2020 Volume 68 Issue 10 Pages 962-970
2020 Volume 68 Issue 10 Pages 962-970
Oleanolic and ursolic acids were used as lead compounds to synthesize a series of pentacyclic triterpenoid derivatives bearing ethylenediamine, butanediamine, or hexanediamine groups at the C-3 position. The potential antiproliferative activity of these compounds was examined in A549 (human non-small cell lung cancer cells), MCF-7 (human breast cancer cells), and HeLa (human cervical carcinoma cells) cells. Methyl 3β-O-[4-(2-aminoethylamino)-4-oxo-butyryl]olean-12-ene-28-oate (DABO-Me) was identified as a promising antiproliferative agent in vitro and in vivo. DABO-Me strongly suppressed the proliferation of A549, MCF-7, and HeLa cells (IC50 = 4–7 µM). In MCF-7 cells, DABO-Me upregulated the pro-apoptotic protein Bax, downregulated the anti-apoptotic protein Bcl-2, promoted the release of cytochrome c, and activated caspase-3/9. Transwell and flow cytometry assays showed that DABO-Me inhibited MCF-7 cell proliferation, migration, and invasion, and induced apoptosis and S phase arrest. In vitro and in vivo experiments indicated that DABO-Me inhibited MCF-7 cell proliferation and suppressed tumor growth. Taken together, these results indicate that DABO-Me could be developed as an effective antitumor drug.
Approximately 20000 triterpenoids have been identified to date from various parts of medicinal plants.1) Oleanolic acid (OA) and its isomer ursolic acid (UA), which are pentacyclic triterpenoids that exist widely in medical plants, inhibit cell proliferation and tumor development, and induce apoptosis in various cancer cells, although their activity is weak.2) OA and UA contain hydroxyl and carboxylic groups, as shown in Fig. 1. Numerous attempts have been made to develop potent, synthetically feasible analogues of pentacyclic triterpenoids with a heteroatom incorporated at different positions of the triterpenoid skeleton. Studies suggest that the activity of these pentacyclic triterpenoids is related to their basic triterpenoid skeletal structure, and the attached functional groups provide opportunities for chemical modification and improvement of activity.2–5)

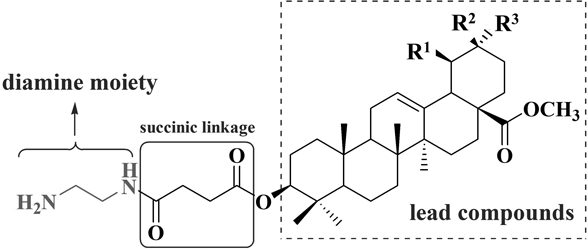
Previous studies from our group that assessed the activity of a series of nitrogen-containing derivatives of OA and UA prepared by modifying the C-28 position showed that these structurally modified compounds inhibit the growth of MCF-7 (human breast cancer cells), HeLa (human cervical carcinoma cells), and A549 (human non-small cell lung cancer (NSCLC) cells) cell lines in vitro.5) We also found that after the introduction of piperazine and butanedioic acid into the hydroxyl group at the C-3 position of OA and UA and conversion of the carboxylic groups at C-28 to a methyl ester, most of the structurally modified compounds showed significant antiproliferative effects compared with the original compounds.6) Based on these results, we selected OA and UA as templates to design a series of nitrogen-containing analogs modified at the C-3 position, i.e., methyl ester groups were introduced at C-28, and ethylenediamine, butanediamine, and hexanediamine were introduced into the C-3 position of OA and UA via a succinic acid linker as shown in Chart 1 and Fig 2. The synthesis of the derivatives was described, and their cytotoxicity was evaluated in A549, MCF-7, and HeLa cells. Methyl 3β-O-[4-(2-aminoethylamino)-4-oxo-butyryl]olean-12-ene-28-oate (DABO-Me) was identified as a promising antiproliferative agent. In the present study, we investigated the effect of DABO-Me on human breast cancer MCF-7 cell proliferation, cell cycle progression, cell migration and invasion in vitro and on tumor inhibition in vivo in preliminary animal models generated using the MCF-7 cell line.
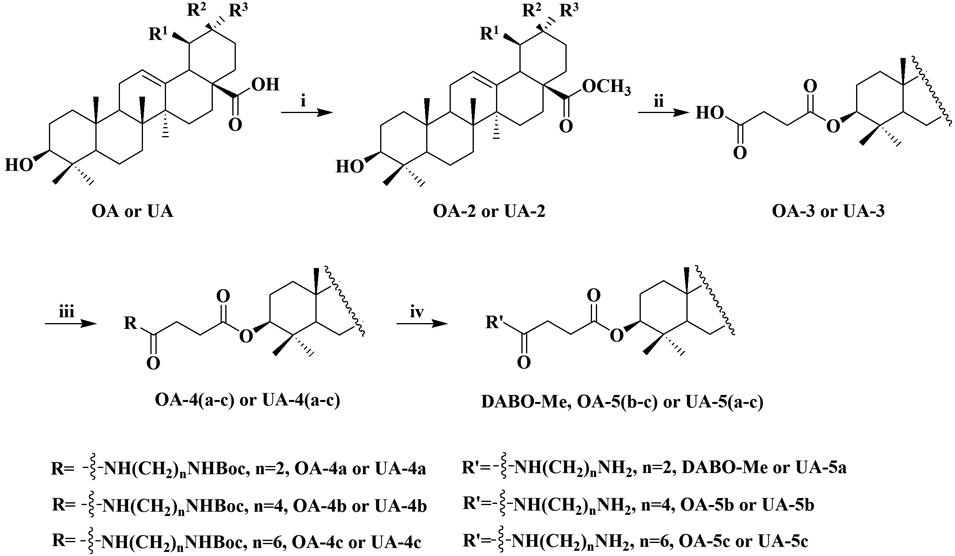
Reagents and conditions: i) CH3I, K2CO3, DMF, room temperature (r.t.); ii) Butanedioic anhydride, DMAP, CH2Cl2, 70°C; iii) N-Boc-Diamine, EDCI, DMAP, CH2Cl2, 0°C to r.t.; iv) TFA, CH2Cl2, 0°C to r.t.
