2020 Volume 68 Issue 11 Pages 1090-1099
2020 Volume 68 Issue 11 Pages 1090-1099
Extensive phytochemical work on the 1-BuOH-soluble fraction of a MeOH extract of the leaves of Symplocos cochinchinensis var. philippinensis resulted in the isolation of 14 new triterpenene saponins, along with four known ones. Their structures were elucidated by comparison of NMR spectroscopic data with related compounds reported in the literature. Three oleanane-type saponins, symplocosins K, M, and P, possessed glucuronic acid as a sugar component, and their carboxyl groups appeared as methyl esters. These are probably formed during extraction and isolation procedures. Symplocosin K (9) showed moderate cytotoxicity toward A549 cells. In addition, all isolated compounds did not show α-glucosidase inhibitory activity.
Symplocos genus contains about 300 species and are mainly found in tropical regions, except for the continent of Africa, and subtropical areas. A small number of the species are in a temperate climate zone. Symplocos cochinchinensis (Loureiro) Spencer Le Marchant Moore var. philippinensis (Brand) Nootboom (Symplocaceae) is an evergreen tall tree distributed in the Amami and Okinawa Islands, Taiwan, southern China, and Indochina. It grows up to about 15 m in height and blooms white flowers in spikes.1) Three species of Symplocos were medicinally used as “lodhra” in the Indian system of traditional Ayurveda medicine. For the treatment of diabetes mellitus in Ayurveda, Symplocos species were given, along with the juice of cucumber,2) and quite recently, hypolipidemic activity was also reported for the hexane extract of an elementary species, S. cochinchinensis.3) In a previous paper, the isolation of two new triterpene saponins were reported,4) and this paper deals with the structural elucidation of 14 new triterpene saponins isolated from the leaves of the title plant.
In a previous paper,4) three megastigmane glycosides, a neolignan glucoside, and two triterpene glycosyl esters were reported from the leaves of S. cochinchinensis var. philippinensis. Further extensive phytochemical investigation resulted in the isolation of 14 new triterpene saponins, named symplocosins C–P (1–14), along with four known saponins, quadranoside IV (15),5) niga-ichigosides F16) and F27) (16 and 17), and 4-epi-niga-ichigoside F18) (18; Fig. 1). This paper deals with structural elucidation of symplocosins C–P (1–14) by precise inspection of NMR spectroscopic data (Fig. 1).

Symplocosin C (1), [α]D21 +18.9, was isolated as an amorphous powder, and its elemental composition was determined to be C41H66O14 by observation of a quasi-molecular ion peak at m/z 805.4329 [M + Na]+ on high-resolution (HR)-electrospray ionization (ESI)-MS. The IR spectrum exhibited absorption peaks assigned to hydroxy and ester groups (3394 and 1733 cm−1) and a double bond (1650 cm−1). In the 1H-NMR spectrum, four singlet methyl (δH 0.98, 1.07, 1.11 × 2), two doublet methyls (δH 0.91 and 0.95), one olefinic proton (δH 5.43), and two anomeric proton [δH 5.16 (d, J = 7.2 Hz) and 6.07 (d, J = 7.2 Hz)] signals were observed (Table 1). The 13C-NMR spectrum displayed 41 signals, of which 11 signals were allotted for those of the sugar moiety, since the sugar analysis of its hydrolyzate revealed the presence of L-arabinose and D-glucose. The remaining signals comprised six methyls, eight methylenes, five methines, and five quaternary carbons, along with one oxygenated methylene, two oxygenated methines, and two olefinic and one carbonyl carbons (Table 2). Two oxygenated methine protons were placed in a vicinal position from 1H–1H correlation spectroscopy (COSY). From the heteronuclear multiple bond correlations (HMBC) between H-2 and H-3; C-4, H3-24, and C-3; and H2-23 and C-4, the diol system and oxygenated methylene must be located on the A-ring (Fig. 2). These findings suggested the structure of the aglycone is asiatic acid, and the 13C-NMR spectrum was superimposable with that of quadranoside IV (15), namely asiatic acid 28-O-β-D-glucopyranosyl ester.5) The terminal sugar was assigned as α-L-arabinopyranose from typical 13C-NMR chemical shifts reported for the terminal arabinopyranoside.4) In the HMBC experiment, the position of the terminal arabinopyranose was confirmed to be on the hydroxy group at the 2′-position (Fig. 2), and the mode of both sugar linkages were β from the coupling constants of the anomeric protons. Therefore, the structure of symplocosin C was elucidated to be asiatic acid 28-O-β-D-(2′-O-α-L-arabinopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
| H | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 | 1.33 m | 1.28 br d 11.5 | 1.28 m | 1.18 m | 1.79 br d 10.4 | 1.26 br d 11.8 | 1.33 m |
| 2.30 dd 12.6 4.4 | 2.26 dd 12.6 4.8 | 2.26 dd 12.6 3.5 | 1.96 br d 12.6 | 1.94 br d 10.4 | 2.26 dd 12.1 3.8 | 2.23 dd 12.3 2.9 | |
