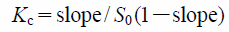Abstract
Zerumbone is a multifunctional compound which shows various biological activities, such as antitumor activity, anti-inflammatory activity, antiulcer activity, etc. However, to use Zerumbone as functional foods or medicines, its pharmaceutical properties such as solubility should be improved. In the present study, we prepared its inclusion complexes with various cyclodextrin (CyD) derivatives, and evaluated their solubility, release profile of the drug and cytotoxic activity. Among 11 CyDs, sulfobutylether (SBE)-β-CyD showed the highest solubilizing effect for Zerumbone. Phase solubility diagrams of SBE-β-CyD/Zerumbone in 10% methanol solution showed AL type, and the stability constant was 756 M−1. SBE-β-CyD also formed the solid complex with Zerumbone by kneading for 90 min. Importantly, the dissolution rate of Zerumbone was improved by complexation with SBE-β- and hydroxypropyl (HP)-β-CyDs, and its supersaturation was maintained for several hours. The solubilizing effects by SBE-β-CyD was greater than that of HP-β-CyD. Moreover, SBE-β-CyD/Zerumbone complex also retained the cytotoxic activity of Zerumbone. These results suggest that CyDs, especially SBE-β-CyD, were useful to improve the solubility of Zerumbone.
Introduction
Zerumbone (Fig. 1A) is a monocyclic sesquiterpenoid reported from the plants of Zingiberaceae family including Zingiber zerumbet Smith1) and Zingiber montanum (J. Koenig) Link ex A. Dietr.2) In recent years, various studies have reported the potent biological activities of Zerumbone such as antioxidant, anti-inflammatory, anti-angiogenesis and antitumor activities.3) However, the low water-solubility of Zerumbone is disadvantageous for the formulation design. Recently, Eid et al.4) reported inclusion complexation of Zerumbone by 2-hydroxypropyl-β-cyclodextrin (HP-β-CyD) in both liquid and solid states; and the solubility of Zerumbone was improved >30 fold by the inclusion complexation with HP-β-CyD. In addition, the complex exhibited cytotoxic activity in human cancer cell lines. However, little is known about utilities of CyD derivatives except for HP-β-CyD for the improvement of pharmaceutical properties of Zerumbone.
In the present study, we have examined the improvement of solubility of Zerumbone by the complexation with various CyDs including parent and modified CyDs. Moreover, solid complexes of Zerumbone with CyDs were prepared by kneading method and characterized by differential scanning calorimetry (DSC) and powder X-ray diffraction. Furthermore, the dissolution behavior and cytotoxic activity of CyD/Zerumbone complexes were evaluated.
