2020 Volume 68 Issue 10 Pages 1001-1007
2020 Volume 68 Issue 10 Pages 1001-1007
By comparing the two types of activated carbon fibers (ACFs), characteristics of adsorption sites for nitrate ion other than quaternary nitrogen (N-Q) were investigated. Using phenol resin as precursors, activation with ZnCl2 was performed, and then heat treatment at 950 °C was carried out to prepare ACFs without N-Q, while ACFs with N-Q was prepared in the same method using polyacrylonitrile-based carbon fiber as precursors. We assessed the amount of functional groups, elemental composition, porous properties, and model of unit crystal size of graphene. For both ACFs with N-Q and without N-Q, equilibrium adsorption amount was not always simply proportional to surface area, but to the average number of benzene rings (Bz-rings) of graphene universally. PhR-5.0Z4 had only 20 benzene rings per graphene unit, but after heat treatment at 950 °C, the number drastically increased to 1088 (PhR-5.0Z4-9.5HT30). However, when the ACFs contained a large amount of oxygen, the number of Bz-rings was limited to 792 (PhR-5.0Z4-Ox-9.5HT30) even after heat treatment at 950 °C, and did not increase sufficiently. Cπ sites are more susceptible to oxygen inhibition than N-Q in adsorbing nitrate ions. For ACFs having Cπ sites as main adsorption sites, the heat treatment at 950 °C without oxidation can enhance the nitrate ion adsorption capacity.
In recent years, groundwater pollution by nitrate/nitrite has gradually attracted attention worldwide.1) In Japan, the number of wells exceeding the environmental standard concentration (10 mg-NO3−/L) has been growing and still remaining high.2) Nitrate contamination comes from excessive fertilizer in agricultural fields and livestock excreta.3) Nitrite ions react with aliphatic amines to form carcinogenic nitrosamines. It has been shown that ingestion of drinking water containing nitrate/nitrite (10 mg-NO3−/L) increased the concentration of nitroso compounds in urine.4,5) High concentrations of nitrate/nitrite in drinking water can cause harmful effect on human health. Nitrate/nitrite also causes eutrophication in lakes and reservoirs,6) resulting in environmental pollution such as algal blooms. Therefore, development of effective removal methods is highly required.
For removing nitrate ion, there are several methods such as reverse osmosis,7) ion exchange,8) adsorption,9) denitrification reaction,10) etc. Among them, adsorption is one of the low cost and an easy method to operate.11) Activated carbon fibers (ACFs) are porous and have been widely used for the adsorption of organic pollutants in water.12) However, ACFs are hydrophobic and not suitable for removing nitrate ions, but there is still a room for improvement. In the present study, various modifications such as chemical activation and heat treatment were performed to improve the adsorption performance of nitrate ions.
In our previous study, various precursors and modification methods have been used to improve the nitrate adsorption capacity of resultant activated carbons (ACs) and ACFs. Equilibrium adsorption amount (Qe) of each AC and ACF was listed in Table 1.13–16) Ammonization at high temperature and thermal chemical vapor deposition (CVD) with acetonitrile enhanced the adsorption capacity. These studies revealed that the quaternary nitrogen (N-Q) could be an effective adsorption site for nitrate ion. The precursor with a high nitrogen content and/or the modification using nitrogen-containing substances were applied to introduce a sufficient amount of N-Q in ACs and ACFs.13–15) There is a need for the development of a method to efficiently introduce N-Q into ACFs. However, in the case of using ACFs, even if N-Q is introduced, adsorption sites other than N-Q were always observed. Then, some ACFs showed a great adsorption amount of nitrate even though the amount of N-Q was small.16) ACFs contain both acidic and basic functional groups in addition to N-Q, and these functional groups have various effects on the adsorption capacity. Therefore, it is important to understand the characteristics of adsorption sites other than N-Q. In this study, ACFs with and without N-Q were prepared and their adsorption properties were examined.