| 2 | 4.21 m | 4.11 m | 4.09 m | 4.30 m | 4.26 m | 4.28 m | 4.21 m |
| 3 | 4.15 m | 3.37 d 9.6 | 3.35 d 9.6 | 4.08 m | 4.15 m | 3.52 d 9.5 | 4.12 m |
| 5 | 1.81 dd 10.4 6.4 | 1.04 m | 1.00 br d 9.9 | 2.01 br d 11.2 | 1.96 dd 12.6 4.0 | 1.10 m | 1.73 m |
| 6 | 1.35 m | 1.37 dd 12.4 8.9 | 1.29 m | 1.30 br d 11.2 | 1.26 m | 1.27 br d 10.9 | 1.30 m |
| 1.62 m | 1.50 br d 12.0 | 1.41 m | 1.50 m | 1.41 m | 1.48 m | 1.66 m | |
| 7 | 0.87 m | 1.67 dd 12.4 4.3 | 1.67 m | 1.69 br d 12.1 | 1.72 m | 1.62 br d 12.2 | 0.87 m |
| 1.57 m | 1.71 br d 12.0 | 1.76 m | 1.81 br d 11.4 | 1.82 dd 14.0 6.0 | 1.74 br d 14.1 | 1.57 m | |
| 9 | 1.73 t 9.4 | 1.95 t 9.0 | 1.94 br d 8.9 | 2.14 m | 2.12 m | 1.93 br d 9.4 | 1.69 m |
| 11 | 1.99 2H m | 2.14 2H m 9.9 4.1 | 2.13 2H dd | 2.13 2H m | 2.13 2H m | 1.24 2H m | 1.99 2H m |
| 12 | 5.43 t 3.2 | 5.57 br s | 5.57 t 4.2 | 5.57 m | 5.55 br s | 5.56 br s | 5.39 m |
| 15 | 1.29 m | 1.46 m | 1.67 m | 1.41 m | 1.58 m | 1.65 br d 11 | 1.34 m |
| 2.32 m | 2.44 dd 12.0 3.7 | 2.36 dd 14.1 4.2 | 2.39 td 12.6 5.4 | 2.32 dd 14.0 4.5 | 2.33 dd 13.9 4.7 | 2.21 dd 12.4 4.0 | |
| 16 | 1.11 br d 11.7 | 2.14 br d 12.0 | 2.30 br d 9.0 | 2.10 m | 2.24 2H | 2.29 br d 13.9 | 1.08 m |
| 2.04 dd 11.7 3.4 | 3.12 td 12.6 3.7 | 3.10 td 12.6 4.2 | 3.08 td 12.6 4.2 | br d 13.5 | 3.10 dd 11.0 4.2 | 2.03 m | |
| 18 | 2.50 br d 11.4 | 2.94 br s | 2.94 m | 2.92 br s | 2.91 br s | 2.93 br s | 3.10 dd 12.4 4.4 |
| 19 | 1.40 m | — | — | — | — | — | 1.23 m |
| 1.70 m | |||||||
| 20 | 0.89 m | 1.48 m | 1.51 dd 10.8 5.2 | 1.46 m | 1.48 m | 1.50 m | — |
| 21 | 1.26 m | 1.29 br d 11.5 | 1.30 dd 9.3 4.7 | 1.28 br d 10.5 | 1.30 br d 14.0 | 1.28 m | 1.32 2H m |
| 1.42 m | 2.05 br d 12.6 | 2.03 br d 11.4 | 2.02 br d 11.5 | 1.99 m | 2.01 br d 13.7 | ||
| 22 | 1.86 2H m | 2.04 2H dd | 1.97 dd 10.5 3.3 | 2.03 2H m | 2.03 2H m | 1.95 br d 10.8 | 1.93 dd 13.9 4.2 |
| 1.30 4.7 | 2.07 br d 12.0 | 2.07 dd 13.7 2.8 | 2.72 m | ||||
| 23 | 3.64 d 10.3 | 1.21 3H s | 1.16 3H s | 3.68 d 11.4 | 3.62 d 10.8 | 1.45 3H s | 3.59 d 10.6 |
| 4.10 d 10.3 | 3.87 d 11.4 | 3.80 d 10.8 | 3.73 d 10.6 | ||||
| 24 | 0.98 3H s | 1.05 3H s | 1.02 3H s | 0.83 3H s | 0.82 3H s | 3.57 d 10.3 | 0.96 3H s |
| 4.40 d 10.3 | |||||||
| 25 | 1.07 3H s | 1.15 3H s | 1.04 3H s | 1.05 3H s | 1.04 3H s | 0.99 3H s | 1.03 3H s |
| 26 | 1.11 3H s | 1.05 3H s | 1.17 3H s | 1.15 3H s | 1.16 3H s | 1.13 3H s | 1.08 3H s |
| 27 | 1.11 3H s | 1.71 3H s | 1.71 3H s | 1.65 3H s | 1.64 3H s | 1.70 3H s | 1.15 3H s |
| 28 | — | — | — | — | — | — | — |
| 29 | 0.95 3H d 6.4 | 1.44 3H s | 1.44 3H s | 1.43 3H s | 1.42 3H s | 1.43 3H s | 0.87 3H s |
| 30 | 0.91 3H d 6.4 | 1.12 3H d 6.6 | 1.11 3H d 6.6 | 1.11 3H d 6.6 | 1.10 3H d 6.6 | 1.11 3H d 6.5 | 0.89 3H s |
| 1′ | 6.07 d 7.2 | 6.19 d 6.6 | 6.15 d 8.4 | 6.16 d 7.2 | 6.13 d 8.4 | 6.15 d 8.2 | 6.08 d 7.5 |
| 2′ | 4.24 dd 8.6 7.2 | 4.32 dd 9.0 6.6 | 4.41 dd 8.6 8.4 | 4.34 m | 4.41 dd 9.2 8.4 | 4.42 m | 4.28 dd 9.2 7.5 |
| 3′ | 4.28 m | 4.33 m | 4.30 m | 4.36 m | 4.32 m | 4.32 m | 4.26 dd 9.2 9.0 |
| 4′ | 4.26 dd 9.0 8.6 | 4.31 br d 9.0 | 4.19 m | 4.29 m | 4.20 m | 4.19 dd 9.2 8.8 | 4.25 m |
| 5′ | 3.91 ddd | 3.99 ddd | 3.99 ddd | 4.17 ddd | 3.99 ddd | 3.93 ddd | 3.89 ddd |
| 8.6 4.4 2.4 | 9.0 4.6 2.5 | 8.6 6.0 2.2 | 9.5 5.7 2.5 | 9.0 4.7 2.2 | 9.2 4.4 2.0 | 9.8 4.4 2.7 | |
| 6′ | 4.32 dd 11.3 4.4 | 4.37 dd 12.0 4.6 | 4.24 m | 4.36 m | 4.21 m | 4.23 br d 11.8 | 4.25 dd 12.2 4.4 |
| 4.43 dd 11.4 2.4 | 4.48 dd 12.0 1.8 | 4.53 dd 11.7 2.2 | 4.50 dd 12.0 2.5 | 4.53 br d 11.9 | 4.54 dd 11.8 2.6 | 4.35 dd 12.2 2.7 | |
| 1″ | 5.16 d 7.2 | 5.22 d 7.5 | 5.44 d 7.2 | 5.22 d 7.2 | 5.42 d 7.6 | 5.44 d 7.8 | 5.19 d 7.5 |
| 2″ | 4.44 m | 4.53 dd 7.8 7.5 | 4.38 m | 4.54 m | 4.37 m | 4.38 m | 4.38 dd 8.0 7.5 |
| 3″ | 4.15 m | 4.18 dd 7.8 3.6 | 4.13 br d 8.6 | 4.21 dd 8.4 3.0 | 4.13 m | 4.14 m | 4.13 m |
| 4″ | 4.17 m | 4.30 m | 4.24 m | 4.33 m | 4.30 m | 4.24 m | 4.16 m |
| 5″ | 3.77 d 11.6 | 3.79 dd 11.6 1.8 | 3.64 d 12.0 | 3.78 d 11.7 | 3.66 dd 12.5 6.4 | 3.64 d 11.2 | 3.70 m |
| 4.30 dd 11.6 3.8 | 4.36 br d 11.6 | 4.25 br d 12.0 | 4.35 br d 11.7 | 4.24 m | 4.25 m | 4.29 m | |
| 6″ | |||||||
| 1‴ | 5.31 d 7.8 | 5.33 d 7.2 | 5.34 d 7.8 | ||||
| 2‴ | 4.04 m | 4.03 br d 8.0 | 4.60 dd 7.8 7.8 | ||||
| 3‴ | 4.19 m | 3.99 m | 4.01 dd 8.6 8.3 | ||||
| 4‴ | 4.14 m | 4.10 dd 8.6 8.6 | 4.15 br d 8.9 | ||||
| 5‴ | 3.91 ddd | 3.93 m | 4.00 ddd | ||||
| 9.2 4.4 2.0 | 8.9 6.0 2.3 | ||||||
| 6‴ | 4.25 m | 4.25 dd 11.4 3.5 | 4.26 dd 12.1 2.3 | ||||
| 4.38 br d 12.3 | 4.37 br d 11.4 | 4.39 dd 12.1 6.0 | |||||
| –OCH3 | |||||||
| CH3CO– | |||||||
| H | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 1 | 1.06 m | 0.80 m | 0.80 m | 0.87 m | 0.85 m | 0.92 m | 0.88 dd 14.0 5.8 |