Target compounds were purified on a silica gel column chromatography (200–300 mesh, Qingdao Marine Chemical Factory, China) using petroleum ether/ethyl acetate or methylene chloride/anhydrous methanol as eluent. Their structures were confirmed using NMR on a AVANCE 500 MHz spectrometer (BRUKER, Switzerland) in CDCl3. Chemical shifts are expressed in ppm and the coupling constants (J) in Hz. IR were recorded in KBr pellets on a WGH-30 (Shanghai Yonggui analysis instrument Co., Ltd.). Melting points were determined on an X-5 fiber melting point detector (temperature-controlled, Beijing Tektronix Instrument Co., Ltd.). Mass spectra were recorded on a GC-TOF high-resolution mass spectrometer (HR-MS) and an Agilent 6540 RRLC/Q-TOF MS (Agilent, Santa Clara, CA, U.S.A.). Most chemicals and solvents were purchased from commercial sources. Further purification and drying by standard methods were employed when necessary. All the reagents and chemicals were of analytical grade or chemically pure. The synthesis routes are presented in Chart 1.
ChemistrySynthesis of OA and UA Derivatives OA and UA derivatives (compounds OA-2, UA-2, OA-3 and UA-3, structures shown in Chart 1) were synthesized as previously described.6)
Synthesis and Characterization of OA DerivativesMethyl 3β-O-[4-(2-N-tert-Butoxycarbonyl (Boc)-aminoethylamino)-4-oxo-butyryl]olean-12-ene-28-oate (OA-4a)To a stirred solution of OA-3 (300.0 mg, 0.53 mmol), N-Boc-ethylenediamine (95.1 mg, 0.63 mmol) and dimethylaminopyridine (DMAP) (72.6 mg, 0.63 mmol) in anhydrous methylene chloride (10 mL) was added 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride (EDCI) (379.5 mg, 2.10 mmol) which dissolved in anhydrous methylene chloride (10 mL) at 0°C. The reaction mixture was stirred for 5 min, and then at room temperature for 3 h. The reaction mixture was extracted with methylene chloride and water, washing to neutral with saturated NaCl solution. The organic layer was dried over anhydrous magnesium sulfate. Filtration and evaporation of solvent at reduced pressure gave light yellow solid, which was purified by silica gel chromatography with a gradient elution of methylene chloride–methanol (20 : 1, v/v) to yield a white solid (365.8 mg, 97.5%), mp 111.9–112.5°C; IR (KBr) νmax 3393, 2949, 2861, 1721, 1375, 1248, 1173 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.39 (s, 1H, –CONH–), 5.29 (s, 1H, H-12), 5.05 (s, 1H, –NH-Boc), 4.50–4.46 (m, 1H, H-3), 3.60 (s, 3H, –COOCH3), 3.31–3.22 (m, 4H, –NHCH2CH2NH–), 2.83 (d, J = 13.3 Hz, 1H, H-18), 2.63–2.62 & 2.45–2.44 (m, 4H, –COCH2CH2CO–), 1.41 (s, 9H, –C(CH3)3), 0.90, 0.87, 0.82, 0.78, 0.74, 0.70 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.26, 172.68, 172.14, 156.71, 143.79, 122.25, 81.37, 79.63, 55.33, 51.48, 47.55, 46.71, 45.85, 41.64, 41.30, 40.58, 40.46, 40.37, 39.29, 38.10, 37.74, 36.92, 33.85, 33.07, 32.59, 32.37, 31.15, 30.66, 29.99, 28.37, 28.04, 27.68, 25.88, 23.62, 23.50, 23.39, 23.21, 18.19, 16.82, 16.69, 15.32. HR-MS Calcd for C42H68N2O7 [M + H]+ 713.5105, Found 713.5100.
Methyl 3β-O-[4-(4-N-Boc-aminobutylamino)-4-oxo-butyryl]olean-12-ene-28-oate (OA-4b)The OA-3 (462.7 mg, 0.75 mmol) was connected with N-Boc-1,4-diaminobutane hydrochloride (201.6 mg, 0.90 mmol) according to the same procedure used in the preparation of OA-4a, to give OA-4b (576.8 mg, 96.0%) as a white solid: mp 101.2–101.9°C; IR (KBr) νmax 3388, 2945, 2864, 1730, 1376, 1249, 1174 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.01 (s, 1H, –CONH–), 5.26 (s, 1H, H-12), 4.64 (s, 1H, –NH-Boc), 4.49–4.46 (m, 1H, H-3), 3.60 (s, 3H, –COOCH3), 3.24–3.10 (m, 4H, –NHCH2CH2CH2CH2NH–), 2.83 (d, J = 12.4 Hz, 1H, H-18), 2.64–2.63 & 2.44–2.43 (m, 4H, –COCH2CH2CO–), 1.42 (s, 9H, –C(CH3)3), 1.11, 0.91, 0.88, 0.83, 0.70 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.25, 172.82, 171.56, 156.12, 143.79, 122.25, 81.37, 79.24, 55.33, 51.48, 47.55, 46.72, 45.86, 41.64, 41.30, 39.29, 39.20, 38.09, 37.75, 36.93, 33.85, 33.07, 32.59, 32.37, 31.23, 30.66, 30.07, 28.41, 28.06, 27.68, 27.53, 26.66, 25.88, 23.62, 23.52, 23.39, 23.06, 18.20, 16.82, 16.70, 15.32. HR-MS Calcd for C44H72N2O7 [M + H]+ 741.5418, Found 741.5418.
