2020 Volume 68 Issue 12 Pages 1131-1142
2020 Volume 68 Issue 12 Pages 1131-1142
Black tea accounts for 70–80% of world tea production, and the polyphenols therein are produced by enzymatic oxidation of four tea catechins during tea fermentation. However, only limited groups of dimeric oxidation products, such as theaflavins, theasinensins, and theacitrins, have been isolated from black tea and chemically characterized. This is largely because of the complexity and heterogeneity of the oxidation products. To determine structures and production mechanisms of uncharacterized black tea polyphenols, in vitro model fermentation experiments using pure catechins and polyphenol oxidase have been applied, and basic oxidation mechanisms have been established. Contemporary methods, such as LC-MS, are also effective to identify catechin oxidation products in black tea. Despite ongoing efforts, almost 60% of the solids in black tea infusion remain uncharacterized. These compounds include the so-called thearubigins, which are a heterogeneous mixture of uncharacterized catechin oxidation products with oligomeric structures. This review summarizes the current knowledge of the production mechanisms of representative black tea polyphenols and presents recent progress in characterization of thearubigins.
Tea is the second most popular beverage after water, and its production has increased by 4.4% annually over the past decade to reach 5.73 million tonnes in 2016.1) Tea is produced from the leaves of Camellia sinensis (L.) O. Kuntze and C. sinensis var. assamica (Masters) Kitamura. Tea plants originated in southwest China, particularly Yunnan Province, and drinking of tea infusion is also considered to have begun in Yunnan Province thousands of years ago.2) It was suggested that the tea plant was originally selected for use as a medicine because of its high caffeine content, similar to the way coffee beans were selected in Africa.3) In the long history of tea, tea drinking and related culture spread worldwide from China, and tea became one of the most important trading materials in 18th and 19th centuries. The expansion of tea consumption prompted the development of different processing methods to produce various types of tea products. Green tea and black tea are the main tea types, with black tea accounting for 70–80% of world tea production.4) At the world level, black tea production has increased annually by 3.0% and green tea by 5.4% percent over the past decade in response to perceived health benefits.1,5,6) Green tea is produced by steaming or pan-frying fresh tea leaves immediately after harvesting to inactivate the leaf enzymes including polyphenol oxidase. Therefore, the composition of green tea polyphenols is similar to those of fresh leaves, and (−)-epigallocatechin (1), (−)-epicatechin (3), and their galloyl esters (2 and 4, respectively) are the major polyphenols (Figs. 1, 2). In black tea production, the living withered leaves are crushed and the tea catechins are oxidized with the aid of polyphenol oxidase and peroxidase,7,8) which are then inactivated by heating and drying at the final stage of tea production. Decreased catechin contents after the enzymic oxidation results in decreased astringency and bitterness.9) Apart from black tea, lowering of catechin levels in green tea is also observed in aerobic and anaerobic microbial fermentation.10–14) Caffeine levels of the tea products are similar to those of the original tea leaves, suggesting that the development of tea processing methods is probably related to decreased astringency through decreased catechin levels. It should be noted here that black tea is not real fermented tea because microorganisms do not participate in black tea production; however, the enzymatic oxidation in the production process is sometimes called “tea-fermentation.”

Sixty percent ethanol extract (0.25 g/15 mL, 70 °C, 30 min) of green tea (Japan) and black tea (Kenya). HPLC conditions: Cosmosil 5C18-ARII (250 × 4.6 mm, i.d.) with gradient elution of 4–30% (39 min) and 30–75% (15 min) CH3CN in 50 mM H3PO4 at 35 °C; flow rate, 0.8 mL/min; photodiode array detection. Caf: caffeine, GA: gallic acid, GC: gallocatechin, Tb: theobromine, TG: theogallin (galloyl quinic acid).

The representative black tea polyphenols are the theasinensins (5–9), theacitrins (10, 11), and theaflavins (12–15) (Fig. 2). Theasinensins (TSs)15–17) and theacitrins (TCs)18–20) are produced by oxidative dimerization of pyrogallol-type catechins 1 and 2, and theaflavins (TFs)21,22) are produced by coupling between catechol-type and pyrogallol-type catechins. In addition to these major catechin dimers, many catechin oxidation products and proanthocyanidins were isolated from black tea and oolong tea by Nonaka and Hashimoto.17,23–27) In Fig. 1, polyphenols detected as a broad hump on HPLC baseline are mainly composed of so-called thearubigins (TRs),28–31) which are a complex mixture of uncharacterized catechin oxidation products with relatively high molecular weights. It is reported that TRs constitute up to 60% of the solids in black tea infusion.32) The concentration of each black tea polyphenol including the four tea catechins (1–4) varies by levels of tea-fermentation; that is, the levels vary according to the levels of enzymatic oxidation of tea catechins.