| 1.42 dd 12.2 4.8 | 1.44 m | 1.38 br d 13.0 | 1.45 m | 1.42 br d 13.5 | 1.44 dd 13.3 2.9 | 1.46 br d 12.7 | |
| 2 | 1.91 dd 13.7 4.3 | 1.82 m | 1.83 br d 13.5 | 1.80 m | 1.84 m | 1.90 m | 1.88 br d 12.7 |
| 2.23 m | 2.07 dd 14.6 4.0 | 2.10 dd 13.5 4.0 | 1.98 dd 14.1 3.9 | 2.12 m | 2.26 m | 2.13 dd 13.5 3.4 | |
| 3 | 3.34 dd 12.0 4.8 | 3.23 dd 11.2 4.0 | 3.19 dd 11.4 4.0 | 3.19 dd 11.7 3.9 | 3.21 dd 12.0 4.8 | 3.42 dd 13.8 4.2 | 3.30 dd 12.0 3.4 |
| 5 | 0.76 d 11.5 | 0.70 d 11.5 | 0.70 d 12.0 | 0.70 d 11.2 | 0.73 d 11.6 | 0.83 d 11.7 | 0.76 d 12.0 |
| 6 | 1.45 dd 12.4 5.2 | 1.43 m | 1.27 m | 1.11 m | 1.27 m | 1.30 m | 1.26 br d 12.5 |
| 1.50 m | 1.52 m | 1.47 m | 1.46 m | 1.45 br d 14.0 | 1.49 br d 13.2 | 1.46 br d 12.7 | |
| 7 | 0.78 m | 0.85 m | 1.24 m | 1.67 m | 1.30 m | 1.36 td 13.2 2.8 | 1.35 m |
| 1.30 m | 1.33 m | 1.46 dd 12.6 7.2 | 1.76 m | 1.58 br d 10.3 | 1.63 dd 12.7 3.8 | 1.60 dd 12.8 11.9 | |
| 9 | 1.75 dd 10.0 7.2 | 1.67 m | 1.58 dd 10.9 7.2 | 1.58 m | 1.71 dd 10.6 6.4 | 1.75 dd 10.7 6.5 | 1.70 br d 9.1 |
| 11 | 1.83 m | 1.80 m | 1.80 2H m | 1.83 2H m | 1.86 2H m | 1.84 2H m | 1.84 2H m |
| 1.91 m | 1.84 m | ||||||
| 12 | 5.38 br s | 5.34 br s | 5.21 br s | 5.23 br s | 5.37 br s | 5.42 br s | 5.42 br s |
| 15 | 1.61 dd 14.6 2.2 | 1.61 m | 1.01 m | 1.01 m | 1.63 br d 14.3 | 1.70 dd 14.4 3.0 | 1.69 m |
| 2.20 m | 2.14 dd 14.0 4.8 | 1.94 dd 13.9 4.0 | 2.80 m | 2.20 dd 14.0 4.3 | 2.22 dd 15.2 4.3 | 2.22 dd 14.9 3.6 | |
| 16 | 4.62 dd 7.9 3.5 | 4.57 m | 1.51 br d 12.5 | 1.51 m | 4.62 m | 4.65 m | 4.65 br s |
| 2.01 br d 13.9 | 3.02 m | ||||||
| 18 | 2.49 td 12.8 3.6 | 2.43 td 12.6 3.5 | 2.28 dd 13.2 3.8 | 2.29 dd 14.0 4.9 | 2.48 br s | 2.59 dd 14.4 3.6 | 2.57 br d 13.5 |
| 19 | 1.29 m | 1.24 m | 1.18 m | 1.88 br d 14.0 | 1.30 m | ||
| 2.72 t 12.6 | 2.70 t 13.6 | 2.70 t 13.8 | 2.73 t 13.5 | 2.73 t 13.5 | |||
| 20 | — | — | — | — | — | — | — |
| 21 | 1.42 m | 1.36 m | 1.25 m | 1.44 dd 14.2 3.2 | 1.43 br d 14.0 | 1.91 dd 11.0 4.0 | 1.89 br d 12.8 |
| 2.33 br d 14.1 | 1.44 dd 13.4 4.4 | 2.40 m | 2.41 dd 14.3 4.2 | 2.27 m | 2.27 dd 11.3 3.8 | ||
| 22 | 1.84 dd 12.6 3.6 | 1.75 m | 1.69 dd 14.0 3.1 | 1.70 dd 14.3 3.2 | 2.20 dd 14.0 4.2 | 2.23 br d 14.0 | 2.29 dd 10.0 3.3 |
| 2.27 m | 2.21 br d 13.8 | 1.95 br d 14.0 | 1.95 br d 14.3 | 2.27 dd 14.3 2.9 | 2.37 m | 2.37 m | |
| 23 | 1.29 3H s | 1.24 3H s | 1.21 3H s | 1.20 3H s | 1.20 3H s | 1.31 3H s | 1.30 3H s |
| 24 | 1.13 3H s | 1.05 3H s | 1.13 3H s | 1.11 3H s | 1.132 3H s | 1.01 3H s | 1.11 3H s |
| 25 | 0.88 3H s | 0.85 3H s | 0.80 3H s | 0.84 3H s | 0.83 3H s | 0.85 3H s | 0.86 3H s |
| 26 | 0.95 3H s | 0.92 3H s | 0.90 3H s | 0.92 3H s | 0.93 3H s | 0.94 3H s | 0.95 3H s |
| 27 | 1.83 3H s | 1.77 3H s | 1.29 3H s | 1.28 3H s | 1.83 3H s | 1.87 3H s | 1.84 3H s |
| 28 | 3.61 d 10.4 | 3.55 d 10.6 | 3.60 d 10.8 | 3.55 d 10.4 | 3.60 d 10.8 | 3.68 d 10.8 | 3.68 d 10.6 |
| 3.72 d 10.4 | 3.68 d 10.6 | 3.73 d 10.8 | 3.84 d 10.4 | 3.73 d 10.8 | 3.73 d 10.8 | 3.73 d 10.6 | |
| 29 | 1.13 3H s | 1.01 3H s | 0.95 3H s | 0.95 3H s | 1.05 3H s | 1.32 3H s | 1.32 3H s |
| 30 | 1.06 3H s | 1.08 3H s | 0.99 3H m | 0.98 3H s | 1.13 3H s | 3.98 d 10.2 | 3.97 d 10.5 |
| 4.03 d 10.2 | 4.03 d 10.5 | ||||||
| 1′ | 5.03 d 7.6 | 4.91 d 7.6 | 4.91 d 7.8 | 4.91 d 7.3 | 4.92 d 7.2 | 5.06 d 8.4 | 4.99 d 7.9 |
| 2′ | 4.29 dd 9.0 7.6 | 4.17 dd 9.0 7.6 | 4.36 br d 8.0 | 4.32 dd 8.8 7.3 | 4.36 m | 4.15 dd 8.9 8.4 | 4.25 dd 8.3 7.9 |
| 3′ | 4.37 dd 9.3 9.0 | 4.24 dd 9.0 8.8 | 5.88 m | 5.82 t 9.2 | 5.88 m | 4.35 dd 9.7 8.9 | 4.31 dd 9.6 8.3 |
| 4′ | 4.16 dd 9.3 9.0 | 4.08 dd 9.5 8.8 | 4.61 m | 4.46 br d 9.3 | 4.60 m | 4.63 br d 9.7 | 4.18 br d 9.6 |
| 5′ | 4.29 d 9.0 | 4.42 d 9.5 | 4.60 m | 4.54 d 9.7 | 4.60 m | 4.73 d 9.7 | 4.52 d 9.6 |
| 6′ | — | — | — | — | — | — | — |
| 1″ | 5.23 d 7.6 | 5.13 d 7.6 | 5.00 d 7.8 | 4.98 d 7.5 | 4.99 d 7.2 | 5.22 d 7.8 | |
| 2″ | 4.56 m | 4.44 br d 8.8 | 4.37 m | 4.35 br d 8.1 | 4.35 m | 4.43 m | |
| 3″ | 4.59 m | 4.48 m | 4.09 dd 9.6 3.0 | 4.07 br d 9.0 | 4.09 dd 9.6 3.0 | 4.58 br d 8.9 | |
| 4″ | 4.69 br d 3.3 | 4.61 br d 2.4 | 4.62 m | 4.59 m | 4.61 br d 3.0 | 4.70 br d 3.0 | |
| 5″ | 4.06 ddd | 3.97 m | 4.05 m | 4.03 m | 4.04 t 6.0 | 4.06 m | |
| 5.0 3.3 2.8 | |||||||
| 6″ | 4.41 dd 11.8 5.0 | 4.31 m | 4.48 dd 10.5 5.4 | 4.44 br d 10.0 | 4.48 br d 10.8 | 4.40 dd 10.7 3.7 | |
| 4.58 dd 11.8 2.8 | 4.48 m | 4.53 br d 10.5 | 4.50 br d 10.0 | 4.53 br d 10.8 | 4.60 br d 10.7 | ||
| 1‴ | 5.89 d 1.8 | 5.50 d 1.5 | 5.89 d 2.4 | ||||
| 2‴ | 4.82 dd 4.2 1.8 | 4.70 m | 4.83 m | ||||
| 3‴ | 4.71 m | 4.71 m | 4.71 m | ||||
| 4‴ | 4.68 m | 4.60 m | 4.68 m | ||||
| 5‴ | 4.18 dd 12.0 4.2 | 4.16 dd 11.0 4.2 | 4.18 dd 12.0 4.2 | ||||
| 4.31 dd 12.0 3.6 | 4.28 m | 4.31 m | |||||
| 6‴ | |||||||
| –OCH3 | 3.69 3H s | 3.80 3H s | 3.72 3H s | ||||
| CH3CO– | 2.41 3H s | 2.40 3H s | 2.41 3H s |
m: multiplet or overlapped signal.