Methyl 3β-O-[4-(6-N-Boc-aminohexylamino)-4-oxo-butyryl]olean-12-ene-28-oate (OA-4c)The OA-3 (500 mg, 0.88 mmol) was connected with N-Boc-hexanediamine hydrochloride (227.2 mg, 1.05 mmol) according to the same procedure used in the preparation of OA-4a, to give OA-4c (579.8 mg, 86.1%) as a white solid: mp 89.8–90.5°C; IR (KBr) νmax 3413, 2946, 2869, 1731, 1377, 1251, 1175 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 5.79 (s, 1H, –CONH–), 5.27 (t, J = 3.5 Hz, 1H, H-12), 4.51–4.48 (m, 2H, H-3 × 1 & –NH-Boc × 1), 3.62 (s, 3H, –COOCH3), 3.23–3.21 & 3.20–3.09 (s, 4H, –NHCH2CH2CH2CH2CH2CH2NH–), 2.84 (d, J = 13.9 Hz, 1H, H-18), 2.67–2.64 & 2.47–2.44 (m, 4H, –COCH2CH2CO–), 1.44 (s, 9H, –C(CH3)3), 1.12, 0.92, 0.88, 0.85, 0.84, 0.72 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.25, 172.82, 171.43, 156.09, 143.79, 122.25, 81.35, 55.34, 51.48, 47.55, 46.71, 45.86, 41.64, 41.30, 39.29, 38.10, 37.74, 36.92, 33.85, 33.07, 32.60, 32.37, 31.28, 30.66, 30.30, 30.12, 29.94, 29.41, 28.42, 28.06, 27.68, 26.49, 26.24, 26.12, 25.88, 23.82, 23.62, 23.51, 23.39, 23.06, 18.19, 16.82, 16.70, 15.32. HR-MS Calcd for C46H76N2O7 [M + H]+ 769.5731, Found 769.5727.
Methyl 3β-O-[4-(2-Aminoethylamino)-4-oxo-butyryl]olean-12-ene-28-oate (DABO-Me)To a stirred solution of OA-4a (200 mg, 0.28 mmol) in anhydrous methylene chloride (5 mL) was added trifluoroacetic acid (TFA) (0.62 mL, 8.42 mmol) at 0°C. The reaction mixture was stirred for 2 h at room temperature. After the reaction, with 0.1 mol L−1 NH3⋅H2O adjust pH to 9 under the ice water bath. The reaction mixture was extracted with methylene chloride and water. The organic layer was dried over anhydrous magnesium sulfate. Filtration and evaporation of solvent at reduced pressure gave light yellow solid, which was purified by silica gel chromatography with a gradient elution of methylene chloride–methanol (10 : 1 v/v) to yield a white solid (158.6 mg, 92.2%), mp 110.5–111.2°C; IR (KBr) νmax 3431, 2925, 2862, 1727, 1385, 1145 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.87 (s, 1H, –CONH–), 5.29 (s, 1H, H-12), 4.50 (s, 1H, H-3), 3.64 (s, 3H, –COOCH3), 3.39–2.94 (m, 4H, –NHCH2CH2NH2), 2.73–2.40 (m, 6H, –COCH2CH2CO– ×4 & –NH2 × 2), 2.16 (d, J = 11.3 Hz, 1H, H-18), 1.14, 0.97, 0.89, 0.84, 0.83, 0.75, 0.72 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 177.33, 138.23, 138.09, 127.97, 127.87, 81.52, 79.00, 55.36, 51.51, 50,76, 48.51, 44.35, 41.41, 41.14, 38.87, 38.52, 37.83, 37.24, 36.87, 35.72, 34.94, 33.09, 32.63, 32.10, 29.92, 28.08, 27.37, 27.10, 25.18, 24.15, 23.63, 21.64, 21.07, 18.33, 17.34, 16.65, 16.35, 15.49. HR-MS Calcd for C37H60N2O5 [M + H]+ 613.4580, Found 613.4580.
Methyl 3β-O-[4-(4-Aminobutylamino)-4-oxo-butyryl]olean-12-ene-28-oate (OA-5b)The OA-4b (401.32 mg, 0.55 mmol) was reacted with TFA (1.23 mL, 16.61 mmol) according to the same procedure used in the preparation of DABO-Me, to give OA-5b (260.6 mg, 75.1%) as a white solid: mp 89.8–90.5°C; IR (KBr) νmax 3419, 2951, 2862, 1736, 1388, 1186 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.92 (s, 1H, –CONH–), 5.23–5.03 (m, 3H, H-12 × 1 & –NH2 × 2), 4.44 (d, J = 4.9 Hz, 1H, H-3), 3.61 (s, 3H, –COOCH3), 3.17–2.66 (m, 4H, –NHCH2CH2CH2CH2NH2), 2.57–2.36 (m, 4H, –COCH2CH2CO–), 2.13 (d, J = 12.3 Hz, 1H, H-18), 1.11, 1.08, 0.88, 0.83, 0.80, 0.68 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.21, 172.88, 172.11, 143.76, 127.90, 122.19, 81.34, 55.29, 51.46, 48.46, 47.51, 46.67, 45.82, 44.30, 41.59, 41.26, 39.25, 38.78, 38.06, 37.70, 36.89, 33.82, 33.07, 32.56, 32.34, 30.63, 29.99, 28.02, 27.65, 26.34, 25.88, 24.13, 23.60, 23.37, 23.03, 18.17, 16.79, 16.68, 15.30. HR-MS Calcd for C39H64N2O5 [M + H]+ 641.4893, Found 641.4899.
Methyl 3β-O-[4-(6-Aminohexylamino)-4-oxo-butyryl]olean-12-ene-28-oate (OA-5c)The OA-4c (369.0 mg, 0.55 mmol) was reacted with TFA (1.64 mL, 22.1 mmol) according to the same procedure used in the preparation of DABO-Me, to give OA-5c (279.2 mg, 87.0%) as a white solid: mp 141.2–141.6°C; IR (KBr) νmax 3422, 2936, 2860, 1722, 1376, 1247, 1183 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.42 (s, 1H, –CONH–), 5.27 (s, 1H, H-12), 4.49–4.46 (m, 1H, H-3), 3.61 (s, 3H, –COOCH3), 3.21 (d, J = 5.8 Hz, 2H, –NH2), 3.01–2.84 (m, 5H, –NHCH2 CH2CH2CH2CH2CH2NH2 × 4 & H–18 × 1), 2.65–2.62 & 2.50–2.47 (m, 4H, -COCH2CH2CO–), 1.25, 1.06, 0.92, 0.87, 0.83, 0.72 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.24, 172.86, 171.57, 143.79, 138.10, 127.93, 122.23, 81.37, 55.33, 51.47, 50.65, 48.49, 47.55, 46.70, 45.85, 44.33, 41.63, 41.39, 39.32, 37.73, 37.23, 36.91, 33.07, 32.61, 31.15, 30.65, 30.08, 29.65, 29.26, 28.05, 27.67, 26.32, 25.88, 24.13, 23.61, 23.05, 18.19, 17.32, 16.81, 16.38, 15.3. HR-MS Calcd for C41H68N2O5 [M + H]+ 669.5206, Found 669.5205.