Results and Discussion
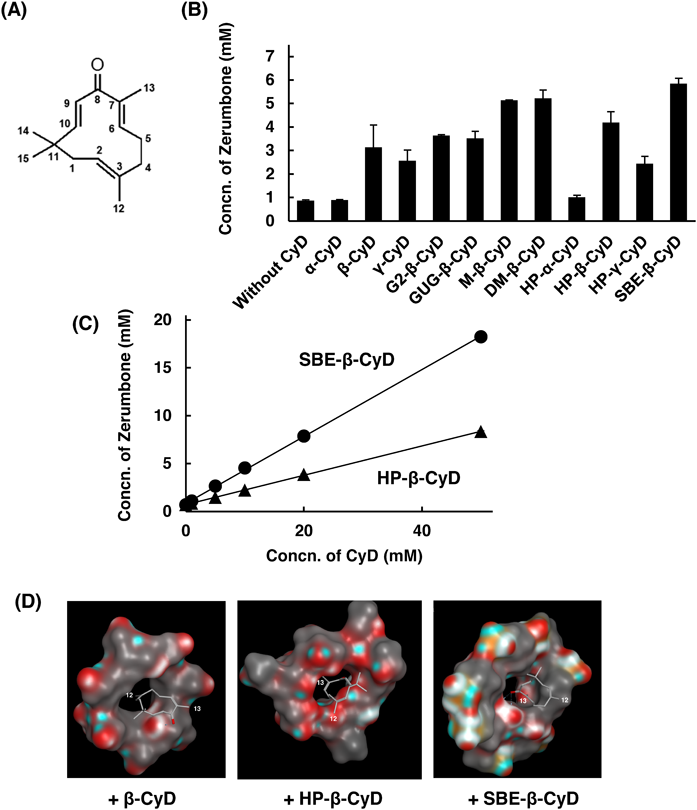
Figure 1B shows the solubility of Zerumbone in the presence of 10 mM of various CyDs. In this study, 10% methanol aqueous solution was used as solvent because of extremely low water-solubility of Zerumbone. The solubility of Zerumbone in 10% methanol was approx. 0.9 mM, and was increased in the presence of CyDs; sulfobutylether (SBE)-β-CyD > dimethyl (DM)-β-CyD > methyl (M)-β-CyD > HP-β-CyD > maltosyl (G2)-β-CyD > glucuronylglucosyl (GUG)-β-CyD > β-CyD > γ-CyD > HP-γ-CyD > HP-α-CyD > α-CyD. Especially, SBE-β-CyD, M-β-CyD and DM-β-CyD showed remarkable solubilizing effect beyond HP-β-CyD. These results indicate that SBE-β-CyD, M-β-CyD and DM-β-CyD are useful to improve the solubility of Zerumbone. However, M-β-CyD and DM-β-CyD showed toxicity compared to HP-β-CyD and SBE-β-CyD5); therefore, we used SBE-β-CyD and HP-β-CyD for following studies.
To examine the solubilizing effects of SBE-β-CyD and HP-β-CyD in detail, phase solubility diagrams of CyDs/Zerumbone were determined (Fig. 1C). Both diagrams showed AL type, suggesting the 1 : 1 complex between CyD and Zerumbone. HP-β-CyD and SBE-β-CyD dissolved 8 mM and 18 mM Zerumbone at 50 mM, respectively, indicating higher solubilizing effect of SBE-β-CyD than HP-β-CyD. Moreover, the stability constant (Kc) of HP-β-CyD/Zerumbone and SBE-β-CyD/Zerumbone were determined as 255 M−1 and 756 M−1, respectively. These results suggest that SBE-β-CyD improves the solubility of Zerumbone compared to HP-β-CyD.
To estimate the interaction modes and complexation binding energies of CyDs/Zerumbone, a molecular operating environment software (MOE, version 2018) was employed according to the Geng’s method.6) SBE-β-CyD and HP-β-CyD interacted with the parts around 13-position of methyl group of Zerumbone, whereas β-CyD did with the parts around 12-position of methyl group (Fig. 1D). Moreover, binding energy of SBE-β-CyD/Zerumbone (−18.72 kcal/mol) was lower than that of HP-β-CyD/Zerumbone (−16.24 kcal/mol) or β-CyD/Zerumbone (−13.66 kcal/mol), indicating that SBE-β-CyD strongly interacts with Zerumbone compared to HP-β-CyD.
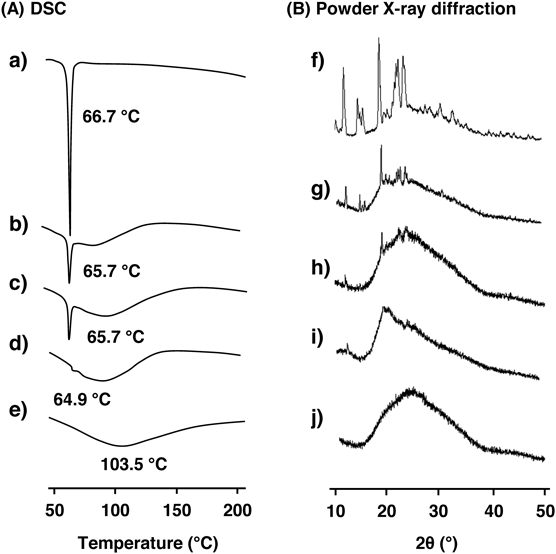
To characterize kneading products of CyDs/Zerumbone, DSC was measured (Fig. 2A). Zerumbone showed endothermic peak at 66.7 °C (Fig. 2a), as same as physical mixtures of CyDs/Zerumbone (Figs. 2b, c). In contrast, the peak was almost disappeared in the kneading products of HP-β-CyD/Zerumbone (Fig. 2d) and SBE-β-CyD/Zerumbone (Fig. 2e), suggesting a breakdown of the crystal lattice of Zerumbone by its complexation with CyDs.