| C | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48.0 | 48.1 | 48.1 | 42.9 | 42.8 | 48.0 | 47.7 | 38.9 | 38.8 | 38.7 | 38.8 | 38.8 | 38.9 | 38.9 |
| 2 | 68.9 | 68.7 | 68.7 | 66.3 | 66.3 | 68.8 | 68.9 | 26.7 | 26.6 | 26.5 | 26.1 | 26.5 | 26.7 | 26.7 |
| 3 | 78.5 | 83.9 | 83.9 | 79.0 | 78.6 | 85.9 | 78.5 | 89.2 | 89.2 | 90.0 | 90.1 | 89.9 | 89.0 | 89.2 |
| 4 | 43.6 | 38.5 | 38.6 | 41.9 | 41.9 | 43.9 | 43.5 | 39.6 | 39.5 | 39.6 | 39.6 | 39.8 | 39.6 | 39.6 |
| 5 | 48.2 | 56.1 | 56.1 | 43.6 | 43.8 | 56.7 | 48.2 | 55.9 | 55.8 | 55.7 | 55.7 | 55.8 | 55.8 | 55.9 |
| 6 | 18.6 | 19.1 | 19.1 | 18.5 | 18.6 | 19.3 | 18.6 | 18.5 | 18.4 | 18.5 | 18.5 | 18.5 | 18.5 | 18.5 |
| 7 | 33.1 | 33.5 | 33.7 | 33.2 | 33.4 | 34.1 | 32.8 | 33.3 | 33.2 | 32.9 | 32.9 | 33.2 | 33.3 | 33.3 |
| 8 | 40.4 | 40.7 | 40.7 | 40.8 | 40.8 | 40.6 | 40.1 | 40.1 | 40.0 | 40.1 | 40.1 | 40.0 | 40.1 | 40.1 |
| 9 | 48.1 | 47.9 | 48.0 | 47.9 | 47.9 | 48.0 | 48.2 | 47.1 | 47.1 | 47.8 | 47.9 | 47.1 | 47.2 | 47.1 |
| 10 | 38.4 | 39.8 | 39.8 | 38.5 | 38.5 | 38.3 | 38.4 | 36.9 | 36.8 | 36.8 | 36.8 | 36.8 | 36.9 | 36.9 |
| 11 | 23.9 | 24.3 | 24.3 | 24.3 | 24.2 | 24.2 | 24.0 | 23.9 | 23.8 | 23.9 | 23.9 | 23.8 | 23.8 | 23.8 |
| 12 | 125.9 | 128.2 | 128.2 | 128.3 | 128.2 | 123.2 | 122.6 | 122.4 | 122.2 | 122.4 | 122.4 | 122.3 | 122.5 | 122.5 |
| 13 | 138.7 | 139.5 | 139.7 | 139.6 | 139.7 | 139.7 | 144.4 | 145.3 | 145.2 | 145.1 | 145.1 | 145.3 | 145.0 | 145.0 |
| 14 | 42.6 | 42.2 | 42.3 | 42.3 | 42.3 | 42.2 | 42.2 | 42.0 | 41.9 | 42.0 | 42.0 | 42.0 | 42.1 | 42.1 |
| 15 | 28.7 | 29.2 | 29.2 | 29.2 | 29.2 | 29.2 | 28.3 | 34.9 | 34.7 | 26.1 | 26.4 | 34.8 | 34.8 | 34.8 |
| 16 | 24.6 | 26.1 | 26.0 | 26.2 | 25.9 | 25.9 | 23.3 | 74.3 | 74.2 | 22.9 | 22.8 | 74.2 | 73.9 | 73.8 |
| 17 | 48.4 | 48.7 | 48.8 | 48.7 | 48.8 | 48.8 | 47.0 | 41.0 | 40.9 | 37.6 | 37.6 | 41.0 | 41.3 | 41.3 |
| 18 | 53.5 | 54.6 | 54.6 | 54.6 | 54.6 | 54.6 | 41.9 | 42.6 | 42.5 | 42.7 | 42.7 | 42.5 | 42.3 | 42.3 |
| 19 | 39.4 | 72.7 | 72.8 | 72.7 | 72.8 | 72.7 | 46.3 | 48.4 | 48.3 | 47.1 | 47.1 | 48.4 | 43.2 | 43.2 |
| 20 | 39.2 | 42.2 | 42.1 | 42.1 | 42.1 | 42.1 | 30.8 | 31.3 | 31.2 | 31.2 | 31.2 | 31.3 | 36.3 | 36.3 |
| 21 | 31.0 | 26.8 | 26.8 | 26.8 | 26.8 | 26.8 | 34.1 | 37.2 | 37.1 | 34.7 | 34.7 | 37.2 | 31.9 | 31.9 |
| 22 | 36.4 | 37.4 | 37.3 | 37.4 | 37.3 | 37.3 | 32.2 | 30.5 | 30.4 | 31.8 | 31.8 | 30.6 | 28.4 | 28.4 |
| 23 | 66.8 | 29.4 | 29.4 | 71.3 | 71.2 | 24.4 | 66.9 | 28.2 | 28.1 | 28.0 | 28.0 | 28.0 | 28.2 | 28.1 |
| 24 | 14.3 | 17.7 | 17.6 | 17.8 | 17.8 | 65.7 | 14.2 | 16.8 | 16.7 | 16.7 | 16.7 | 16.8 | 17.0 | 16.8 |
| 25 | 17.6 | 17.1 | 17.1 | 17.2 | 17.2 | 17.5 | 17.5 | 15.8 | 15.7 | 15.6 | 15.6 | 15.7 | 15.8 | 15.8 |
| 26 | 17.6 | 17.4 | 17.5 | 17.4 | 17.6 | 17.3 | 17.6 | 17.1 | 17.0 | 16.9 | 17.0 | 17.0 | 17.2 | 17.2 |
| 27 | 23.8 | 24.5 | 24.5 | 24.5 | 24.4 | 24.5 | 26.1 | 27.4 | 27.3 | 26.2 | 26.2 | 27.4 | 27.5 | 27.4 |
| 28 | 176.3 | 176.9 | 177.0 | 176.9 | 177.7 | 177.1 | 176.5 | 70.2 | 70.1 | 68.7 | 68.7 | 70.2 | 69.6 | 69.6 |
| 29 | 17.4 | 27.1 | 27.2 | 27.1 | 27.1 | 27.1 | 33.2 | 33.5 | 33.4 | 33.5 | 33.5 | 33.5 | 28.2 | 28.2 |
| 30 | 21.3 | 16.8 | 16.7 | 16.7 | 16.7 | 16.8 | 23.8 | 24.9 | 24.8 | 23.8 | 23.9 | 24.8 | 67.3 | 67.3 |
| 1′ | 93.9 | 94.1 | 93.6 | 94.2 | 93.6 | 93.6 | 93.8 | 105.3 | 105.1 | 104.9 | 104.7 | 104.8 | 107.3 | 105.3 |
| 2′ | 81.4 | 81.4 | 77.5 | 81.5 | 77.6 | 77.5 | 81.2 | 83.9 | 83.5 | 78.4 | 78.05 | 78.3 | 75.6 | 83.7 |
| 3′ | 78.3 | 78.5 | 89.0 | 78.4 | 88.8 | 88.9 | 78.5 | 77.3 | 76.8 | 75.3 | 75.1 | 75.2 | 78.2 | 77.5 |
| 4′ | 71.0 | 71.0 | 69.9 | 71.1 | 69.9 | 69.9 | 70.8 | 75.0 | 74.5 | 77.7 | 77.9 | 77.7 | 73.5 | 75.0 |
| 5′ | 78.9 | 79.0 | 78.6 | 78.9 | 78.7 | 78.8 | 78.8 | 77.7 | 77.4 | 76.0 | 74.9 | 76.0 | 77.9 | 76.7 |
| 6′ | 62.4 | 62.3 | 62.4 | 62.4 | 62.4 | 62.4 | 62.2 | 172.5 | 170.4 | 171.8 | 169.8 | 171.9 | 172.8 | 170.5 |
| 1″ | 106.5 | 106.5 | 104.9 | 106.6 | 104.9 | 105.0 | 106.3 | 107.2 | 106.9 | 105.5 | 105.4 | 105.5 | 107.1 | |
| 2″ | 73.4 | 73.5 | 73.2 | 73.6 | 73.2 | 73.1 | 73.4 | 73.1 | 72.7 | 72.7 | 72.6 | 72.6 | 72.8 | |
| 3″ | 74.5 | 74.5 | 45.0 | 74.5 | 75.1 | 75.1 | 74.5 | 74.7 | 74.8 | 75.4 | 75.4 | 75.4 | 74.7 | |
| 4″ | 69.4 | 69.4 | 69.7 | 69.4 | 69.6 | 69.6 | 69.4 | 69.6 | 69.5 | 69.7 | 69.7 | 69.7 | 69.6 | |
| 5″ | 67.4 | 67.4 | 67.5 | 67.4 | 67.5 | 67.5 | 67.3 | 77.0 | 76.6 | 76.9 | 77.0 | 76.9 | 77.0 | |
| 6″ | 61.4 | 61.3 | 61.9 | 62.0 | 61.9 | 61.3 | ||||||||
| 1‴ | 104.8 | 104.8 | 104.8 | 110.6 | 110.6 | 110.5 | ||||||||
| 2‴ | 75.5 | 75.5 | 75.5 | 83.4 | 83.4 | 83.4 | ||||||||
| 3‴ | 78.7 | 78.7 | 78.8 | 78.4 | 78.14 | 78.3 | ||||||||
| 4‴ | 71.7 | 71.7 | 71.6 | 86.2 | 85.9 | 86.1 | ||||||||
| 5‴ | 78.8 | 78.7 | 78.7 | 62.7 | 62.6 | 62.7 | ||||||||
| 6‴ | 62.1 | 62.2 | 62.0 | |||||||||||
| –OCH3 | 52.0 | 52.5 | 52.1 | |||||||||||
| CH3CO– | 22.0 | 21.9 | 22.0 | |||||||||||
| CH3CO– | 170.9 | 170.8 | 170.9 |

Symplocosin D (2), [α]D25 −7.92, was isolated as an amorphous powder, and its elemental composition was determined to be C41H66O14 by HR-ESI-MS. The IR spectrum showed similar absorptions to those of 1, and 13C-NMR resonances were also similar to those of 1, except for the disappearance of oxygenated methylene and one methine signals. Instead, one more singlet methyl and oxygenated tertiary carbon (δC 72.7) singles were observed. The HMBC correlation cross peaks between singlet methyl protons (δH 1.44) and C-18, C-19 (δC 72.7), and C-20 suggested the oxygenated tertiary carbon was placed on the E-ring. The above evidence suggested the aglycone was tormentic acid, whose 28-O-glucosyl ester was isolated from Rubus pinfaensis.9) The 13C-NMR resonances of the aglycone of 2 were essentially the same as those of tormentic acid 28-O-glucosyl ester, and the sugar moiety was the same as that of 1. Therefore, the structure of 2 was elucidated to be tormentic acid 28-O-β-D-(2′-O-α-L-arabinopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