| Precursors | Methods | pHe | Qe [mmol/g] | References |
|---|---|---|---|---|
| A-BAC LP (Kureha Corp.) | Oxidized and treated by ammonium gas at 950°C | 3 | 0.54 | 13) |
| Melamine sponge (Fuji Gomu Co., Ltd.) | Activated with ZnCl2 at 500°C and treated by CH3I | 3–4 | 0.60 | 14) |
| KF1500 (Toyobo Co., Ltd.) | CVD at 800°C using acetonitrie, heat treatment at 950°C and steam activation at 800°C | 3 | 0.71 | 15) |
| KF1500 (Toyobo Co., Ltd.) | Steam activation at 800°C, CVD at 800°C using acetonitrie, heat treatment at 950°C and steam activation at 800°C | 3 | 0.74 | 16) |
| Phenol resin (Gun Ei Chemical Industry Co., Ltd.) | Steam activation at 800°C, heat treatment at 950°C, and steam activation at 800°C | 3 | 0.49 | 16) |
Phenol resin (PhR) and air-stabilized polyacrylonitrile (PAN)-based carbon fiber (Py) were used as precursors. PhR, which has a low nitrogen content, was purchased from Gun Ei Chemical Industry Co., Ltd., Japan. Py with high nitrogen content was obtained from Teijin Ltd., Japan. In order to activate the precursors, raw materials and ZnCl2 were impregnated at a weight ratio of 1 to 4 g/g. The mixture was dried at 110 °C in an oven overnight. Then, the impregnated sample was placed in a boat, put it into a tubular reactor and activated with the heating program depicted in Fig. 1 under nitrogen flow.14) When cooling the sample after activation, the flow rate of nitrogen was increased to prevent backflow of water and oxidation by air. The PhR impregnated at the ratio of 1 to 4 g/g and activated at A °C (A = a × 100) was referred to as PhR-aZ4. For example, when PhR was activated at 500 °C at the ratio of 4, it was named as PhR-5.0Z4. After the activation, PhR-aZ4 was washed with 0.1 M hydrochloric acid repeatedly and hot distilled water to remove ZnCl2, and then washed with Soxhlet extractor until pH of the solution was no longer changed. The PhR-aZ4 was oxidized with 2 M ammonium peroxodisulfate (APS) at 30 °C for 3 h, and then the oxidized PhR-aZ4 was washed with hot distilled water. The washed sample was referred to as PhR-aZ4-Ox. The PhR-aZ4-Ox was loaded on ceramic boat, placed in quartz tube and heat-treated (HT) at B °C (B = 300, 500, 700, 950) for c min under helium flow.17) Heat treatment at B °C (B = b × 100) was referred to as PhR-aZ4-Ox-bHTc. Likewise, ACFs prepared from Py were named as Py-aZ4-bHTc. For comparison, HP555, which was purchased from Sumika Chemtex Co., Ltd., Japan, was used as adsorbent after being dried at 40 °C for 24 h. HP555 was anion exchange resin and has only trimethylammonium (N-Q) function (salt, -N+(CH3)3Cl−) as an effective adsorption sites for anions. Chemicals such as hydrochloric acid, sodium hydroxide, zinc chloride, and sodium nitrate were purchased from Kanto Chemical Co., Ltd., Japan, and reagent grade.

In the Boehm titration, acidic functional groups and basic sites on the activated carbon surface can be determined.18) The mixture of 50 mg of sample and 25 mL of each 0.1 M NaOH, 0.05 M Na2CO3 and 0.1 M NaHCO3 solution were added in 100 mL Erlenmeyer flask, and stirred at 100 rpm at 25 °C for more than 48 h. Each solution was filtered and neutralized by 0.05 M HCl. NaOH reacts with carboxy, lactone and hydroxy groups on the carbon surface, Na2CO3 does carboxy and lactone, and NaHCO3 neutralizes only carboxy groups. As described later section quaternary nitrogen (N-Q) in place of carboxy groups can be detected because positively charged nitrogen (N-Q) principally behaves like a proton (H+) released from carboxy groups in the titration. From the difference between the measured values of each solution, the amount of carboxy and/or N-Q, lactone, and hydroxy groups can be determined. In order to quantify the basic sites including Cπ sites that can accommodate protons, the mixture of 50 mg of sample and 25 mL of 0.1 M HCl solution was added in 100 mL Erlenmeyer flask, and the same operation was performed. The solution was filtered, 0.1 M NaOH was added, and the solution was titrated with 0.05 M HCl. Neutralization points were determined using methyl red and phenolphthalein. Methyl red was used when the solution was a weak base, while phenolphthalein was used when the solution was a strong base.