Taking world consumption into account, black tea is the most important source of dietary polyphenols for humans. However, based on the SciFinder® database, the number of scientific studies of black tea is about one third of those on green tea. This is because the polyphenol composition of green tea is much simpler than that of black tea, and pure samples of the major green tea catechins are now commercially available. The total polyphenol content of black tea is comparable to that of green tea,33) despite decreases in catechins by oxidation during black tea production. The low concentration of tea catechins and presence of catechin oxidation products including TSs, TCs, TFs, and TRs in black tea affect its biological activities. Epidemiological studies have suggested that green tea consumption is inversely associated with cardiovascular disease and all-cause mortality, whereas black tea consumption is significantly inversely associated with all cancer and all-cause mortality.34) Prevention of diabetes mellitus was suggested by in vivo experiments that showed dietary supplementation of black tea induces glucose transporter type 4 (GLUT4) translocation to the plasma membrane and increases the expression of insulin receptor to improve glucose intolerance.35) Other health-promoting and cancer prevention effects of black tea based on their antioxidant and anti-inflammatory activities as their main underlying mechanisms are also reported.36–41) However, clinical investigations regarding black tea applications in humans are still limited,42) because many biological studies have indicated that the main functional components in tea are monomeric catechins and the total catechin contents in black tea are much lower than those in green tea. In addition, the concentrations of individual polyphenols that are characteristic to black tea, such as TFs and TSs, are significantly varied by conditions of black tea production; that is, by commercial brand. Some black teas contain tea catechins as major polyphenols, while TRs are major constituents in other black teas. The lower bioavailability of black tea polyphenols compared with tea catechins is also of particular importance, and there is almost no information about their microbial degradation in the colon.43) To clarify the biological activities of black tea, an understanding of the chemical composition of black tea polyphenols, especially TRs, is necessary.
Black tea polyphenols are usually analyzed by reversed-phase HPLC (Fig. 1). Methods for extraction and separation of black tea polyphenols are basically the same as those used to separate tannins.17,24–27) Aqueous acetone or aqueous ethanol are effective to extract total polyphenols. While extraction with hot water reflects normal tea infusion, some non-polar polyphenols, such as TFs and hydrophobic fractions of high-molecular-weight TRs, remain in the tea leaf. Solvent partitioning of the extract with ethyl acetate is sometimes useful; galloyl esters of catechin and TSs and TFs are extracted to the ethyl acetate phase, and flavonol glycosides, a portion of 1, and most of the TRs remain in the aqueous phase. Various combinations of adsorption chromatography using Sephadex LH-20 or Diaion HP20SS or column chromatography using various octadecylsilyl (ODS) columns with water and varying proportions of methanol are effective for separation on a large scale.17,24–26) In addition, size-exclusion column chromatography using Sephadex LH-20 or Toyopearl HW40 with a mixture of 8 M urea and acetone as eluent,44) which was originally developed for separation of oligomeric proanthocyanidins, is applicable to separation of high- and low-molecular-weight polyphenols.
The basic reaction mechanism for the production of black tea polyphenols is the coupling of electron-deficient o-quinones with electron-rich phenol rings45) (Chart 1). Enzymes, such as polyphenol oxidase, only catalyze the oxidation of catechol or pyrogallol rings to the respective o-quinones, and most of the subsequent coupling reactions of the quinones are non-enzymatic processes. This was suggested by stereoselective production of theasinensins by non-enzymatic oxidation of 1 and 2 with CuCl2.46) This reaction is a mimic of oxidation with polyphenol oxidase, which has two Cu atoms at the catalytic center,47,48) and the reaction can be applied to large-scale preparation of theasinensin A (5).46) Interestingly, homogenates prepared from various fruits such as Japanese pear, loquat, and banana also oxidize tea catechins to produce black tea polyphenols.49) These fruits do not contain catechins; however, enzymes of the fruits preferentially use catechins as electron donors to reduce molecular oxygen. Although the reactions are not completely identical to those observed in tea leaves, Japanese pear homogenate is well suited to investigate catechin oxidation on a large scale because the homogenate shows no substantial background peaks on HPLC analysis.