Symplocosin E (3), [α]D25 +4.35, was isolated as an amorphous powder, and its elemental composition was determined to be C47H76O19. Spectroscopic data indicated that 3 was analogous to 2, with three anomeric protons and carbons (δH 5.31 on δC 104.8, δH 6.15 on δC 93.6, and δH 5.44 on δC 104.9). Sugar analysis revealed the presence of L-arabinose and D-glucose, and 13C-NMR signals for terminal arabinopyranoside were clearly observed. Thus, the remaining two hexoses were expected be the inner and terminal glucopyranose units. The HMBC correlations between H-1′ (δH 6.15) and C-28 (δC 177.0), H-1″ (δH 5.44) and δC 77.5 (C-2′) with δH 4.41, and H-1‴ (δH 5.31) and C-3′ (δC 89.0), together with the COSY correlation from H-1′ (δH 6.15) to H-2′ (δH 4.41) on C-3′, established the sugar linkage to be 2′-O-α-L-arabinopyranosyl and 3′-O-β-D-glucopyranosyl. Therefore, the structure of 3 was elucidated to be tormentic acid 28-O-β-D-(2′-O-α-L-arabinopyranosyl, 3′-O-β-D-glucopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
Symplocosin F (4), [α]D25 −2.14, was isolated as an amorphous powder, and its elemental composition was C41H66O15. The 13C-NMR spectroscopic data for the sugar moiety were essentially the same as those of 1 and 2. Five singlet methyl and one doublet methyl signals, along with one primary alcohol signal (δH 3.68 and 3.87), as shown in 1, were observed in the 1H-NMR spectrum. From COSY and HMBC spectra, a vicinal alcohol system was expected to be retained on the A-ring. However, the geometry of two hydroxy groups was apparently different from the aforementioned compounds (Tables 1, 2). The 13C-NMR spectroscopic data of the aglycone were essentially the same as those of niga-ichigoside F2 (16), which co-occurred in this plant and possessed a 2α,3α-cis-diol system.7) Therefore, the aglycone of 4 was myrianthic acid,10) and the sugar moiety was 28-O-β-D-(2′-O-α-L-arabinopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
Symplocosin G (5), [α]D25 −10.8, was isolated as an amorphous powder, and its elemental composition was C47H76O20. Symplocosin G (5) was an analogous compound to 4 with three anomeric protons. The 13C-NMR spectroscopic data of the sugar region were the same as those of 3. Therefore, the structure of 5 was elucidated to be myrianthic acid 28-O-β-D-(2′-O-α-L-arabinopyranosyl, 3′-O-β-D-glucopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
Symplocosin H (6), [α]D26 +1.36, was isolated as an amorphous powder, and its elemental composition was C47H76O20. The 13C-NMR spectroscopic data indicated that functionalities of the aglycone moiety were the same as those of 4 and 5, and those of the sugar region were the same as 3 and 5. The vicinal diol system must have been trans from the coupling constant of H-3 (d, J = 9.5 Hz). Since singlet methyl protons (δH 1.45) at the 4-position showed significant correlation peak with H-3 in the rotating Overhauser enhancement and exchange spectroscopy spectrum (ROESY), this methyl was assigned to the 23-position. Thus, the aglycone was expected to be 24-hydroxytormentic acid,11) and its 28-O-glucosy ester co-occurred in this plant as 4-epi-niga-ichigoside F1 (18).12) Therefore, the structure of symplocosin H (6) was elucidated to be 24-hydroxytormentic acid 28-O-β-D-(2′-O-α-L-arabinopyranosyl, 3′-O-β-D-glucopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
Symplocosin I (7), [α]D26 +21.4, was isolated as an amorphous powder, and its elemental composition was determined to be C41H66O14 by HR-ESI-MS. In the 1H-NMR spectrum, six singlet methyl, one olefinic proton, and two anomeric proton signals were observed. The 13C-NMR spectrum indicated that signals assignable to A and B rings were virtually coincident with those of 1, and the presence of six singlet methyls indicated the aglycone was an oleanene-type triterpene, namely arjunoric acid,13,14) whose 28-O-glycosyl esters were isolated as asteryunnanosides A and B from Aster yunnanensis.15) With spectroscopic similarity to 1 in the sugar region, the structure of 7 was elucidated to be arjunoric acid 28-O-β-D-(2′-O-α-L-arabinopyranosyl)glucopyranosyl ester, as shown in Fig. 1.
Symplocosin J (8), [α]D23 −11.0, was isolated as an amorphous powder, and its elemental composition was determined to be C42H68O14 by HR-ESI-MS. The IR spectrum exhibited absorptions assignable to hydroxy, ester, and olefinic functional groups (3388, 1734, and 1650 cm−1, respectively). In the NMR spectrum, two anomeric protons (δH 5.03 on δC 105.3 and δH 5.23 on δC 107.2) were observed, and the sugar analysis of its hydrolysate revealed the presence of D-glucuronolactone and D-galactose. The 13C-NMR spectrum was comprised of seven methyl, nine methylene, three methine, six quaternary, and two olefinic carbon signals, together with two oxymethine and one primary alcohol groups. All the methyl signals appeared as singlets in the 1H-NMR spectrum. HMBC correlations from the geminal methyl groups (δH 1.13 and 1.29) to C-3 (δC 89.2) placed one of the oxymethines at the 3-postion. Those of H-18 (δH 2.49) to C-17, geminal protons of the primary alcohol (δH 3.61 and 3.72) to C-16 (δC 74.3), C-17 and C-22 and H-16 (δH 4.62) to C-17 allowed for placement of the other oxymethines at the C-16 position (Fig. 3). From above evidence, the aglycone was expected to be primulagenin A,16) and its 3-O-glycosidic derivatives were also reported.16) On the other hand, 13C-NMR spectroscopic data of the sugar region exhibited six signals typical for terminal β-D-galactopyranoside,17) and then, its anomeric proton (δH 5.23) and that (δH 5.03) of D-glucuronic acid showed cross peaks with C-2′ (δC 83.9) and C-3 (δC 89.2) carbons in the HMBC spectrum, respectively. The linkage mode was determined to be β from coupling constants (Table 1). Therefore, the structure of 8 was elucidated to be primulagenin A 3-O-β-D-(2′-O-β-D-galactopyranosyl)glucuronide, as shown in Fig. 1.

Symplocosin K (9), [α]D26 +1.79, was isolated as an amorphous powder with a positive optical rotation, and its elemental composition was C43H70O14. NMR spectroscopic data showed close similarity to 8. A methoxy signal (δH 3.69), observed in the 1H-NMR spectrum of 9, showed a cross peak with the carbonyl signal (δC 170.4) in the HMBC spectrum, which resonated at a slightly higher frequency than that of 8. Therefore, the structure of 9 was concluded to be a methyl ester of 8.