Shafeeyan et al. showed that acidic functional groups were decomposed as CO/CO2 and H2O by heat treatment.19) In particular, carboxy groups were decomposed in the range of 100–400 °C.19) Complete decomposition of carboxy groups can be expected when we perform heat treatment at 950 °C. HP555 has only N-Q (trimethylammonium) as an adsorption site. Although HP555 does not have carboxy groups, they showed a high value corresponding to carboxy groups in case of Boehm titration. This result indicated that the N-Q reacted with NaOH, Na2CO3 and NaHCO3 as a strong adsorption site instead of carboxy groups. Therefore, in this study, the value of the carboxy groups of the heat-treated samples measured by Boehm titration was regarded as N-Q.
Elemental CompositionElemental composition (carbon, hydrogen and nitrogen) was measured by Pekin-Elimer PE2400 CHN analyzer. In calculating the elemental composition, it was assumed that the prepared sample was composed of only C, H, N and O. The oxygen content was determined by subtracting the total value of C, H and N from 100 wt%.
The Surface Area and Pore Size DistributionThe surface area and pore size distribution were measured through BELSORP-mini II surface analyzer (MicrotracBEL Corp., Japan). The surface area (SBET), average pore diameter (Davg) and total pore volume (Vtotal) were calculated by Brunauer–Emmet–Teller (BET) method. The micropore volume (Vmicro) was determined by t-plot method. The mesopore volume (Vmeso) was determined by subtracting Vmicro from Vtotal.
Fourier Transform (FT)-IR AnalysisThe surface functional groups of prepared ACFs were examined by FT-IR spectroscopy (IRAffinity-1S, Shimadzu Corp., Japan) in the range of wave numbers from 3500 to 700 cm−1 in the resolution of 1 cm−1. After mixing the samples and KBr at a ratio of 1 to 9 g/g, the measurement was made by using diffuse reflectance method.
Model of Unit Crystal Size of Activated Carbon (AC)AC is composed of many graphene sheets with various crystal sizes. In this study, the crystal size of AC was estimated using the relational expression in Fig. 2 and the value of elemental analysis.20) For example, when the elemental composition of C and H was 99 and 1%, the chemical formula and the number of benzene-rings (Bz-rings) of AC can be estimated to be C408H50 and 180, respectively.

NaNO3 dissolved in aqueous solution was used as adsorbate and the initial concentration was 200 mg-NO3−/L. In 30 mL Erlenmeyer flask, 30 mg samples and 15 mL nitrate solution were added, and equilibrium solution pH (pHe) was adjusted to 3 ± 0.2 by hydrochloric acid and sodium hydroxide solutions. The mixture was shaken for more than 12 h at 100 rpm and 25 ± 2 °C. Nitrate concentration was measured by ion chromatograph (ICS-1100, Nippon Dionex KK, Japan), and adsorption amount was determined by the following equation:
 | (1) |
where Qe is the equilibrium adsorption amount (mmol/g), C0 and Ce are the initial and equilibrium concentration of nitrate solution (mmol/L), respectively, V is the solution volume (L) and W is the weight of sample (g). Using the prepared activated carbon, the adsorption experiment was performed by changing the pHe ranging from 2 to 11 and the initial concentration from 20 to 500 mg/L.
The obtained results were fitted to the Langmuir and Freundlich isotherm models. The Langmuir isotherm model is obtained by the following equation for linear analysis:
 | (2) |
where Ce means the equilibrium concentration of nitrate ions (mmol/L). Qe and Xm are the equilibrium adsorption amount and the maximum adsorption capacity (mmol/g), respectively. Ke represents the Langmuir isotherm constant representing adsorption affinity (L/mmol).
Freundlich isotherm model can be expressed by the following equation:
 | (3) |
where KF is Freundlich isotherm constant [(mmol/g) (L/mmol)1/n], and 1/n represents the heterogeneity of the surface.