To make sure the complexation between CyDs and Zerumbone in the solid state, powder X-ray diffraction was measured (Fig. 2B). The Zerumbone (Fig. 2f) and physical mixtures of CyDs/Zerumbone (Figs. 2g, h) showed the peaks derived from its crystal structure. On the other hand, the peaks were disappeared in kneading products of HP-β-CyD/Zerumbone (Fig. 2i) and SBE-β-CyD/Zerumbone (Fig. 2j), suggesting the dispersion of Zerumbone in a monomolecular state in kneading products. These results suggest that Zerumbone forms inclusion complexes with SBE-β-CyD and HP-β-CyD in the solid state.
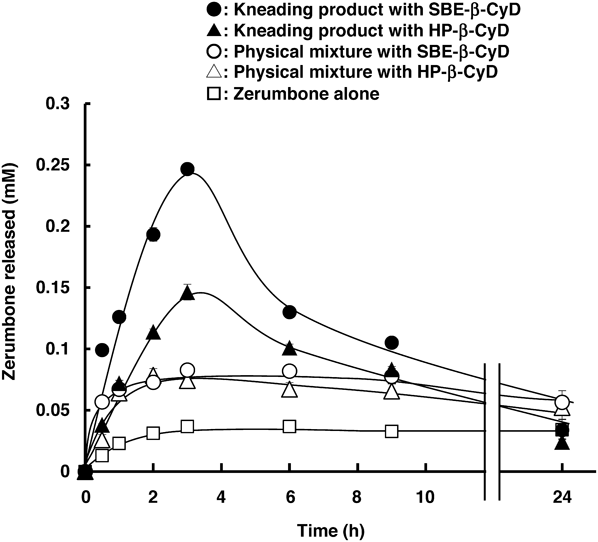
We next performed dissolution test using the kneading products containing 1.98 mg of Zerumbone in 10 mL of phosphate buffered saline (PBS, pH 7.4) at 37 °C, an experimental condition in which insoluble particles are presence (Fig. 3). The release rate of Zerumbone from the kneading product with SBE-β-CyD dramatically increased at 3 h when compared to that of the physical mixture which dissolved and reached equilibrium of Zerumbone in 0.9 mM CyD solution, and was higher than that of the kneading product with HP-β-CyD. Interestingly, the release rate of both kneading products decreased after 3 h, suggesting the supersaturation state of Zerumbone. In this connection, the dissolution of Zerumbone from the kneading products of HP-β-CyD and SBE-β-CyD was slightly decreased compared to the corresponding physical mixtures at 24 h. This is probably within error margin because of measurement accuracy. Anyhow, these results suggest that CyD derivatives are useful to maintain the supersaturation state of Zerumbone. The supersaturating drug delivery systems have been acknowledged as a promising concept to obtain adequate oral bioavailability.7) Therefore, the results indicate the great potential of SBE-β-CyD as a solubilizing agent for Zerumbone.
