 40 mL·min−1. About 6 mg of FEL or cocrystal was sealed in an aluminum pan, following with thermal scanning at a heating rate of 10°C·min−1 over the interval 20 to 300°C. FT-IR (KBr pellet) spectra were recorded on a FTIR-8400S spectrometer (Shimadzu, Japan). The spectra were scanned in the range of 400–4000 cm−1 with a resolution of 2 cm−1 and the total number of scans was 40.
Sample PreparationAn equimolar mixture of FEL (384.3 mg) and βRA (154.1 mg) was dissolved in 20 mL anhydrous ethanol by sonication, followed by heating in a water bath at 65°C for 30 min. Then, the obtained solution was placed at ambient temperature for solvent evaporation slowly. The precipitates were collected and dried in a vacuum condition to remove the residual solvent. The obtained white solid was stored in desiccator for further analysis. The thermal stability of FEL and FEL–βRA cocrystal was examined by thermogravimetric analysis, which indicated that no chemical degradation occurred during the preparative steps (provided in Fig. S5).
Fluorescence Spectral Studies on Interaction between FEL–βRA and BSAThe BSA stocking solution (5 × 10−5 mol·L−1) was prepared by dissolving BSA powder in Tris–HCl buffer solution (pH 7.4). The stock solutions of FEL and FEL–βRA cocrystal were prepared by dissolving them in a small amount of ethanol, then diluted by the Tris–HCl buffer solution to the concentration of 1 × 10−3 mol·L−1. Fluorescence emission spectra were recorded on the F-380 spectrofluorophotometer (Tianjin, China) equipped with 1.0 cm quartz cells. 1.0 mL BSA stocking solution and various amount of FEL (or FEL–βRA cocrystal) were added to 5 mL volumetric flasks and the concentrations of FEL were ranged from 4.0 × 10−6 to 3.2 × 10−5 mol·L−1, following by shocking and incubation in air bath for 1h. Afterward, fluorescence emission spectra were measured from 200 to 450 nm at an excitation wavelength of 289 nm with 2.5 nm slit widths at three temperatures of 298, 304, and 310 K, respectively. Every case of all experiments was in the same buffer solution mentioned above.
Simulation on Optimization Molecular StructureThe supramolecular structure of FEL–βRA cocrystal was obtained by simulated calculations using the Gaussian 09 program package.37) The initial structures of FEL (CCDC No.: 1144175) and βRA (CCDC No.: 747944) were obtained from the Cambridge crystal database and then optimized at the DFT(B3LYP) theoretical level combined with the 6–31 G (d, p) basis set. The geometries of the FEL–βRA cocrystal were constructed and optimized at the same computational level.
Molecular DockingThe interactions of FEL and its cocrystals with BSA were investigated by molecular docking. To clarify the possible binding mechanism of cocrystal with BSA protein, four binding patterns were designed, including (1) the binding between FEL and BSA; (2) the binding between βRA and BSA; (3) the binding between physical mixture of FEL and βRA (no weak interaction force between FEL and βRA) and BSA; (4) the binding between supramolecular FEL–βRA cocrystal and BSA (existing weak interaction force between FEL and βRA).
The crystal three-dimensional structure of BSA (PDB ID: 4JK4) was downloaded from the PDB database (https://www.rcsb.org/). In order to avoid unnecessary calculations, we chose to retain the A chain of the structure. The structure of BSA was optimized by the molecular dynamics method using Amber16 in ff14SB force field. The whole molecular system was placed in the TIP3P water box model (10 × 10 × 10 Å) through adding Cl− and Na+ as the stable ions maintain charge neutrality in the system.
The whole optimization involves three steps: (I) binding proteins, optimizing water molecules and balancing ions; (II) binding protein backbone, optimizing protein side chains, water molecules, and stable ions; (III) releasing all restrictions and optimizing the whole system. The entire optimization process used the 2000 steps steepest descent method and the 2000 steps conjugate gradients method. The temperature and the convergence standard were set at 300 K and 0.2 kcal·mol−1, respectively.
The initial structures of FEL (CCDC No.: 1144175) and βRA (CCDC No.: 747944) were obtained from the Cambridge crystal database and then were optimized using the Tripos force field using Sybyl 2.0 software. DFT simulated calculations were performed to obtain the supramolecular structure of cocrystal FEL–βRA dimer (as mentioned above). The docking software Autodock 4.2.2 along with the Autodock Tools 1.5.6 was used to simulate the binding interaction between FEL and BSA. Since the specific binding sites for FEL and BSA are not explicitly reported in the literature, we first evaluated and selected three possible binding sites (site I, site II, site II′) mentioned in the literature. FEL small molecules was docked into above three possible binding sites using AutoDock 4.2.2. The gridpoint spacing was set to 0.375 Å and the box size was 60 × 60 × 60. Using the Lamarkian genetic algorithm (LGA) for conformational search, each molecule was given 256 docking conformations and we chose combined conformation with the highest score as final conformation. For the recognition of the binding sites on BSA, the energy of three different combination modes were optimized using the sander module of Amber16 through the 3000 step steepest descent and the 3000 step conjugate gradient methods. The temperature and the convergence standard were set at 300 K and 0.1 kcal·mol−1, respectively. Based on the optimized structure, the binding free energy of the three possible combinations was calculated using the molecular mechanics/Poisson–Boltzmann surface area module of Amber16. The specific binding sites for FEL and BSA was obtained through the binding free energy. Similarly, the binding assembly of βRA and FEL–βRA dimer with BSA were performed with the same binding site of BSA using AutoDock 4.2.2. The parameter settings were consistent with the previous ones, and the top conformation was selected as the final binding conformation.
This work was supported by the Natural Science Foundation of Hebei province (Grant numbers: H2017206214, H2016206096); The College Student Innovation Project of Hebei Medical University (Grant number: USIP2018189); and Education Department of Hebei province of China through innovative hundred talents support program (Grant numbers: SLRC2017047).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.