Chemical activation is an operation of generating micropores inside carbon materials, and it can impart an adsorption capacity to the carbon material.21) Zinc chloride (ZnCl2) has a strong dehydration effect and is excellent as an activator. It is believed that as the activation temperature increases, zinc chloride changes and activates the precursor by the following processes exhibited in equations.
 | (4) |
 | (5) |
 | (6) |
These reactions were described based on the paper by Chen et al.21) Mochida et al. reported that the ACFs and ACs after activation have structures as shown in Fig. 3.22)

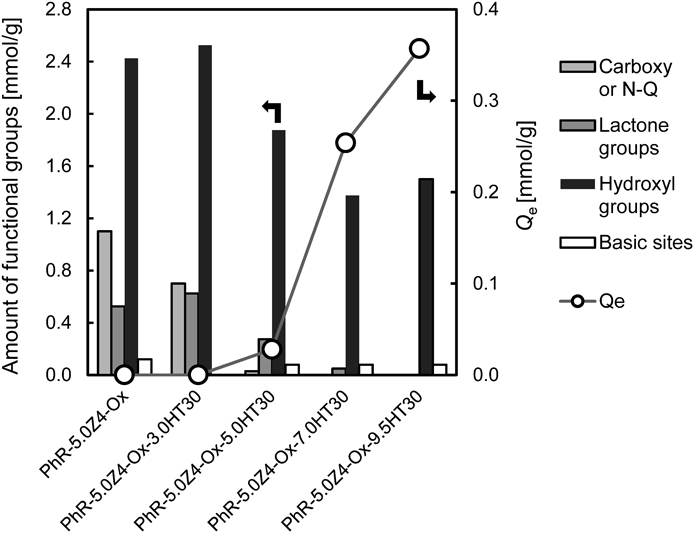
The result of the Boehm titration is listed in Table 2. The value of the carboxy and/or N-Q of the HP555 was 2.13 mmol/g. The amount of N-Q can be estimated by Boehm titration. Samples prepared from PhR had neither carboxy nor N-Q function. However, carboxy and/or N-Q value was detected for all samples prepared from Py. These results meant that the samples prepared from PhR and Py were ACFs without N-Q and ACFs with N-Q, respectively. In Py-7.0Z4, the carboxy group was also detected because the high temperature treatment was not performed. When the heat treatment time was increased, the amounts of basic sites increased, but the N-Q decreased.
| Sample | Carboxy or N-Q [mmol/g] | Lactone groups [mmol/g] | Hydroxyl groups [mmol/g] | Basic sites [mmol/g] |
|---|---|---|---|---|
| HP555 | 2.13 | 0.00 | 0.41 | 0.13 |
| PhR-5.0Z4-Ox | 1.10 | 0.53 | 2.42 | 0.12 |
| PhR-5.0Z4-Ox-3.0HT30 | 0.70 | 0.63 | 2.52 | 0.00 |
| PhR-5.0Z4-Ox-5.0HT30 | 0.03 | 0.27 | 1.88 | 0.08 |
| PhR-5.0Z4-Ox-7.0HT30 | 0.00 | 0.05 | 1.38 | 0.08 |
| PhR-5.0Z4-Ox-9.5HT30 | 0.00 | 0.00 | 1.50 | 0.08 |
| PhR-5.0Z4 | 0.00 | 0.00 | 4.23 | 0.33 |
| PhR-5.0Z4-9.5HT30 | 0.00 | 0.00 | 1.15 | 0.03 |
| PhR-6.0Z4 | 0.00 | 0.00 | 2.75 | 0.01 |
| PhR-6.0Z4-9.5HT20 | 0.00 | 0.00 | 0.87 | 0.10 |
| PhR-6.0Z4-9.5HT30 | 0.00 | 0.00 | 1.35 | 0.52 |
| Py-7.0Z4 | 1.48 | 0.70 | 2.47 | 0.32 |
| Py-7.0Z4-9.5HT5 | 0.55 | 0.00 | 2.60 | 0.12 |
| Py-7.0Z4-9.5HT10 | 0.67 | 0.29 | 3.41 | 0.37 |
| Py-7.0Z4-9.5HT20 | 0.52 | 0.33 | 2.65 | 0.75 |
| Py-7.0Z4-9.5HT30 | 0.27 | 0.00 | 3.60 | 1.13 |
In order to investigate the characteristics of the adsorption sites, adsorption experiments were performed by changing the solution pH. By comparing HP555, PhR-6.0Z4-9.5HT30, and Py-7.0Z4-9.5HT10, the influence of the presence and absence of N-Q on the adsorption capacity was examined. The result is represented in Fig. 4. The equilibrium adsorption amount (Qe) of HP555 increased from pH 2.0 to 3.0, and showed constant values when the solution pH was in the range of 3.0 to 10. It can be seen that the N-Q was a good adsorption site even when the solution was adjusted to a neutral or basic state. The Qe of PhR-6.0Z4-9.5HT30 increased from pH 2.0 to 3.0 and maximized at pH 3.0. In case of pH >3.0, Qe decreased as pH increased. At pH <3.0, adsorption was inhibited by chloride ions, and at pH >4.0, adsorption was inhibited by hydroxide ions.16) Cπ sites adsorb nitrate ions by accommodating protons in advance in the solution to become positively charged surface.18) The result implied that the ACFs with Cπ sites as the main adsorption sites were inhibited by not only the increase of hydroxide ions but also the decrease of protons. The Qe of Py-7.0Z4-9.5HT10 behaved similar to PhR-6.0Z4-9.5HT30, despite being rich in N-Q. If the adsorption site of Py-7.0Z4-9.5HT10 was only N-Q, it is considered that the adsorption amounts were constant at pH 3–10 like HP555. This indicated that the Py-7.0Z4-9.5HT10 had another adsorption site other than N-Q.