Synthesis and Characterization of UA DerivativesMethyl 3β-O-[4-(2-N-Boc-aminoethylamino)-4-oxo-butyryl]urs-12-ene-28-oate (UA-4a)The UA-3 (582 mg, 1.02 mmol) was connected with N-Boc-ethylenediamine (196 mg, 1.22 mmol) according to the same procedure used in the preparation of OA-4a, to give UA-4a (659.8 mg, 82.5%) as a white solid: mp 87.3–87.9°C; IR (KBr) νmax 3374, 2931, 2868, 1729, 1375, 1239, 1176 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.24 (s, 1H, –CONH–), 5.23 (t, J = 3.6 Hz1H, H-12), 4.94 (s, 1H, –NH-Boc), 4.52–4.49 (m, 1H, H-3), 3.60 (s, 3H, –COOCH3), 3.35–3.24 (m, 4H, –NHCH2CH2NH–), 2.69–2.64 & 2.47–2.44 (m, 4H, –COCH2CH2CO–), 2.22 (d, J = 11.3 Hz, 1H, H-18), 1.44 (s, 9H, –C(CH3)3), 1.07, 0.94, 0.93, 0.86, 0.85, 0.84, 0.74 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.03, 172.69, 172.16, 156.72, 138.19, 125.45, 81.40, 79.68, 55.33, 52.66, 51.41, 48.09, 47.49, 41.59, 40.63, 40.35, 39.51, 39.04, 38.87, 38.28, 37.75, 36.87, 36.63, 32.89, 31.17, 30.64, 30.00, 28.37, 28.08, 28.01, 24.22, 23.56, 23.29, 21.15, 18.19, 17.03, 16.90, 16.74, 15.46. HR-MS Calcd for C42H68N2O7 [M + H]+ 713.5105, Found 713.5101.
Methyl 3β-O-[4-(4-N-Boc-aminobutylamino)-4-oxo-butyryl]-urs-12-ene-28-oate (UA-4b)The UA-3 (600 mg, 1.05 mmol) was connected with N-Boc-1,4-diaminobutane hydrochloride (283.5 mg, 1.26 mmol) according to the same procedure used in the preparation of OA-4a, to give UA-4b (699.8 mg, 89.8%) as a white solid: mp 82.1–82.9°C; IR (KBr) νmax 3403, 2935, 2872, 1711, 1376, 1239, 1175 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.23–6.18 (m, 1H, –CONH–), 5.17 (s, 1H, H-12), 4.77–4.70 (m, 1H, –NH-Boc), 4.43–4.42 (m, 1H, H-3), 3.54 (s, 3H, –COOCH3), 3.18–3.05 (s, 4H, –NHCH2CH2 CH2CH2NH–), 2.82 (d, J = 13.3 Hz, 1H, H-18), 2.68–2.42 (m, 4H, –COCH2CH2CO–), 1.36 (s, 9H, –C(CH3)3), 1.11, 1.10, 0.88, 0.79, 0.78, 0.68, 0.67 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 177.21, 172.72, 171.53, 155.95, 138.12, 125.37, 81.21, 78.96, 55.26, 53.41, 52.82, 51.34, 48.01, 47.41, 41.92, 39.44, 38.96, 38.81, 38.21, 37.67, 36.80, 36.56, 32.82, 31.06, 29.99, 29.59, 28.92, 28.38, 28.10, 27.94, 27.41, 27.37, 24.93, 24.15, 23.52, 23.48, 23.23, 22.98, 21.10, 18.13, 17.13, 16.99, 16.70, 15.40. HR-MS Calcd for C44H72N2O7 [M + H]+ 741.5418, Found 741.5420.
Methyl 3β-O-[4-(6-N-Boc-aminohexylamino)-4-oxo-butyryl]urs-12-ene-28-oate (UA-4c)The UA-3 (600 mg, 1.05 mmol) was connected with N-Boc-hexanediamine hydrochloride (318.6 mg, 1.26 mmol) according to the same procedure used in the preparation of OA-4a, to give UA-4c (774.9 mg, 95.9%) as a white solid: mp 71.3–71.9°C; IR (KBr) νmax 3387, 2933, 2865, 1720, 1373, 1244, 1168 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 5.90(s, 1H, –CONH–), 5.22 (s, 1H, H-12), 4.58 (s, 1H, –NH-Boc), 4.48 (t, J = 7.5 Hz, 1H, H-3), 3.58 (s, 3H, –COOCH3), 3.20–3.07 (m, 4H, –NHCH2CH2CH2CH2CH2CH2NH–), 2.65–2.62 & 2.45–2.43 (m, 4H, –COCH2CH2CO–), 2.21 (d, J = 11.3 Hz, 1H, H-18), 1.41 (s, 9H, –C(CH3)3), 1.05, 0.92, 0.86, 0.83, 0.82, 0.71 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.00, 172.78, 171.42, 156.08, 138.17, 125.43, 81.30, 79.01, 55.31, 53.39, 52.86, 51.38, 48.06, 47.47, 41.97, 39.49, 39.27, 39.01, 38.85, 38.26, 37.72, 36.84, 36.61, 32.87, 31.22, 30.62, 30.08, 29.91, 29.40, 28.41, 28.08, 27.99, 26.11, 24.20, 23.55, 23.52, 23.28, 21.14, 18.17, 17.03, 16.88, 16.74, 15.44. HR-MS Calcd for C46H76N2O7 [M + H]+ 769.5731, Found 769.5724.