Epigallocatechin (1) and its 3-O-gallate (2) account for about 70% of green tea catechins,50) and the oxidation–reduction potential of their pyrogallol-type B-rings is lower than that of catechol-type B-rings51); thus, 1 and 2 are important sources of black tea polyphenols. Theasinensins (5–9) are simple B–B′ linked dimers of these pyrogallol-type catechins,28–31) and the contents are comparable to those of TFs in some black teas. Interestingly, when fresh tea leaves were cut and crushed, TFs were produced as the major oxidation products; however, TSs were not detected in the crushed leaves and appeared after the leaves were heated. This result suggests that TSs are produced via heat-sensitive precursors. Treatment of the crushed leaves with 1,2-phenylenediamine afforded phenazine derivatives, such as 16a as a representative, indicating that the precursors are quinone dimers (16 and analogues).52) Although isolation of the precursors from the tea leaves failed, 16 was prepared by oxidation of 2 with Japanese pear homogenate and named dehydrotheasinensin A.53) Non-enzymatic oxidation of 2 with CuCl2 also affords 16 as the major product.46) The production mechanism of 16 was proposed as shown in Chart 2. The phenazine derivatives obtained from crushed tea leaves have an R-biphenyl bond, and production of the diastereomer of 16 was not detected in enzymatic or CuCl2 oxidation of 2. This suggests that the stereoselectivity of coupling between o-quinone 2a and 2 is regulated by the presence of bulky galloyl groups and probably by hydrophobic and/or π–π interactions between two A-rings.
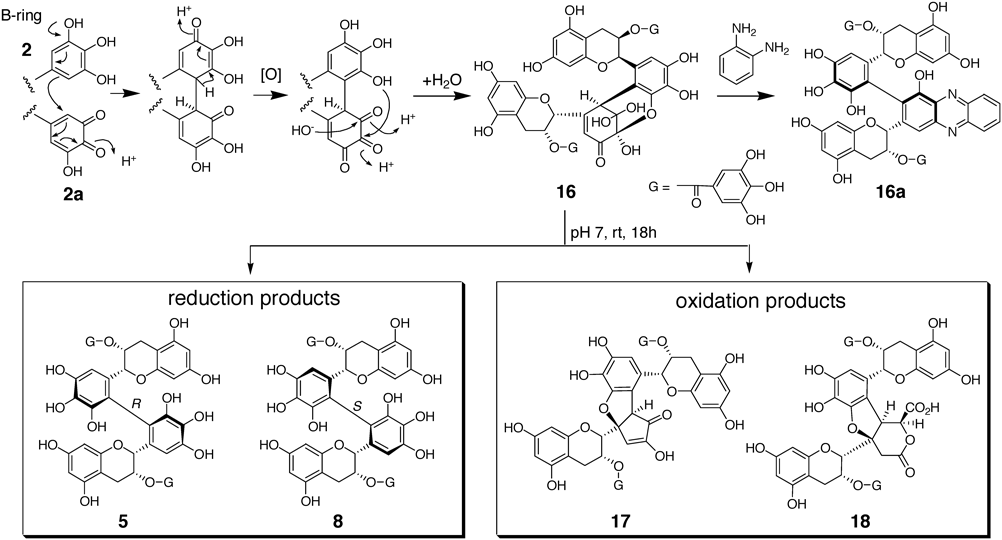
Reduction of the purified 16 with ascorbic acid or mercaptoethanol yielded 5 with R biphenyl bond. In contrast, heating of 16 afforded the atropisomer 8 with S-biphenyl bond together with a complex mixture of uncharacterized products. In a pH 7 buffer solution at room temperature, 16 gradually decomposed to give reduction products 5 and 8, together with oxidation products: galloyl oolongtheanin (17),17,54,55) and a ring cleavage product 1856) (Chart 2). These results strongly suggest that production of the black tea polyphenols 5, 8 and 17 from 16 is a reduction–oxidation disproportionation. Surprisingly, in 1957, Roberts had already presumed that theasinensin-type oxidation products might be formed by oxidation–reduction disproportionation of dimer quinones.57,58) Hashimoto et al. synthesized galloyl oolongtheanin (17) by oxidation of 2 with K3Fe(CN)6,17) and recently a detailed production mechanism of 17 was proposed by Hirose et al.59)