Symplocosin L (10), [α]D24 −3.89, was isolated as an amorphous powder, and its elemental composition was determined to be C49H78O18 by HR-ESI-MS. In the NMR spectrum, three anomeric protons (δH 4.91 on δC 104.9, δH 5.00 on δC 105.5, and δH 4.48 δC 110.6) were observed, and sugar analysis of its hydrolyzate revealed the presence of D-glucuronolactone, D-galactose, and L-arabinose. The 13C-NMR spectrum exhibited 30 signals for the aglycone, two signals for the acetyl group, six signals for the inner glucuronic acid, and six signals for the terminal galactopyranose, but the remaining five signals could not be assigned for the terminal arabinopyranoside found in symplocosins C–H (1–6). A highly deshielded anomeric carbon signal at δC 110.6 suggested that arabinose existed in a furanose form.18,19) The aglycone was expected to be similar to that of 8 and 9, except for the absence of a hydroxy group at the C-16 position, namely erythrodiol.20) In the HMBC spectrum, the anomeric proton at δH 4.91 showed a cross peak with C-3 and H-1″ (δH 5.00), correlated with δC 78.4 (C-2′), whose proton was found to be adjacent to the anomeric proton of C-1′ in the COSY spectrum. A highly deshielded signal at δH 5.88 on C-3′ indicated that acetylation occurred at this position, which was supported by a cross peak between H-3′ and the carbonyl carbon of the acetyl group in the HMBC spectrum. Finally, only the remaining arabinofuranosylation site was at the hydroxy group of C-4′, and the anomeric proton correlated with C-4′ (δC 77.7) in the HMBC spectrum. Therefore, the structure of 10 was elucidated to be erythrodiol 3-O-β-D-(2′-O-β-D-galactopyranosyl, 3′-O-acetyl, 4′-O-α-L-arabinofuranosyl)glucopyranoside, as shown in Fig. 1.
Symplocosin M (11) was isolated as an amorphous power with a negative optical rotation, and its elemental composition was C50H80O18. NMR spectroscopic data showed significant similarity to 10. A methoxy signal (δH 3.80) observed in the 1H-NMR spectrum of 11 and showed a cross peak with the carbonyl signal (δC 169.8) in the HMBC spectrum, which resonated at slightly higher frequency than that of 10. Therefore, the structure of 11 was concluded to be a methyl ester of 10.
Symplocosin N (12), [α]D25 −24.3, was isolated as an amorphous powder, and its elemental composition was determined to be C49H78O19 by HR-ESI-MS. The NMR spectra indicated that the aglycone was primulagenin A, as found in 8 and 9, and the signals assignable to sugar region were superimposable with those of 10, including the acetyl group. Therefore, the structure of 12 was elucidated, as shown in Fig. 1.
Symplocosin O (13), [α]D26 −12.9, was isolated as an amorphous powder, and its elemental composition was C36H58O10. In the 1H-NMR spectrum, only one anomeric proton was observed, and the sugar component was analyzed to be D-glucuronic acid. The NMR spectra were similar to those of 8 and 9; however, one of the singlet methyl groups must be oxidized to a primary alcohol [δH 3.98 (d, J = 10.2 Hz) and 4.03 (d, J = 10.2 Hz)]. In the HMBC spectrum, since two methyl proton signals were correlated with C-3, the geminal dimethyl groups were retained at the C-4 position (Fig. 4), and one of the methyl groups showed significant correlation cross peaks with the primary alcohol carbon and vice versa. Other HMBC correlations from H-18 to C-17, C-19, and C-28 and H2-30 to C-19 substantiated that one of the methyl groups on the C-20 position was oxidized to the primary alcohol. From ROESY correlation between H-18 and the carbinol protons (H2-30), the carbinol carbon must be on the same face as H-18 and, thus, was assigned to be C-30. The position of the sugar unit was also clarified by HMBC correlation of the anomeric proton with C-3. This aglycone was known as an artifactual sapogenin, predentigenin E,21) formed from opening of the original 13β,28-epoxy derivatives by drastic acid hydrolysis. However, its glycoside was first isolated from the natural source in this experiment, and predentigenin E in symplocosin O must not be an artifact, since symplocosin O was obtained under usual isolation conditions.

Symplocosin P (14) was isolated as an amorphous powder with a negative optical rotation, and its elemental composition was C43H70O15. The NMR spectroscopic data of the aglycone region were superimposable to those of 13 and the sugar range in 9. The structure of 14 is shown in Fig. 1.
From the leaves of S. cochinchinensis var. philippinensis, 14 new ursane- and oleanane-type triterpene saponins, named symplocosins C–P (1–14), were isolated. Some of oleanane-type triterpene saponins (9, 11, and 14) possess D-glucuronic acid as their methyl ester forms. These compounds are probably artifacts formed during extraction and isolation procedures. The 3′-position of D-glucuronic acid in symplocosins L–M (10–12) was acetylated. Since only the specific position was acetylated, these compounds (10 and 12) were considered to be genuine natural compounds. Saikosaponins and primulasaponins, which possess 11-en-13,28-epoxy rings, are known to slightly degrade to 11-hydroxy (methoxy)-12-en-28-hydroxy derivatives during extraction and further transform to 11,13-diene compounds in acidic conditions.22) In our experiment, corresponding probable mother compounds, 11-en-13,28-epoxy-type aglycones, were not found, and the C-11 position did not carry a hydroxy functional group. A related artifactual aglycone, predentigenin E (aglycone of 13), was obtained from the 13,18-epoxy derivative, ardisicrenoside A, by heating at 100 °C for 2 h in a mixture of MeOH–2 M HCl (1 : 1) in a sealed tube.21) During conventional extraction and isolation procedures, such a transformation may not occur, and probably, 12-en-28-hydroxy forms may have a role as biosynthetic precursors of 11-en-13,28-epoxy-type triterpenes.
Biological assessments, such as cytotoxicity toward the human aveolar adenocarcinoma cell line, A549 cells; anti-Leishmania activity; and α-glucosidase inhibitory activity were performed. Only symplocosin K (9) showed moderate cytotoxicity toward A549 cells at IC50 73.8 ± 2.31 µM, where that of doxorubicin was 0.4 ± 0.1 µM. Other compounds, including known ones, did not show significant activities.
Optical rotations were measured on a JASCO P-1030 digital polarimeter. IR spectra were measured on Horiba FT-710 spectrophotometers. 1H- and 13C-NMR spectra were taken on a Bruker Avance III 600 spectrometer at 600 and 150 MHz, respectively, with tetramethylsilane as an internal standard. Positive-ion HR-ESI-MS was performed with an Applied Biosystems QSTAR® XL NanoSpray™ System.
A highly-porous synthetic resin (Diaion HP-20) was purchased from Mitsubishi Kagaku (Tokyo, Japan). Silica gel column chromatography (CC) was performed on silica gel 60 (E. Merck, Darmstadt, Germany) and octadecylsilanized (ODS) open CC on Cosmosil 75C18-OPN (Nacalai Tesque, Kyoto, Japan; Φ = 50 mm, L = 25 cm, linear gradient: MeOH–H2O (1 : 9, 1 L)→(1 : 1, 1 L), 10 g fractions being collected). The droplet counter-current chromatograph (DCCC) (Tokyo Rikakikai, Tokyo, Japan) was equipped with 500 glass columns (Φ = 2 mm, L = 40 cm), with the lower and upper layers of the solvent mixture (CHCl3–MeOH–H2O–n-PrOH; 9 : 12 : 8 : 2) being used as the stationary and mobile phases, respectively. Five gram fractions were collected and numbered according to their order of elution with the mobile phase. HPLC was performed on an ODS column (Inertsil, GL Science, Tokyo, Japan or Develosil, Nomura Chemical, Aichi Japan; Φ = 6 mm, L = 250 mm, 1.0 mL/min), and the eluate was monitored with a UV detector at 254 nm and a refractive index monitor.
Plant MaterialLeaves of S. cochinchinensis var. philippinensis were collected in Taketomi-cho, Yaeyama-gun, Okinawa, Japan in November 2003, and a voucher specimen was deposited in the Herbarium of Pharmaceutical Sciences, Graduate School of Biomedical and Health Sciences, Hiroshima University (03-SC-Okinawa-1105). The plant was identified by Prof. T. Shinzato of Faculty of Agriculture, the University of the Ryukyus, whom the authors acknowledge.