The effect of acidic functional groups on nitrate ion adsorption was investigated by using ACFs with different amounts of acidic functional groups. Figure 5 represented the relationship between the amount of surface functional groups and Qe depending on the heat treatment temperature. When heat treatment was performed at 500, 700 and 950 °C, and Qe increased, while the carboxy and the lactone groups decreased as well. Our previous study showed similar results with different precursors.17) Ota et al.18) reported that the π-electrons on the graphene layer (Cπ) can attract some protons resulting in a slight increase of adsorption site under acidic conditions. The carboxy and lactone groups, which are electron withdrawing groups, lower the π-electron (Cπ) density on the graphene layer, thereby reducing the amount of adsorption. The oxygen on the carboxy and lactone groups has a high electronegativity and is negatively charged. It can be assumed that the oxygen inhibited the adsorption of nitrate ions. In addition, the presence of an acidic functional group in the micropores of the ACFs could cause steric hindrance and reduce Qe.
In this experiment, the properties of functional groups on the carbon surface were investigated using samples without and with N-Q and samples after adsorption of nitrate ion (200 mg-NO3−/L). Figure 6 displays the FT-IR spectra of prepared samples. The samples after the adsorption of nitrate were named as PhR-6.0Z4-9.5HT30-ads and Py-7.0Z4-9.5HT10-ads. The peak at 1760–1690 cm−1 was related to C=O functional groups.23) The band around 1500 cm−1 was assigned to C=C bonds in benzene rings.24) The peak at 1380 cm−1 of PhR-6.0Z4-9.5HT30-ads and Py-7.0Z4-9.5HT10-ads was attributed to N=O stretching, attributed to the nitrate adsorption.14) Only Py-7.0Z4-9.5HT10 had a peak located around 1000 cm−1. The functional group observed around 1000 cm−1 could be involved in nitrate ion adsorption and could be presence of N-Q as well.

Characterization of ACFs without and with N-Q is listed in Table 3. The relationships between Qe and SBET, and between Qe and the number of Bz-rings of ACFs without and with N-Q are also shown in Figs. 7 and 8, respectively. Figure 7 and Table 3 indicated that the SBET was not proportional to Qe. No significant relationship between Vmeso (or Vmicro) and Qe also could be observed. However, from Fig. 8, the number of Bz-rings showed a proportional relationship with Qe. The slope value (Qe/number of Bz-rings) for both ACFs without and with N-Q was 0.0002, which was comparable. The results implied that the larger the number of Bz-rings on the carbon skeleton, the larger the π-electron and the more protons can be attracted. In the case of ACFs without N-Q, three plots located at the lower left in Fig. 8 attributed to the samples having an oxygen content of 16% or more, and the influence of oxygen is too large as compared with the π electrons (Cπ), thereby they were excluded from the approximate line. From Table 3 and Fig. 8, the ACF with N-Q contained oxygen of 10% or more in all samples, but the coefficient of determination (R2) was 0.930, which was larger than that of ACFs without N-Q (R2 = 0.690). ACF with N-Q showed a higher R2 because N-Q in the Bz-rings might suppress oxygen inhibition, whereas ACFs without N-Q were more susceptible to oxygen than ACFs with N-Q.