Theacitrins A (10) and C (11) are yellow pigments of black tea that were first reported in 1997 by Davis et al.18) Theacitrin C (11) is obtained as a minor product of enzymatic oxidation of 2 together with 16.19) The stereochemistry was determined in 2016 by Matsuo et al. by density functional theory (DFT) calculations of NMR chemical shifts of 11 and time-dependent DFT calculations of the electronic circular dichroism spectra of theacitrinin A (19), which is a degradation product of 1120) (Chart 3). The degradation of 11 gives 2053) along with 19, and both are detected in black tea and a fermented tea related to black tea.60) The pigments 10 and 11 are produced from 2 and/or 1 via a bicyclo[3,2,1]octane-type intermediate.19) The presence of galloyl ester at C-3′ hydroxy group is important for production of 10 and 11: an acetyl analogue of 11 is unstable and spontaneously decomposes to give an acetyl analogue of 19, suggesting that π–π interaction of the galloyl group with the B-ring moiety is important for the stability of 10 and 11.19)

Absence of galloyl ester at the C-3 position produces different results. Enzymatic oxidation of 1 yields dehydrotheasinensin C (21) as the major product by the same mechanism as observed for the oxidation of 2. However, theacitrin-type products were not detected in the reaction mixture; instead, proepitheaflagallin (22) with a bicyclo[3,2,1]octane moiety was generated19,61,62) (Chart 4). The production mechanism is related to that of theacitrins, and formation of a hemiacetal ring between C-3′ hydroxy group and C-g in 22 is the critical part of the mechanism. Interestingly, oxidation of gallocatechin, a 2,3-trans isomer of 1, proceeds much more slowly and predominantly affords a proepitheaflagallin-type product.63) Proepitheaflagallin (22) is unstable and has not been detected in black tea. Heating of 22 gives epitheaflagallin (23), hydroxytheaflavin (24),61) and a yellow pigment 25.64)

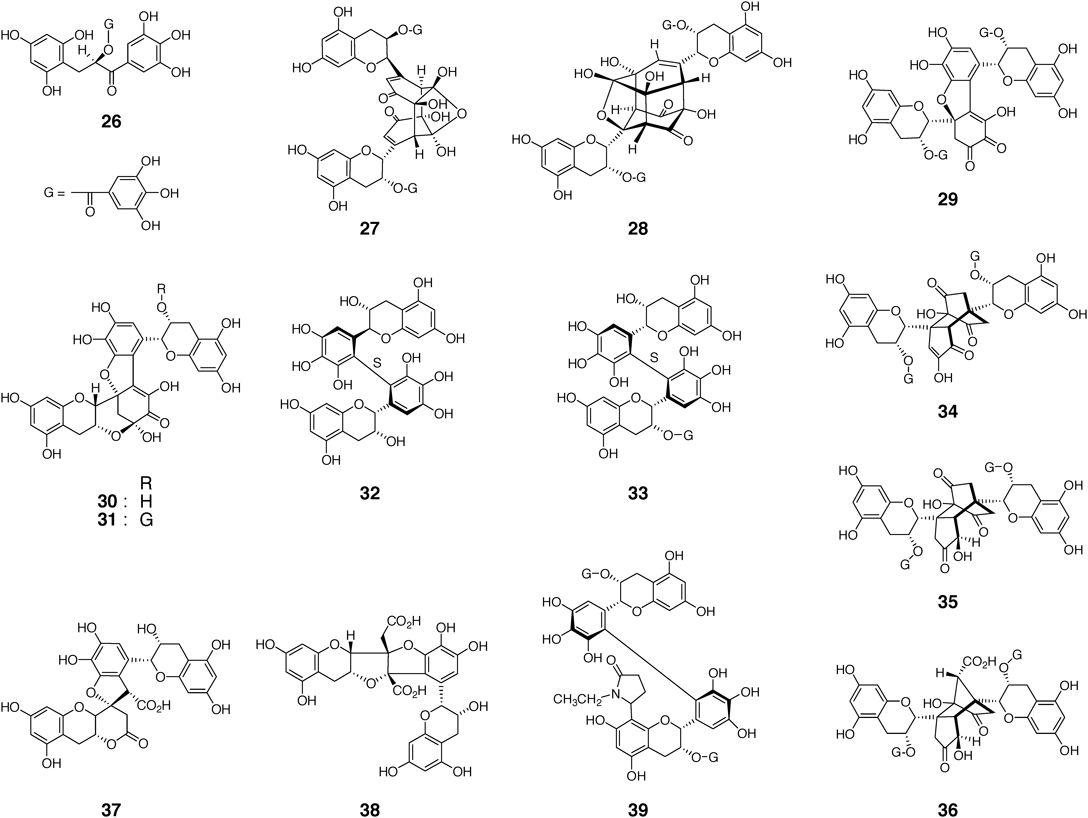
In addition to the abovementioned compounds, various oxidation products of 1 and 2 were obtained by model fermentation experiments or isolated from black tea (Fig. 3). Compounds 27,53,65) 28,66) 29,66) 34–3667,68) were produced by in vitro enzymatic oxidation of 2, and 30,49) 32,64) 37,64) and 3864) were obtained by similar oxidation of 1. Compounds 26, 31, and 33 were isolated from a fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves,60,69–72) and 29, 34, and 3973) were isolated from commercial black tea leaf. Compound 39 is a condensation product of theasinensin A (5) with Strecker degradation product of L-theanine (γ-L-glutamyl ethyl amide), an amino acid characteristic to tea leaf. Later, similar metabolites of monomeric catechins were found in fresh tea leaves and some tea products.74–77)
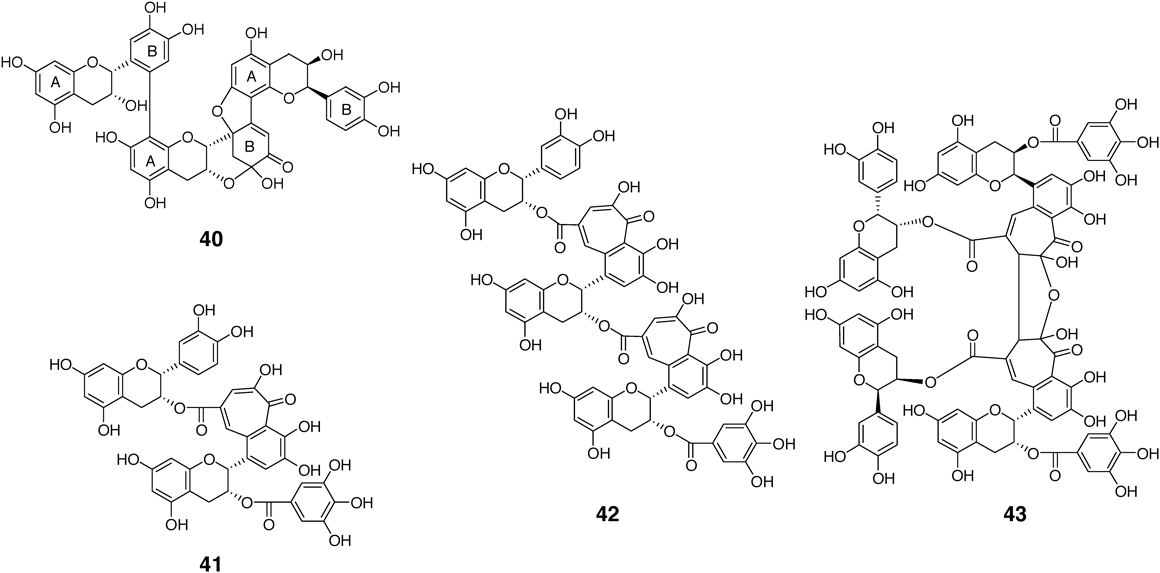