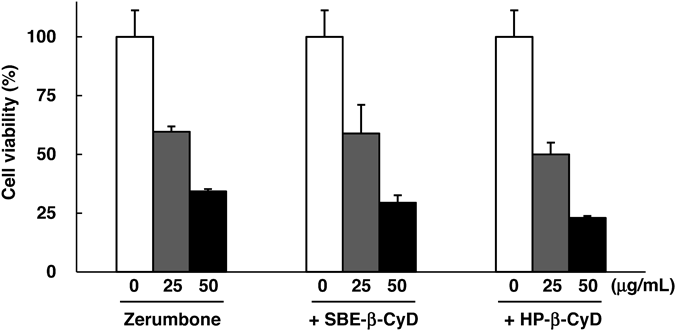
Zerumbone shows cytotoxic activity after its cellular uptake and subsequent induction of apoptosis. In general, CyD complex is negligibly taken into the cells because of its high hydrophilicity and high-molecular weight; thus, to confirm the activity of CyD/Zerumbone complexes is essential. Therefore, we next evaluated cytotoxic activities of CyD/Zerumbone complexes in KB cells (Fig. 4). In this study, to assure the soluble condition of the complexes, 0.75–1.5% dimethyl sulfoxide (DMSO) was added in the samples, and preliminary confirmed the negligible cytotoxic activity of DMSO alone (data not shown). Zerumbone complexes with SBE-β-CyD and HP-β-CyD showed cytotoxic activities, and were equivalent to that of Zerumbone alone. Thus, the cytotoxic activities of Zerumbone in the complexes with SBE-β-CyD and HP-β-CyD were retained in KB cells. As future efforts, we should examine the in vivo antitumor activity of CyD/Zerumbone complexes.
Conclusion
In this study, we evaluated various CyD derivatives as solubilizing agents for Zerumbone, and demonstrated that SBE-β-CyD shows the most potent solubilizing effect and sustainability of supersaturation state for Zerumbone. SBE-β-CyD formed inclusion complex with Zerumbone in both solid and liquid states, and cytotoxic activity of Zerumbone in the complex with SBE-β-CyD was completely retained in KB cells. These results suggest the potential of SBE-β-CyD as a promising pharmaceutical material for Zerumbone.
Experimental
Materials and MethodsZerumbone was isolated from the dried rhizomes of Zingiber montanum (J. Koenig) Link ex A. Dietr as previously described.2) α-CyD, β-CyD, γ-CyD and HP-CyDs in which hydroxypropyl groups were mainly grafted at 2-position of secondary hydroxy groups (degree of substitution: DS 4.4) were supplied by Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan). SBE-β-CyD (DS 6.2–6.9) was supplied by CyDing Co., Ltd. (Kumamoto, Japan). G2-β-CyD and GUG-β-CyD were supplied by Ensuiko Sugar Refining (Tokyo, Japan). M-β-CyD (DS 12.2) and DM-β-CyD (DS 14.0) were purchased from Tokyo Kasei (Tokyo, Japan) and Wako Pure Chemical Corporation (Osaka, Japan), respectively.
Solubility StudiesAn excess amount of Zerumbone (5 mg) was added to 1 mL of 10% methanol aqueous solution containing various concentration of CyDs (0–50 mM). Then the solutions were shaken at 25 °C for 24 h. After filtration of each solution by syringe filter (0.2 µm, Advantec, Tokyo, Japan), the concentration of dissolved Zerumbone was determined by absorbance at 242 nm by using Spectrophotometer (JASCO V-650 UV-visible Spectrophotometer, Tokyo, Japan). The solubility of Zerumbone was plotted to CyD concentrations, and Kc was calculated using the slope and intercept according to the following equation.
Where, intercept represents S0, the intrinsic solubility of Zerumbone in 10% methanol.