Extraction and IsolationLeaves of S. cochinchinensis var. phillipinensis (10.8 kg) were extracted three times with MeOH (45 L × 3) at room temperature for one week and, then, concentrated to 6 L in vacuo. The concentrated extract was washed with n-hexane (6 L, 150 g), and then, the MeOH layer was concentrated to a gummy mass. The latter was suspended in H2O (6 L) and extracted with EtOAc (6 L) to give 107 g of an EtOAc-soluble fraction. The aqueous layer was extracted with 1-BuOH (6 L) to give a 1-BuOH-soluble fraction (247 g), and the remaining H2O-layer was concentrated to furnish 494 g of a H2O-soluble fraction. The 1-BuOH-soluble fraction (119 g) was subjected to a Diaion HP-20 CC (Φ = 50 mm, L = 50 cm), using H2O–MeOH (4 : 1, 4 L), (3 : 2, 4 L), (2 : 3, 4 L), and (1 : 4, 4 L), and MeOH (3 L), with 500 mL fractions being collected. The residue (28.4 g) in fractions 26–33 of the 80% MeOH eluent was subjected to silica gel (600 g) CC and elution with CHCl3 (3 L), CHCl3–MeOH [(49 : 1, 3 L), (24 : 1, 3 L), (47 : 3, 3 L), (23 : 2, 3 L), (9 : 1, 3 L), (7 : 1, 3 L), (4 : 1, 3 L), (3 : 1, 3 L), and (7 : 3, 3 L)], and CHCl3–MeOH–H2O (35 : 15 : 2, 3 L), with 500 mL fractions being collected. The residue (2.56 g) in fractions 26–46 of the 8–20% MeOH eluate was separated by ODS open CC, giving four residues. The first residue (897 mg) in fractions 152–169 was purified by DCCC, and the residue (223 mg) in fractions 59–78 was separated again by silica gel (10 g) CC by elution with CHCl3–MeOH [(15 : 1, 150 mL), (9 : 1, 100 mL), (7 : 1, 100 mL), and (5 : 1, 100 mL)] and CHCl3–MeOH–H2O (15 : 6 : 1, 100 mL), with 5 g fractions being collected. The residue (70.9 mg) in fractions 58–72 of the 10–14% MeOH eluate was finally purified by HPLC [H2O–MeOH (1 : 1), Develosil], giving 15.5 mg of 16 from the peak at 21 min. The second residue (514 mg) in fractions 170–190 was first purified by HPLC [H2O–MeOH (1 : 1), Inertsil] to give the peak (94.4 mg) at 28 min, which was purified again by HPLC [H2O–MeOH (11 : 9), Inertsil] to give 4.7 mg of 18 from the peak at 102 min. The third residue (238 mg) in fractions 191–206 was purified by HPLC [H2O–MeOH (2 : 3), Inertsil], yielding 17.8 mg of 2 from the peak at 11 min. The last residue (355 mg) in fractions 207–216 was separated by DCCC, and the residue (98.2 mg) in fractions 65–112 was purified by HPLC [H2O–MeOH (3 : 7), Inertsil], giving 7.4 mg of 15 from the peak at 36 min. The residue (3.01 g) in fractions 47–57 of the 20–30% eluate was separated by ODS open CC, giving three residues. The first residue (994 mg) in fractions 134–174 was subjected to silica gel (30 g) CC and elution with CHCl3 (200 mL) and CHCl3–MeOH [(9 : 1, 300 mL), (17 : 3, 300 mL), (4 : 1, 300 mL), and (7 : 3, 300 mL)], with 5 g fractions being collected. The residue (176 mg) in fractions 156–183 of the 15–20% MeOH eluate was purified by HPLC [H2O–MeOH (11 : 9), Develosil], giving 7.0 mg of 4 from the peak at 40 min. The residue (95.7 mg) in fractions 190–199 of the 20% MeOH eluate was purified by HPLC [H2O–MeOH–(CH3)2CO (12 : 5 : 3), Inertsil], giving 8.0 mg of 17 from the peak at 46 min. The residue (106 mg) in fractions 206–236 of the 20–30% MeOH eluate was purified by HPLC [H2O–MeOH (1 : 1), Inertsil], giving 6.1 mg of 5 and 7.4 mg of 6 from the peaks at 44 and 60 min, respectively. The second residue (552 mg) in fractions 175–192 was subjected to DCCC, and the residue (99.0 mg) in fractions 6–21 was purified by HPLC [H2O–MeOH (2 : 3), Inertsil], giving 13.8 mg of 3 from the peak at 20 min. The residue (128 mg) in fractions 22–40 was subjected to silica gel (6 g) CC with elution with CHCl3 (100 mL) and CHCl3–MeO [(9 : 1, 100 mL), (4 : 1, 100 mL), and (7 : 3, 100 mL)], with 5 g fractions being collected. The residue (55.1 mg) in fractions 36–54 of the 10–20% MeOH eluate was purified by HPLC [H2O–MeOH (2 : 3), Inertsil], yielding 7.8 mg of 14 from the peak at 22 min. The third residue (588 mg) in fractions 193–216 was separated by DCCC, and the residue (159 mg) in fractions 16–36 was purified by HPLC [H2O–MeOH (41 : 56), Develosil], giving 15.2 mg of 7 and 14.2 mg of 1 from the peaks at 24 and 27 min, respectively. The residue (2.80 g) in fractions 58–70 of the 30% MeOH eluate was separated by ODS open CC and gave three residues. The first residue (335 mg) in fractions 89–133 was subjected to silica gel (10 g) CC and elution with CHCl3 (50 mL), CHCl3–MeOH (7 : 3, 70 mL), and CHCl3–MeOH–H2O (15 : 6 : 1, 480 mL), with 10 g fractions being collected. The residue (88.9 mg) in fractions 16–27 was purified by HPLC [H2O–MeOH (3 : 2), Develosil] to give 4.8 mg of 13 from the peak at 24 min. The second residue (673 mg) in fractions 134–173 was subjected to silica gel (30 g) CC and elution with CHCl3 (50 mL), CHCl3–MeOH (7 : 3, 150 mL), CHCl3-MeOH–H2O (15 : 6 : 1, 270 mL), and MeOH (500 mL), with 10 g fractions being collected. The residue (432 mg) in fraction 28 of the 100% eluate was purified by HPLC [H2O–MeOH (1 : 1), Develosil] to give 32.2 mg of 10 from the peak at 11 min. The third residue (730 mg) in fractions 174–190 was separated by DCCC, and the residue (376 mg) in fractions 12–26 was subjected to silica gel (50 g) CC with elution with CHCl3 (50 mL), CHCl3–MeOH (7 : 3, 110 mL), CHCl3–MeOH–H2O (15 : 6 : 1, 60 mL), and MeOH (500 mL), with 10 g fractions being collected. The residue (126 mg) in fraction 18 of the 100% MeOH eluate was purified by HPLC [H2O–MeOH (1 : 1), Develosil] to give 10.5 mg of 8 from the peak at 11 min.
The residue (11.9 g) in fractions 34–36, obtained on Diaion HP-20 CC, was subjected to silica gel (500 g) CC and elution with CHCl3 (3 L), CHCl3–MeOH [(49 : 1, 3 L), (24 : 1, 3 L), (23 : 2, 3 L), (9 : 1, 3 L), (7 : 1, 3 L), (17 : 3, 3 L), (4 : 1. 3 L), (3 : 1, 3 L), and (7 : 3, 110 mL)], and CHCl3–MeOH–H2O (35 : 15 : 2, 3 L), with 3 L fractions being collected. The residue (1.51 g) in fraction 7 of the 10–12.5% MeOH eluate was separated by ODS open CC to give 96.4 mg of 9 in fractions 310–316. The residue (80.7 mg) in fractions 327–341, which was purified by HPLC [H2O–MeOH (3 : 7), Develosil], gave 11.3 mg of 12 from the peak at 20 min. The residue (2.15 g) in fraction 8 of the 12.5–15% MeOH eluate was separated by ODS open CC. The residue (105 mg) in fractions 206–217 was subjected to DCCC, and the residue (48.4 mg) in fractions 31–36 was purified by HPLC [H2O–MeOH (1 : 1), Develosil] to give 20.1 mg of 11 from the peak at 32 min.
Symplocosin C (1) amorphous powder, [α]D21 +18.9 (c = 0.95, MeOH); IR νmax (film) cm−1: 3394, 2927, 1733, 1650, 1454, 1075, 1032; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 805.4329 [M + Na]+ (Calcd for C41H66O14Na: 805.4350).