Oxidation of epicatechin (3) with catechol-type B-ring yields products generated by oxidative coupling between A- and B-rings, such as 4049,78,79) (Fig. 4). Enzymes oxidize the B-ring to an electrophilic o-quinone, and the quinone reacts with electron-rich A-ring C-8 or C-6 to form a C–C bond. Given that the coupling products still possess reactive A- and B-rings, the major products of oxidation of 3 are polymeric substances. In contrast, oxidation of epicatechin-3-O-gallate (4) affords products having a benzotropolone moiety produced by oxidative coupling between a B-ring and a galloyl group, such as 41 and 42.80–82) Tetramer 43 is a dimer generated by oxidative coupling of two molecules of 41. The predominance of B-ring–galloyl coupling in the oxidation of 4 may reflect stronger self-association of 4 than that found in 3.83)
Unlike the model fermentation reactions with single substrates explained above, enzymatic oxidation of mixtures of catechol-type and pyrogallol-type catechins produces TFs, which are the important reddish-orange black tea pigments21,22) (Fig. 2). In vitro enzymatic oxidation of a mixture of 1 and 3 suggested that the enzymes preferentially oxidize 3 to its o-quinone (3a),49) and 3a plays two roles: an oxidant and an electrophilic enone. As an oxidant, 3a oxidizes the electron-rich pyrogallol-type B-ring of 1, and simultaneously 3a is reduced to 3 (Chart 5). The resulting o-quinone 1a reacts with 1 to give dehydrotheasinensin C (21) as mentioned before. However, as an electrophile, 3a undergoes a cycloaddition reaction with the B-ring of 1 to afford a bicyclo[3.2.1]octane-type intermediate. The subsequent oxidation and decarboxylation of the intermediate give theaflavin (12). The production of the bicyclo[3.2.1] intermediate has been confirmed by Yanase et al.,84) and non-enzymatic synthesis of 12 via the bicyclo[3.2.1] intermediate has been reported by Matsuo et al.85) A theaflavin-related benzotropolone pigment was also prepared from procyanidin B-1 and 1.86) The condensation occurred regioselectively at the extension (upper) unit of the procyanidin, probably because of a steric effect. In Chart 5, when 1 is completely used up to produce 12 and 21, the quinone 3a begins to oxidize 12 to afford theanaphthoquinone (44).87) Furthermore, reaction of 3a with the benzotropolone ring of 12 affords flavanotheaflavin A (45).88) The reactions disclosed in these studies are now applied to structural revision and biomimetic synthesis of biologically active polyphenols, goupiolones A and B.89)

Other compounds produced by oxidation of benzotropolone moiety are shown in Fig. 5. Dehydrotheaflavin (46) was produced by oxidation of a mixture of 1 and 2 with tea leaf enzymes,67) and theacoumarin A (47) was obtained by treatment of 12 with peroxidase.90) Bistheaflavin A (48) was produced by oxidation of a mixture of 1 and 2 using banana polyphenol oxidase, whereas bistheaflavin B (49) was an autoxidation product of 12.91)