Molecular Operating Environment (MOE) SimulationThe 3D structure of β-CyD was extracted by MOE from the protein data bank (PDB ID: 1Z0N). Then, HP-β-CyD and SBE-β-CyD were constructed by the incorporation of 2-hydroxypropyl groups and sulfobutylether groups at 2-position of secondary hydroxy groups and 6-position of primary hydroxy groups of β-CyD, respectively, using ChemDraw professional version 16.0.1.4 (61) molecular builder. To estimate interaction modes and binding energies of CyDs/Zerumbone, MOE was performed as reported previously.6)
Preparation of Kneading Product or Physical MixtureTo obtain the kneading product, the powder of HP-β-CyD (0.3473 g) or SBE-β-CyD (0.5425 g) was mixed with Zerumbone (0.0545 g) at a molar ratio 1 : 1, and was kneaded for 90 min with appropriate volume of water during the kneading process. Then, the samples were dried under the reduced pressure for 24 h. To obtain the physical mixture, the same amounts of powders of Zerumbone and CyDs were mixed using a mortar and pestle for 5 min.
Differential Scanning Calorimetry (DSC)In measurement of DSC (AutoQ20, TA Instruments, Tokyo, Japan), the solid samples (approx. 3 mg) were placed in a flat-bottomed aluminum pan, heated at 10°C/min and scanned at 0–300°C under a constant flow of dry nitrogen.
Powder X-Ray DiffractionPowder X-ray diffraction patterns were measured by Rigaku Ultima IV X-ray diffractometer (Tokyo, Japan). The voltage was 40 kV and 30 mA. The scanning rate was 2°/min at 5–50°.
Release ProfileThe solid samples including 1.98 mg of Zerumbone were added in 10 mL of PBS (pH 7.4) at 37 °C. At appropriate intervals, 1 mL of the medium was collected and filtered (0.2 µm, DISMIC-25 CS, Toyo Roshi, Tokyo, Japan). The fresh 1 mL of PBS was added in the medium to adjust the total volume to 10 mL. The concentration of Zerumbone in the filtrate was determined by absorbance at 242 nm.
Cytotoxic ActivityKB cells were obtained from the Institute of Development, Aging and Cancer (IDAC), Tohoku University (Sendai, Japan). The cells were grown in RPMI-1640 culture medium containing penicillin (1 × 105 mU/mL) and streptomycin (0.1 mg/mL) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified 5% CO2 and 95% air atmosphere. KB cells were seeded in 96 well plate at (2 × 104 cell/well) and incubated for 24 h. Cells were treated with 0.75 and 1.5% DMSO solutions containing 25 and 50 µg/mL of Zerumbone and CyDs at a molar ratio 1 : 1, respectively. After the incubation at 37 °C for 36 h, cell viability was determined by the WST-1 method (Wako Pure Chemical Corporation, Osaka, Japan). WST-1 formazan was monitored by microplate reader Multiskan FC (Thermo Scientific, Waltham, MA, U.S.A.).
Acknowledgments
This work was partially supported by Program for Leading Graduate Schools “HIGO (Health life science: Interdisciplinary and Glocal Oriented) Program,” Kumamoto University.
Conflict of Interest
Dr. Motoyama and Dr. Higashi are technical advisors of CyDing Co., Ltd. (Kumamoto, Japan).
References
- 1) Dev S., Tetrahedron, 8, 171–180 (1960).
- 2) Hassan M. M., Adhikari-Devkota A., Imai T., Devkota H. P., Separations, 6, 31 (2019).
- 3) Kalantari K., Moniri M., Moghaddam B. A., Abdul Rahim R., Ariff B. A., Izadiyan Z., Mohamad R., Molecules, 22, 1645 (2017).
- 4) Eid E. E. M., Abdul A. B., Suliman F. E. O., Sukari M. A., Rasedee A., Fatah S. S., Carbohydr. Polym., 83, 1707–1714 (2011).
- 5) Irie T., Uekama K., J. Pharm. Sci., 86, 147–162 (1997).
- 6) Geng Q., Li T., Wang X., Chu W., Cai M., Xie J., Ni H., Sci. Rep., 9, 1882 (2019).
- 7) Brouwers J., Brewster M. E., Augustijns P., J. Pharm. Sci., 98, 2549–2572 (2009).