Methyl 3β-O-[4-(2-Aminoethylamino)-4-oxo-butyryl]urs-12-ene-28-oate (UA-5a)The UA-4a (320 mg, 0.45 mmol) was reacted with TFA (1.33 mL, 17.95 mmol) according to the same procedure used in the preparation of DABO-Me, to give UA-5b (248.6 mg, 90.4%) as a white solid: mp 98.7–99.3°C; IR (KBr) νmax 3416, 2936, 2872, 1727, 1388, 1234, 1186 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.41 (s, 1H, –CONH–), 5.25–5.23 (m, 1H, H-12), 4.51–4.48 (m, 1H, H-3), 3.60 (s, 3H, –COOCH3), 3.35–3.30 & 2.88–2.86 (m, 4H, –NHCH2CH2NH2), 2.67–2.47 (m, 4H, –COCH2CH2CO–), 2.34 (s, 2H, –NH2), 2.22 (d, J = 11.3 Hz, 1H, H-18), 1.07, 0.94, 0.93, 0.86, 0.85, 0.74 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.64, 173.14, 172.31, 156.79, 138.16, 125.58, 80.93, 79.05, 55.24, 52.90, 51.41, 48.10, 47.58, 42.01, 39.51, 39.06, 38.88, 38.75, 38.63, 36.98, 36.64, 32.99, 30.66, 29.67, 28.13, 28.04, 27.24, 24.24, 23.60, 23.30, 21.16, 18.32, 17.01, 16.91, 15.60, 15.42. HR-MS Calcd for C37H60N2O5 [M + H]+ 613.4580, Found 613.4576.
Methyl 3β-O-[4-(4-Aminobutylamino)-4-oxo-butyryl]urs-12-ene-28-oate (UA-5b)The UA-4b (500 mg, 0.68 mmol) was reacted with TFA (1.5 mL, 20.24 mmol) according to the same procedure used in the preparation of DABO-Me, to give UA-5b (341.6 mg, 79.0%) as a white solid: mp 115.3–115.9°C; IR (KBr) νmax 3433, 2931, 2867, 1386, 1210 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 7.70 (s, 2H, –NH2), 7.16 (s, 1H, –CONH–), 5.23 (s, 1H, H-12), 4.46 (s, 1H, H-3), 3.60 (s, 3H, –COOCH3), 3.17–2.04 (m, 4H, –NHCH2CH2CH2CH2NH2), 2.60–2.47 (m, 4H, –COCH2CH2CO–), 2.22 (d, J = 13.1 Hz, 1H, H-18), 1.07, 0.94, 0.93, 0.86, 0.84, 0.81, 0.74 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.05, 173.57, 155.33, 138.20, 125.44, 81.91, 55.23, 52.88, 51.42, 48.08, 47.44, 41.97, 39.50, 39.04, 38.87, 38.20, 37.65, 36.82, 36.63, 32.87, 31.90, 30.64, 30.01, 29.67, 29.33, 28.03, 27.92, 25.74, 24.21, 23.56, 23.29, 22.66, 21.17, 18.18, 17.06, 16.87, 16.61, 15.42. HR-MS Calcd for C39H64N2O5 [M + H]+ 641.4893, Found 641.4896.
Methyl 3β-O-[4-(6-Aminohexylamino)-4-oxo-butyryl]urs-12-ene-28-oate (UA-5c)The UA-4c (300 mg, 0.39 mmol) was reacted with TFA (1.2 mL, 15.61 mmol) according to the same procedure used in the preparation of DABO-Me, to give UA-5c (232.1 mg, 89.0%) as a white solid: mp 99.1–99.6°C; IR (KBr) νmax 3439, 2925, 2862, 1719, 1208 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 7.81 (s, 2H, –NH2), 6.63 (s, 1H, –CONH–), 5.28 (s, 1H, H-12), 4.48–4.45 (m, 1H, H-3), 3.59 (s, 3H, –COOCH3), 3.16–2.92 (m, 4H, –NHCH2CH2CH2CH2CH2CH2 NH2), 2.61–2.46 (m, 4H, –COCH2CH2CO–), 2.22 (d, J = 11.1 Hz, 1H, H-18), 1.06, 0.93, 0.92, 0.85,0.84, 0.82, 0.73 (each s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 178.03, 173.11, 158.74, 138.20, 125.42, 81.58, 65.80, 55.30, 55.38, 52.88, 51.40, 48.07, 47.47, 41.97, 39.50, 38.86, 38.24, 37.69, 36.84, 36.62, 32.87, 30.63, 30.06, 29.66, 28.68, 28.02, 25.66, 24.21, 23.56, 23.28, 21.15, 18.18, 17.04, 16.88, 16.68, 15.43. HR-MS Calcd for C41H68N2O5 [M + H]+ 669.5206, Found 669.5205.
Biological ActivityReagentsThiazolyl blue tetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The Annexin V-propidium iodide (PI) staining kit and cell cycle detection kit were purchased from Nanjing KeyGen Biotech. Co., Ltd., China.
Cell CultureHuman breast cancer MCF-7 cells, human cervical carcinoma HeLa cells, human NSCLC A549 cells, and a non-malignant cell line (Chang liver) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured and maintained in RPMI 1640 medium (Hyclone) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (0.1 mg/mL) at 37°C in a humidified atmosphere with 5% CO2.
Cell Viability AssayThe proliferative activities of A549, MCF-7, Hela, and Chang liver cells exposed to different treatments were assessed using the MTT assay. Cells were seeded in 96-well plates at a density of 2 × 103 cells per well in complete medium and cultured for 24 h, after which media were replaced with RPMI-1640 containing 10% FBS with or without pentacyclic triterpenoid derivatives. Gefitinib and test compounds were dissolved in dimethyl sulfoxide (DMSO) at a final concentration of <0.1% before addition to cell culture assays. Following incubation at 37°C for 48 h, 20 µL MTT (5 mg/mL) was added to each well, and the plates were incubated for 4 h. The reaction was terminated by adding 150 µL DMSO, and the optical density at 492 nm was measured on a microplate reader after 10 min of low-speed shaking. The cell viability after exposure to each test compound was calculated as follows: (OD570 treated cells − OD570 blank control)/(OD570 control − OD570 blank control) × 100%. Dose response curves were plotted for the samples, and the IC50 values were calculated as the concentrations of the test compounds resulting in 50% reduction of absorption compared with the control cells. More than three independent experiments were performed.