| Sample | Qe [mmol/g] | Elemental composition | Surface area and pore volumes | Model of unit crystal size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C [wt%] | H [wt%] | N [wt%] | O [wt%] | SBET [m2/g] | Vtotal [cm3/g] | Vmeso [cm3/g] | Vmicro [cm3/g] | Davg [nm] | CxHy | Bz-rings | ||
| PhR-5.0Z4-Ox | 0.000 | 69 | 2.1 | 0.5 | 29 | 1080 | 0.75 | 0.14 | 0.61 | 2.78 | C47H17 | 16 |
| PhR-5.0Z4-Ox-3.0HT30 | 0.000 | 73 | 1.8 | 0.5 | 25 | 1020 | 0.70 | 0.07 | 0.63 | 2.74 | C70H21 | 26 |
| PhR-5.0Z4-Ox-5.0HT30 | 0.028 | 82 | 1.8 | 0.6 | 16 | 1000 | 0.61 | 0.01 | 0.60 | 2.45 | C84H22 | 32 |
| PhR-5.0Z4-Ox-7.0HT30 | 0.254 | 91 | 1.3 | 0.5 | 7.5 | 918 | 0.55 | 0.01 | 0.54 | 2.38 | C216H36 | 91 |
| PhR-5.0Z4-Ox-9.5HT30 | 0.357 | 92 | 0.5 | 0.4 | 6.7 | 1060 | 0.77 | 0.13 | 0.65 | 2.91 | C1683H100 | 792 |
| PhR-5.0Z4 | 0.238 | 88 | 2.4 | 0.2 | 9.7 | 1560 | 1.32 | 0.17 | 1.15 | 3.37 | C58H19 | 20 |
| PhR-5.0Z4-9.5HT30 | 0.378 | 92 | 0.4 | 0.2 | 7.9 | 1020 | 0.70 | 0.18 | 0.52 | 2.74 | C2291H117 | 1088 |
| PhR-6.0Z4 | 0.351 | 89 | 1.9 | 0.2 | 9.0 | 1120 | 1.08 | 0.53 | 0.56 | 3.62 | C137H29 | 55 |
| PhR-6.0Z4-9.5HT20 | 0.483 | 95 | 0.4 | 0.3 | 4.4 | 1190 | 0.92 | 0.36 | 0.56 | 3.09 | C2232H116 | 1059 |
| PhR-6.0Z4-9.5HT30 | 0.569 | 96 | 0.4 | 0.7 | 3.1 | 1060 | 0.74 | 0.21 | 0.52 | 2.79 | C2520H123 | 1199 |
| Py-7.0Z4 | 0.540 | 68 | 1.4 | 15 | 16 | 1070 | 0.49 | 0.00 | 0.49 | 1.80 | C98H24 | 38 |
| Py-7.0Z4-9.5HT5 | 0.690 | 84 | 0.4 | 4.7 | 11 | 1240 | 0.62 | 0.06 | 0.56 | 2.00 | C1838H105 | 867 |
| Py-7.0Z4-9.5HT10 | 0.750 | 85 | 0.4 | 4.3 | 10 | 1140 | 0.55 | 0.02 | 0.53 | 2.00 | C1882H106 | 889 |
| Py-7.0Z4-9.5HT20 | 0.700 | 81 | 0.4 | 5.4 | 13 | 1260 | 0.59 | 0.05 | 0.54 | 1.90 | C1709H101 | 805 |
| Py-7.0Z4-9.5HT30 | 0.630 | 84 | 0.5 | 4.4 | 11 | 1000 | 0.55 | 0.04 | 0.51 | 2.20 | C1176H84 | 547 |


From Table 3, comparing PhR-5.0Z4-9.5HT30 and PhR-6.0Z4-9.5HT20, the number of Bz-rings showed 1088 and 1059, but Qe was 0.378 and 0.483 mmol/g, respectively. Although the number of Bz-rings was almost same, there was a difference in Qe. PhR-5.0Z4-9.5HT30 had a higher oxygen content (6.7 wt%) than PhR-6.0Z4-9.5HT20 (4.4 wt%), suggesting that the Qe was reduced by inhibition of oxygen. The number of Bz-rings in PhR-5.0Z4 and PhR-5.0Z4-9.5HT30 was 20 and 1088, respectively. The number of Bz-rings increased sharply when the heat treatment was conducted at 950 °C. However, comparing PhR-5.0Z4-Ox-9.5HT30 and PhR-5.0Z4-9.5HT30, the number of Bz-rings was 792 and 1088, respectively, and there was a large difference despite the same heat treatment. These results showed that the oxygen increased by the oxidation inhibited the increase of the Bz-rings at the post heat treatments. When heat treatment is performed, the crystallites are arranged in parallel and graphitization tends to proceed.25) On the other hand, if the crystallite orientation is random, graphitization is difficult to progress.25) The oxygen containing groups remained at the edge of graphene sheet could be assumed to inhibit the progress of condensation of small graphene sheets to transform large flat graphene sheet at higher temperature.