Presence of a galloyl group at the C-3 positions affects the oxidation of theaflavins. Enzymatic oxidation of galloyl theaflavins (13–15) in the presence of 3 affords oligomeric products with multiple benzotropolone units, such as theadibenzotropolone A (50) and theatribenzotropolone A (51)92,93) (Fig. 6). This reaction is similar to those observed for oxidation of 4.

Thearubigins (TRs)28–31) are reddish-brown pigments of black tea that were designated by Roberts in 1958.29) They were originally characterized as a substance showing a broad streak on two-dimensional paper chromatography. Roberts fractionated the TRs into the three fractions of SI, SIa, and SII based on solubility in ethyl acetate and n-butanol.28,29) Since then, many efforts have been made to prepare “pure” TRs and disclose their chemical structures, because TRs account for an estimated 60–70% of the solids in a typical black tea infusion.32) Now, it is generally accepted that TRs are heterogeneous mixtures of oligomeric or polymeric polyphenols derived from tea catechins and their oxidation products, such as theaflavins and theasinensins.31,94) However, because of a lack of rigorous chemical definition and the difficulties in isolating “pure” TRs, the structures are still confusing and ambiguous. In HPLC analysis (Fig. 7), TRs are detected as a broad Gaussian shape on the baseline, and the difficulty in separating TRs is removal of low-molecular-weight contaminants, the so-called floating peaks on the hump of the HPLC profile (e.g., peaks at tR 22 min in Fig. 7D). To prepare TRs, combinations of Roberts’s liquid–liquid phase separation, Sephadex LH-20,95,96) ODS gel,97,98) and Amberlite XAD-7 chromatography, high-speed counter-current chromatography,99,100) and caffeine precipitation101) have been applied. The caffeine precipitation method is based on the formation of a complex of TRs and caffeine as the main components of so-called tea cream down, which forms as a collection of insoluble particles when a strong infusion of black tea cools. Size-exclusion chromatography using Sephadex LH-20 or Toyopearl HW40F with acidic aqueous acetone containing high concentration of urea was developed for fractionation of proanthocyanidin oligomers and can also be applied to separation of TRs.44,71,82,96) As shown in Fig. 7, almost pure TRs can be obtained by this method, although the yields are not always satisfactory because dimeric oxidation products, such as theasinensins, overlap with TRs with relatively low molecular weights. The TRs from n-butanol and ethyl acetate phases shown in Fig. 7 may correspond to SII and SI TRs, respectively, as designated by Roberts in 1957.28) The UV absorption at 275 nm of the TRs is similar to that of theasinensin A (5), indicating the presence of galloyl moieties (Fig. 7).