Analysis of Apoptosis and Cell Cycle Progression by Flow CytometryApoptosis and cell cycle progression in MCF-7 cells were analyzed after incubating cells with DABO-Me (15 µM) for 48 h on a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, U.S.A.). For cell cycle analysis, MCF-7 cells were harvested using trypsin in ethylenediaminetetraacetic acid (EDTA), washed twice with phosphate buffered saline (PBS), resuspended with 70% cold ethanol, and incubated at 4°C overnight in the dark. Cells were then treated with RNaseA for 30 min at 37°C and stained with PI at 4°C in the dark for 30 min. Samples were then put on ice for analysis by flow cytometry. For apoptosis analysis, the cells were collected with EDTA-free trypsin and washed twice with cold PBS. Cells were then resuspended in binding buffer and stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide for 15 min. The number of annexin V-FITC-positive apoptotic cells was expressed as the percentage of the total number of cells counted.
Western Blotting AnalysisTotal protein was extracted from cells using RIPA buffer (Sigma). After treatment with 15 µM DABO-Me for 48 h, MCF-7 cells were washed with ice-cold PBS solution and lysed in lysis buffer at 0°C for 20 min. Cell lysates were scraped and centrifuged at 14000 rpm for 30 min at 4°C, and the supernatant was collected. Equivalent amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking for 1 h in 5% skim milk in Tris-buffered saline, the membrane was incubated with the indicated primary antibodies (1 : 2000 dilution) overnight at 4°C, followed by treatment with horseradish peroxidase-coupled secondary antibody (1 : 5000 dilution) for 1 h at room temperature. Immunoreactive proteins were detected using an enhanced chemiluminescent substrate kit (Thermo Scientific, MA, U.S.A) and visualized using the ChemiDoc MP imaging system (Bio-Rad, Hercules, CA, U.S.A.). Antibodies against the following proteins were used for Western blotting: caspase-3, caspase-9, cytochrome-c, Bcl-2, Bax, cyclin D1, CDK4, TIMP-1, matrix metalloproteinase (MMP)-2, MMP-9, and β-actin (all from Proteintech Group, SanYing Biotechnology, Wuhan, China). β-Actin was used as an internal control.
Cell Migration and Invasion AssaysMCF-7 cells were plated at a density of 2 × 105 cells per well in 6-well plates and incubated overnight in complete medium. After 24 h of treatment with 15 µM DABO-Me, cells were digested by trypsinization, and 2 × 104 cells were seeded in 200 µL medium with 1% FBS in the upper chambers of a 24-well Transwell plate containing polycarbonate filters with 8 µm pores (Corning Inc., Corning, NY, U.S.A.). In the lower chambers, 500 µL medium containing 10% FBS was added as the chemoattractant. The cells were incubated for 24 h, the chamber was washed with PBS, and the cells on the upper surface of the insert were wiped away gently with a cotton swab. Cells adhering to the lower surface were fixed with methanol, stained with Giemsa solution, and counted under an inverted microscope in nine random fields of view (40× objective).
The cell invasion assay procedure was similar to that of the migration assay, except that the Transwell membranes were coated with Matrigel (BD Biosciences, San Jose, CA, U.S.A.) at a dilution of 1 : 4 and plates were incubated for 1 h at 37°C. Cells adhering to the lower surface were counted as described for the cell migration assay.
Animal ExperimentsAll animal experiments were performed according to the rules approved by the Animal Experimental Ethics Committee of Dalian Medical University. Female nude mice (BALB/c, 4–6 weeks old, 15–20g) were provided by Dalian Medical University Experimental Animal Center. Mice were bred and housed in laminar-flow cabinets under specific pathogen-free conditions at room temperature. MCF-7 cells (5 × 106 cells in 0.1 mL PBS) at log growth-phase were injected subcutaneously into the athymic nude mice on day 0 to establish a tumor-bearing mouse model. On day 5 after implantation, DABO-Me were injected into the tumor daily with sterile PBS as a negative control. Tumor size was measured every 5 d. On day 30, all tumors of significant size were harvested and weighed to determine the tumor burden. Tumor volume was calculated with the following formula: (length × width2)/2.
Statistical AnalysisData were analyzed using statistical methods and expressed as the mean ± standard deviation (S.D.). Statistical comparisons were made using the Student’s t-test. Differences were considered statistically significant at p ≤ 0.05 (*), p ≤ 0.01 (**) and p ≤ 0.0001 (****). Three independent experiments were performed to confirm data and results.
A series of 12 novel OA and UA derivatives were designed and synthesized. The derivatives had structural modifications at positions C-3 and C-28 of OA and UA, respectively. The procedures used to synthesize derivatives of OA and UA were similar, and the synthetic pathways are presented in Chart 1. The carboxylic groups (C-28 of OA and UA) were subjected to methyl iodide treatment in the presence of potassium carbonate in dimethyl formamide (DMF) to create methyl ester moieties with retention of stereochemistry (OA-2, and UA-2). The hydroxyl groups at C-3 of OA-2 and UA-2 were esterified with butanedioic anhydride, and amidated with ethylenediamine, butanediamine, and hexanediamine in the presence of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) to obtain OA-4, OA-5, UA-4, and UA-5, respectively. The compounds were characterized by IR, 1H-NMR, 13C-NMR, and HR-MS. IR spectra showed typical absorption peaks for the functional groups present. 1H-NMR and 13C-NMR spectra were as expected. The HR-MS measured values were consistent with theoretical values. IR, 1H-NMR, 13C-NMR, and HR-MS data of newly synthesized compounds are summarized in the “Chemistry” section.