Comparing Py-7.0Z4-9.5HT10 with PhR-6.0Z4-9.5HT30, the number of Bz-rings showed 889 and 1199, but Qe was 0.750 and 0.569 mmol/g, respectively, as could be seen in Table 3. Py-7.0Z4-9.5HT10 has abundant N-Q, showing high Qe even with a small number of Bz-rings. The N-Q can be formed inside the Bz-rings, and the development of Bz-rings was inhibited by oxygen groups.
Adsorption IsothermsAn adsorption experiment was performed at the equilibrium solution pH (pHe) 3. Figure 9 displays the adsorption isotherms for prepared samples. The calculated Langmuir and Freundlich parameters are listed in Table 4. The coefficient of determination (R2) was higher for Langmuir isotherm than Freundlich isotherm in all samples, suggesting that the nitrate adsorption could occur on a monolayer. Py-7.0Z4-9.5HT10 showed higher Qe than PhR-6.0Z4-9.5HT30 and PhR-5.0Z4-Ox-9.5HT30 at low and high nitrate concentrations. Comparing ACFs with N-Q and without N-Q, the latter ACFs reached the maximum adsorption capacity at lower nitrate concentration. Ke is used as a parameter exhibiting adsorption affinity that can be assumed to be average value of Ke′ (N-Q sites) and Ke″ (Cπ sites).26) However, from Table 4, comparing the three samples, the lowest value of Ke (1.41 L/mmol) showed the highest adsorption capacity (Py-7.0Z4-9.5HT10). In case of equiliburium solution pH (pHe) 3, it was estimated that Ke″ (Cπ sites) was greater than Ke′ (N-Q sites) because the Ke value of Py-7.0Z4-9.5HT10 at pHe 3 was the lowest among the three samples.

| Sample | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| Xm [mmol/g] | Ke [L/mmol] | R2 | KF [(mmol/g) (L/mmol)1/n] | 1/n | R2 | |
| Py-7.0Z4-9.5HT10 | 0.982 | 1.41 | 0.962 | 0.451 | 0.403 | 0.880 |
| PhR-6.0Z4-9.5HT30 | 0.599 | 2.61 | 0.991 | 0.331 | 0.373 | 0.968 |
| PhR-5.0Z4-Ox-9.5HT30 | 0.534 | 4.84 | 0.989 | 0.334 | 0.340 | 0.957 |
In this study, using phenol resin (PhR) and air-stabilized polyacrylonitrile (PAN)-based carbon fiber (Py) as precursors, the characteristics of adsorption sites other than N-Q were examined by comparing the nitrate ion adsorption capacity and structure of each other. The main conclusions were summarized as follows:
This work was supported in part by Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (KAKENHI Grant No. JP20K05187). The authors thank the center for analytical instrumentation Chiba University for supporting elemental analysis. They are also grateful to Prof. Dr. Fumio Imazeki, the head of Safety and Health Organization, Chiba University, for his financial support on our study.
The authors declare no conflict of interest.