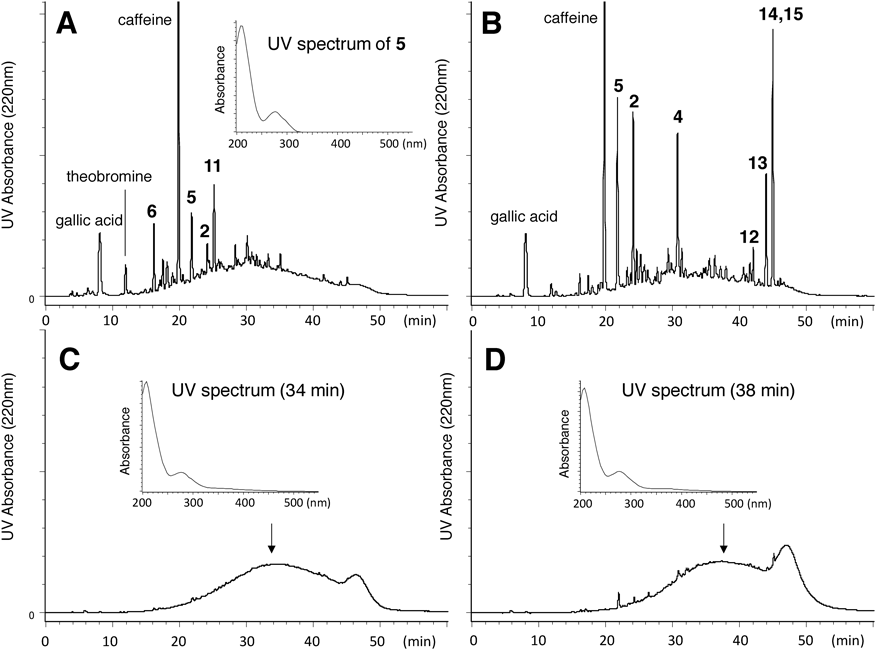
Size-exclusion column chromatography: Sephadex LH-20 with 7 M urea–acetone (4 : 6, v/v; 5 mL/L conc. HCl) followed by removal of urea by Diaion HP20 column chromatography. HPLC conditions are the same as those of Fig. 1. UV spectra obtained by photodiode array detector.
Studies on structures of TRs are also in progress. Ozawa et al. proposed a partial structure containing theasinensins, theaflavins, and proanthocyanidins based on chemical degradation.102) Tea leaves contain dimeric and trimeric proanthocyanidins as minor polyphenols17,27) and thiol degradation of TRs yields thioethers of tea catechins96); therefore, proanthocyanidins may be incorporated into TR structures. However, proanthocyanidins are shown to be stable during tea fermentation and their reactivity is limited during enzymatic oxidation.27) Rather, in vitro experiments strongly suggested that oxidative couplings between B–B, B–A, and B–galloyl rings of four catechins 1–4 are mainly responsible for TR formation. This was supported by LC-MS analysis of fractions of black tea extract, which revealed trimers and tetramers produced by oxidative couplings of 3 with galloyl groups of theaflavins and theasinensins (such as 50–52)103) (Fig. 6 and Chart 6). In addition, trimer 53 having a C–C bond between galloyl and B-ring was produced by enzymatic oxidation of 2.66) Production of the C–C bond via dehydrotheasinensin-type intermediate (53a) was confirmed by isolation of 54 from reaction mixture treated with 1,2-phenylenediamine45) (Chart 6). These trimers and tetramers with molecular weights of 1500–2000 Da are probably components of low-molecular-weight TRs.