Antiproliferative ActivityWe compared the inhibitory effects of the two pentacyclic triterpenoids (OA and UA) and the 12 analogues against three cancer cell lines: MCF-7, HeLa, and A549. Gefitinib was used as the positive control, and the results are summarized in Table 1. The derivatives containing ethylenediamine, butanediamine, and hexanediamine moieties (DABO-Me, OA-5b, UA-5a, UA-5b, and UA-5c) had the strongest growth inhibitory effects against three human cancer cell lines, followed by OA-4a and OA-5c compounds. In particular, DABO-Me showed the strongest cytotoxic activity against MCF-7, HeLa, and A549 cells (IC50 = 4.87 ± 0.77 , 5.71 ± 0.32 , and 6.03 ± 0.82 µM, respectively). However, their intermediates, OA-4b, OA-4c, UA-4a, UA-4b, and UA-4c, displayed poor cytotoxicity against the cancer cell lines, with IC50 values of >100 µM; increasing carbon chain length did not improve the antiproliferative activity of OA and UA derivatives. The effects of nine compounds (OA-3, OA-4a, DABO-Me, OA-5b, OA-5c, UA-3, UA-5a, UA-5b, and UA-5c) against Chang liver cells, an immortalized non-tumor cell line derived from normal liver tissue, were also evaluated. Those derivatives did not cause any detectable toxic effect at a dose of 100 µM on Chang liver cells. The cell morphology after treatment was also normal as the control (Figure S1). The pronounced influence of the incorporated diamines on the antiproliferative effect could be explained by their ability to form hydrogen bonds, improvement of water-solubility, and modified physicochemical properties.
| Compounds | IC50 (µM)a) | ||
|---|---|---|---|
| MCF-7 | Hela | A549 | |
| OA | >100 | >100 | >100 |
| OA-3 | 55.71 ± 4.81 | 57.81 ± 6.62 | 51.84 ± 4.94b) |
| OA-4a | 55.03 ± 3.54 | 50.12 ±2.87 | 59.27 ± 3.17 |
| OA-4b | >100 | >100 | >100 |
| OA-4c | >100 | >100 | >100 |
| OA-5a (DABO-Me) | 4.87 ± 0.77 | 5.71 ± 0.32 | 6.03 ± 0.82 |
| OA-5b | 9.51 ± 0.72 | 8.46 ± 1.38 | 9.66 ± 1.03 |
| OA-5c | 50.55 ± 2.96 | 61.60 ± 1.51 | 51.10 ± 2.44 |
| UA | >100 | >100 | >100 |
| UA-3 | 36.04 ± 3.25 | 46.68 ± 3.68 | 74.24 ± 8.77b) |
| UA-4a | >100 | >100 | >100 |
| UA-4b | >100 | >100 | >100 |
| UA-4c | >100 | >100 | >100 |
| UA-5a | 8.26 ± 0.49 | 9.18 ± 0.86 | 9.34 ± 0.74 |
| UA-5b | 13.69 ± 0.89 | 14.77 ± 1.65 | 17.10 ± 1.05 |
| UA-5c | 12.21 ± 1.07 | 10.37 ± 1.44 | 15.92 ± 1.28 |
| Gefitinib | 17.83 ± 7.85 | 15.40 ± 4.65 | 11.02 ± 3.27b) |
Human tumor cells were treated with different concentrations of samples for 48 h (n = 3 independent experiments). a) Data are presented as IC50 (µM, the concentration of 50% cytotoxic effect). b) Cited from ref. 6.
To investigate the mechanism of apoptosis induced by DABO-Me in MCF-7 cells, the expression of apoptosis-related proteins (caspase-3, caspase-9, cytochrome-c, Bax, and Bcl-2) was detected by Western blotting. As shown in Figs. 3A and B, DABO-Me markedly upregulated the expression of caspase-3, caspase-9, cytochrome-c, and Bax and downregulated Bcl-2. DABO-Me caused a 46.88% increase in the levels of early and late apoptotic MCF-7 cells (Figs. 3C, D). These results indicate that DABO-Me induced apoptosis in MCF-7 cells through the intrinsic mitochondrial pathway.

(A) Expression levels of caspase-3, -9, and cytochrome-c in MCF-7 cells treated without (NC) and with DABO-Me (15 µM) for 48 h were monitored by Western blotting. β-Actin was used as a loading control. (B) Expression levels of Bcl-2 and Bax in MCF-7 cells treated without (NC) and with DABO-Me (15 µM) for 48 h were monitored by Western blotting. β-Actin was used as a loading control. (C) Cell apoptosis was evaluated by flow cytometry at 48 h post-treatment. All experiments were performed in triplicate. * p < 0.05; ** p < 0.01.
The effect of DABO-Me on cell cycle-related proteins was assessed, and the results showed that the expression levels of CDK4 and cyclin D1 decreased after treatment with DABO-Me for 48 h compared with the control group (Figs. 4A, B). Flow cytometry analysis showed that DABO-Me decreased the percentage of cells at the G1 stage (Figs. 4C, D) and increased the number of cells in S stage to 37.07%. By contrast, the percentage of cells in S phase was 27.35% in the control. These results suggest that DABO-Me causes cell cycle arrest at S phase.

(A) Expression levels of CDK4 and cyclin D1 in cells treated without (NC) and with DABO-Me (15 µM) for 48 h were monitored by Western blotting. β-Actin was used as a loading control. (B) Relative quantitative analysis of CDK4 and cyclin D1 expression. (C) Cell cycle phase distribution by flow cytometry. (D) Quantitative analysis of G1, G2, and S phases. * p < 0.05; ** p < 0.01.
The effect of DABO-Me treatment on the migratory and invasive potential of MCF-7 cells was evaluated by Transwell migration and Matrigel invasion assays, respectively. The MMP/tissue inhibitor of metalloproteinases (TIMP) system is responsible for cancer invasion, progression, and metastasis. DABO-Me upregulated the expression of TIMP-1 and downregulated the expression of MMP-2 and MMP-9 in MCF-7 cells compared with the controls (Fig. 5A). Moreover, migration and invasion were effectively inhibited by DABO-Me in MCF-7 cells (Figs. 5B, C).