Symplocosin D (2) amorphous powder, [α]D25 −7.92 (c = 1.19, MeOH); IR νmax (film) cm−1: 3302, 3019, 1736, 1650, 1541, 1457, 1075; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 805.4344 [M + Na]+ (Calcd for C41H66O14Na: 805.4350).
Symplocosin E (3) amorphous powder, [α]D25 +4.35 (c = 0.92, MeOH); IR νmax (film) cm−1: 3354, 2935, 1729, 1664, 1447, 1077, 1029; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 967.4866 [M + Na]+ (Calcd for C47H76O19Na: 967.4878).
Symplocosin F (4) amorphous powder, [α]D25 −2.14 (c = 0.47, MeOH); IR νmax (film) cm−1: 3303, 2933, 1729, 1664, 1448, 1078, 1044; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 821.4291 [M + Na]+ (Calcd for C41H66O15Na: 821.4299).
Symplocosin G (5) amorphous powder, [α]D25 −10.8 (c = 0.41, MeOH); IR νmax (film) cm−1: 3393, 2930, 1730, 1638, 1511, 1076, 1033; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 983.4822 [M + Na]+ (Calcd for C47H76O20Na: 983.4828).
Symplocosin H (6) amorphous powder, [α]D26 +1.36 (c = 0.59, MeOH); IR νmax (film) cm−1: 3362, 2926, 1733, 1650, 1457, 1076; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 983.4818 [M + Na]+ (Calcd for C47H76O20Na: 983.4828).
Symplocosin I (7) amorphous powder, [α]D26 +21.4 (c = 1.52, MeOH); IR νmax (film) cm−1: 3393, 2942, 1734, 1650, 1455, 1076, 1031; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 805.4323 [M + Na]+ (Calcd for C41H66O14Na: 805.4350).
Symplocosin J (8) amorphous powder, [α]D23 −11.0 (c = 0.70, MeOH); IR νmax (film) cm−1: 3388, 2946, 1734, 1650, 1457 1078, 1040; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 819.4489 [M + Na]+ (Calcd for C42H68O14Na: 819.4507).
Symplocosin K (9) amorphous powder, [α]D26 +1.79 (c = 6.47, MeOH); IR νmax (film) cm−1: 3395, 2944, 1742, 1649, 1546, 1443, 1048; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 833.4641 [M + Na]+ (Calcd for C43H70O14Na: 833.4663).
Symplocosin L (10) amorphous powder, [α]D24 −3.89 (c = 1.05, MeOH); IR νmax (film) cm−1: 3374, 2944, 1733, 1648, 1559, 1245, 1073; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 977.5066 [M + Na]+ (Calcd for C49H78O18Na: 977.5086).
Symplocosin M (11) amorphous powder, [α]D21 −3.49 (c = 0.77, MeOH); IR νmax (film) cm−1: 3392, 2945, 1746, 1650, 1457, 1368, 1047; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 991.5215 [M + Na]+ (Calcd for C50H80O18Na: 991.5242).
Symplocosin N (12) amorphous powder, [α]D25 −24.3 (c = 0.95, MeOH); IR νmax (film) cm−1: 3321, 2942, 1770, 1736, 1512, 1457, 1075; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 993.5018 [M + Na]+ (Calcd for C49H78O19Na: 993.5035).
Symplocosin O (13) amorphous powder, [α]D26 −12.9 (c = 0.31, MeOH); IR νmax (film) cm−1: 3384, 2944, 2872, 1726, 1635, 1448, 1024; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 673.3920 [M + Na]+ (Calcd for C36H58O10Na: 673.3928).
Symplocosin P (14) amorphous powder, [α]D25 −4.20 (c = 0.50, MeOH); IR νmax (film) cm−1: 3384, 2946, 2875, 1740, 1650, 1371, 1078; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 849.4603 [M + Na]+ (Calcd for C43H70O15Na: 849.4612).
Acid Hydrolysis and Sugar Analyses About 1 mg, each, of compounds 1–8, 10, 11, and 13 were hydrolyzed with 1 M HCl (1.0 mL) at 80 °C for 2 h. After the reaction mixtures were neutralized with Amberlite IRA-96SB, they were partitioned with an equal amount of EtOAc (1.0 mL), and the H2O layers were analyzed by HPLC, equipped with a chiral detector (JASCO OR-2090plus) on an amino column [Asahipak NH2P-50 4E, CH3CN–H2O (3 : 1), 1 mL/min]. The peak was identified by co-chromatography with an authentic sample. The hydrolysates of compounds 1–7 gave peaks for L-arabinose with a positive optical rotation and D-glucose with a positive rotation sign at 5.7 and 7.3 min, respectively. The hydrolysate of 8 gave peaks for D-glucuronic acid and D-galactose with positive optical rotation values at 4.2 and 7.2 min, respectively. The hydrolysates of compounds 10 and 11 gave peaks for D-glucuronolactone, L-arabinose, and D-galactose. The hydrolysate of 13 gave a peak for D-glucuronic acid. Peaks were identified by co-chromatography with authentic D-glucuronic acid, L-arabinose, D-galactose, and D-glucose.
About 1 mg, each, of 9, 11, and 14 were hydrolyzed with 1 M HCl (1.0 mL) at 90 °C for 2 h. The reaction mixtures were washed with equal amounts of EtOAc and, then, passed through Amberlite IRA-96SB. The pass-through fractions were evaporated to dryness to give residues. The residues were dissolved in 0.1 mL of dry pyridine, and then, 0.5 mg of L-cysteine methyl ester was added. To these mixtures, 1.4 mg of o-tolylthioisocyanate in 70 µL of pyridine was added, followed by standing at 60°C for 1 h. The reaction mixtures were analyzed by HPLC [ODS: Cosmosil 5C18ARII (Φ = 4.6 mm, L = 250 mm), CH3CN–50 mM H3PO4 (1 : 3), 0.8 mL/min, UV detector at 250 nm]. The hydrozates of 9 and 14 gave peaks for thiocarbamoylthiazolidine derivatives of D-galactose and D-glucuronic acid at 26.0 and 31.0 min, respectively. The hydrolyzate of 11 gave peaks for thiocarbamoylthiazolidine derivatives of D-galactose, D-glucuronic acid, and L-arabinose at 26, 31, and 37 min, respectively. The peaks were identified by co-chromatography with thiocarbamoylthiazolidine derivatives of authentic D-galactose, D-glucuronic acid, and L-arabinose.
Cytotoxic Activity toward Lung Adenocarcinoma A549 CellsCytotoxic activity toward lung adenocarcinoma cells was determined by a colorimetric cell viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Lung adenocarcinoma cell line A549 was purchased from the JCRB Cell Bank, Japan. A549 cells were cultured in Dulbecco's modified Eagle medium, supplemented with 10% heat-inactivated fetus calf serum, and kanamycin (100 µg/mL) and amphotericin B (5.6 µg/mL).
In a 96-well plate, 1 µL aliquots of sample solutions and cancer cells (5 × 103 cells/well) in 100 µL medium were added to each well, and then, the plate incubated at 37°C under a 5% CO2 atmosphere for 72 h. A solution of MTT (100 µL) was then added to each well, and the incubation continued for an additional 1 h. The absorbance of each well was measured at 540 nm using a Molecular Devices Versamax tunable microplate reader. Dimethyl sulfoxide (DMSO) was used as a negative control and doxorubicin as a positive control. The cytotoxic activity was calculated as:
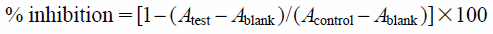 |
where Acontrol is the absorbance of the control (DMSO) well, Atest the absorbance of the test wells, and Ablank the absorbance of the cell-free wells.
Anti-Leishmania Activity and α-Glucosidase Inhibitory ActivityAssays were performed according to the procedures previously reported.23,24)
The authors are grateful for access to the superconducting NMR instrument (Bruker Avance 600 III) at the Analytical Center of Molecular Medicine of Hiroshima University, Faculty of Medicine, and an Applied Biosystem QSTAR XL system ESI (Nano Spray)-MS at the Analysis Center of Life Science of the Graduate School of Biomedical and Health Sciences, Hiroshima University. This work was supported, in part, by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 22590006, 23590130, 25860078, and 15H04651), the Japan Society for the Promotion of Science, and the Ministry of Health, Labour and Welfare. Thanks are also due to the Research Foundation for Pharmaceutical Sciences and the Takeda Science Foundation for financial support.
The authors declare no conflict of interest.