The 13C-NMR spectra of TRs obtained by abovementioned size-exclusion chromatography were related to the spectrum of the polymer of 2 prepared by enzymatic oxidation10,104) (Fig. 8). A decrease of pyrogallol-type B-ring methine signals at δC 108 suggests that the oxidative coupling of pyrogallol-type B-ring is mainly responsible for the production of TRs. Strong A-ring (δC 94–99 and δC 153–158) and galloyl signals (δC 109, 120, 138, and 145) probably indicate that most of the A-rings and galloyl groups remained intact. In addition, appearance of the signals attributable to the aromatic methine signals of catechol-type B-ring (δC 115–120, and δC 132) may indicate the incorporation of 3 and 4 by coupling at the A-ring or galloyl ring (Fig. 4). These spectral features are consistent with the production mechanisms for dimeric and trimeric products as described before. However, apparent signals attributable to benzotropolone moieties were not observed.

A: A-ring, B: pyrogallol-type B-ring, C: C-ring; G: galloyl group, and cat-B: catechol-type B-ring.
In addition to oxidative coupling reactions, oxidative cleavage of A-ring with H2O2 has been reported105) (55 in Fig. 9). This reaction possibly participates in thearubigin formation because H2O2 is generated by reduction of oxygen molecule during enzymatic oxidation.106,107) More recently, Kuhnert et al. applied high-resolution mass spectrometric methods to TRs prepared by caffeine precipitation, and proposed that TRs are generated by oxidative cascade-type reactions of catechin dimers, such as theaflavins and theasinensins.108–111) The reactions are mainly oxygenation reactions at aromatic methine carbons of A- and B-rings that increase phenolic hydroxy groups, such as 56 and 57 in Fig. 9, and the cascade-type complex reactions explain the production of numerous inseparable mixtures. However, the hypothetical structures with additional hydroxy groups at A-ring 6 and 8 positions are not consistent with the 13C-NMR spectrum in Fig. 8, which shows large signals attributable to the A-ring methine carbons. Kuhnert reported that the molecular weights of TRs are less than 2000 Da108); however, this is also still contentious,96,112) and the discrepancy may be caused by the different extraction solvents and preparation methods that are used for preparation of TRs.

In considering the biological activities of TRs, the inhibition of digestive enzymes that are related to lifestyle disease, such as lipase and amylase, is an important function of black tea.96) In addition, protection against acetaminophen-induced hepatic and renal injury,113) improvement of the sildenafil-induced delayed gut motility,114) inhibition of benzo[a]pyrene-induced mutagenicity and genotoxicity,115) counteracting the effects of botulinum neurotoxins,116) and a protective effect against the neuromuscular blocking action of botulinum neurotoxins117) have been reported. Furthermore, recent studies have shown that black tea high-molecular-weight polyphenol fraction increases mitochondrial membrane potential and stimulates exercise training-induced improvement of endurance capacity in mice.118–120) Intestinal absorption of TRs is probably not high and it had been hypothesized that TRs might be converted to other metabolites during the intestinal passage.121)
This review summarizes the results obtained from the two major strategies used to disclose the chemistry of black tea polyphenols. One was the use of model fermentation experiments using pure catechins with enzymes or reagents and subsequent analysis of the products and reaction mechanisms. The other was separation of polyphenol components from black tea and subsequent analysis using contemporary technologies, such as LC-MS. Many structures of catechin oxidation products have been determined and the production mechanisms have been clarified; however, TRs, the major polyphenols of black tea, have not been properly characterized to date, and definition of TRs remains an obvious goal. Total flavonoid intake in high black tea-consuming countries may be greatly influenced by TRs. It has been estimated that TRs comprise 48% of the total flavonoids in the diet of the U.K. general population.122) Thus, future efforts using new methodology will be necessary to understand black tea polyphenols.
Our work mentioned in this review were supported by the Japan Society for the Promotion of Science KAKENHI Grant Numbers 17K08338, 26460125, 13J06608, 16K07741, and 25870532.
The authors declare no conflict of interest.