(A) Expression levels of TIMP-1, MMP-2, and MMP-9 in cells treated without (NC) and with DABO-Me (15 µM) for 48 h were monitored by Western blotting. β-Actin was used as a loading control. (B) Migration and invasion of MCF-7 cells treated with DABO-Me was determined by Transwell and Matrigel assays. (C) Quantitative analysis of the migration and invasion in (B). * p < 0.05; ** p < 0.01; **** p < 0.0001.
To investigate the effect of DABO-Me on tumorigenesis in vivo, a xenograft model of breast cancer was generated. As shown in Fig. 6, treatment with DABO-Me delayed tumor growth compared with that in the control. The average tumor volume of the DABO-Me, and control groups was 206 ± 23 and 360 ± 35 mm3 (n = 3), respectively, after 30 d. The results indicate that DABO-Me effectively inhibited breast tumor growth in vivo.
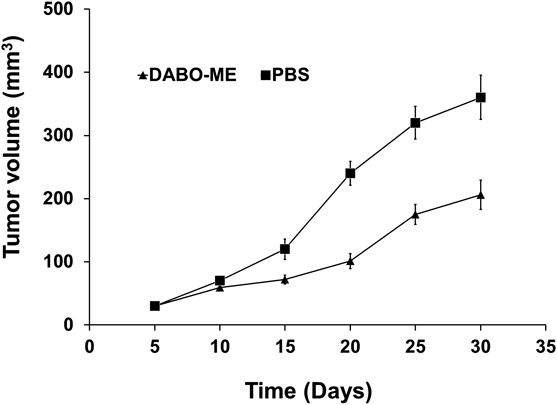
Tumor volumes were calculated depending on the sizes of neoplasms every 5 d after DABO-Me injection.
Naturally occurring pentacyclic triterpenoids, which are widely distributed in plants, fungi, marine invertebrates, and other organisms, show a wide range of biological activities, including antiviral,7) antitumor,8,9) anti-inflammatory,10,11) antiparasitic,12) and wound healing properties.13) OA and UA are pentacyclic triterpenoids that can inhibit tumor initiation and growth, and induce apoptosis in various cancer cells.2,14) The low water solubility of OA and UA results in low bioavailability. Efforts have been made to improve their water solubility by chemical modification. For instance, earlier study has found that introduction of a moiety at the C-3 position of OA and UA, such as a carboxyl group, significantly improved the IC50 value.15) To improve the cytotoxic or antitumor activity of triterpenoids, various semisynthetic OA and UA derivatives were generated by modifying the A or E ring,5,16) by introducing piperazine and butanedioic acid at the C-3 position,6) or by introducing nitrogen- and sulfur-containing heterocycles.17) The introduction of nitrogen-containing fragments into drug structure can regulate the acid-base balance and enhance the water solubility of drug molecules. In this study, we generated a series of pentacyclic triterpenoid derivatives bearing ethylenediamine, butanediamine, or hexanediamine groups at the C-3 position and investigated their cytotoxic potential against MCF-7, HeLa, and A549 cells. Most of these compounds showed significant antiproliferative effects compared with their parent compounds. In particular, DABO-Me showed the highest inhibitory activity against the three human cancer cell lines.
Apoptosis is a complex cellular process, and the caspase and Bcl-2 protein families play crucial roles in the induction of apoptosis. Tritepenoids can induce mitochondrial apoptosis by targeting the permeability transition pore complex, by suppressing the inhibitor of apoptosis proteins, or by inhibiting anti-apoptotic Bcl-2 proteins.2) OA and UA inhibit the proliferation of a variety of cancer cells through a caspase-dependent apoptotic pathway.14,18) In the present study, the results of flow cytometric assays showed that DABO-Me treatment induced apoptosis in MCF-7 cells through the activation and cleavage of caspases 3 and 9. DABO-Me upregulated the pro-apoptotic protein Bax and downregulated the anti-apoptotic protein Bcl-2. The balance between these anti-apoptotic (e.g., Bcl-2) and pro-apoptotic proteins (e.g., Bax) profoundly affects the apoptotic cellular response.19) Analysis of apoptosis-related proteins in MCF-7 cells revealed that DABO-Me activated the mitochondria-mediated apoptosis pathway, as shown by an increased Bax/Bcl-2 ratio, the release of cytochrome c, and the cleavage of caspase-3 and caspase-9. In addition, DABO-Me caused cell cycle arrest at the S phase.
MMPs and their inhibitors, TIMPs, are responsible for tissue remodeling and degradation of the extracellular matrix. MMPs are promising targets in cancer because of their involvement in malignant pathologies, and inhibiting MMPs could be developed as a powerful strategy for the treatment of cancer.20) As previously reported, UA inhibits the transcriptional expression of gelatinases A and B (MMP-2 and MMP-9, respectively) by inhibiting the extracellular signal-regulated kinase (ERK) and cAMP response element binding protein (CREB) signaling pathways in non-small cell lung cancer,21) and a co-drug of aspirin and UA blocks adhesion, invasion, and the migration of breast cancer MCF-7 and MDA-MB-231 cells to the vascular endothelium by downregulating MMP-2, MMP-9, and other MMP-related proteins.22) In this study, DABO-Me downregulated MMP-2 and MMP-9 in MCF-7 cells and inhibited cell invasion by upregulating TIMP-1. These results indicate that DABO-Me suppressed the invasive abilities of MCF-7 cells.
In this study, we showed that DABO-Me inhibited MCF-7 cell proliferation and decreased the tumor burden in MCF-7 cell models. The mechanism underlying the antiproliferative activity of DABO-Me remains unknown. Additional studies are necessary to identify the receptor that mediates its antiproliferative activity.
Taken together, the in vitro and in vivo results indicate that DABO-Me can be developed as a promising drug with significant anticancer activity, and it shows advantages such as low-cost and ease of preparation. The compound DABO-Me therefore warrants further investigation as a novel chemotherapeutic or chemopreventive agent against cancer.
This work was supported by the National Natural Science Foundation of China (No. 81371676).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.