2020 年 68 巻 12 号 p. 1184-1192
2020 年 68 巻 12 号 p. 1184-1192
A novel series of 4-(4-methoxynaphthalen-1-yl)-5-arylpyrimidin-2-amines were designed, synthesized, and evaluated for their anticancer activities. Most of the synthesized compounds exhibited moderate to high antiproliferative activity in comparison to the standard drug cisplatin. Among them, 5i bearing ethoxy at the 4-position of the phenyl was found to be the most active on MCF-7 and HepG2 cancer cell lines, with IC50 values of 3.77 ± 0.36 and 3.83 ± 0.26 µM, respectively. Further mechanism study shown that 5i potently inhibited tubulin polymerization, induced cell cycle arrest at G2/M phase and cell apoptosis in MCF-7 cell line. Furthermore, molecular modeling study suggested that 5i probably binds to the colchicine site of tubulin.
Microtubules are key components of the cytoskeleton in eukaryotic cells, which consist of α- and β-tubulin heterodimers, and are involved in many essential cellular processes, such as formation and maintenance of cell shape, cell signaling, secretion, intracellular transport, and cell division.1–3) During eukaryotic cell division, the microtubule-formed mitotic spindle guide replicated chromosomes separate equally to the two daughter cells. Interruption of the microtubule dynamics can arrest the dividing cell in G2/M phase of the cell cycle and finally result in apoptotic cell death.4) Therefore, tubulin has been recognized as an important target for developing anticancer drugs.5) Despite some antimitotic agents already used in the clinic for treatment of various kinds of cancer, drug resistance, toxicity and side effects remain problems to be solved,6) hence discovery and development of new anticancer agents as tubulin polymerization inhibitors are still in progress.7–9)
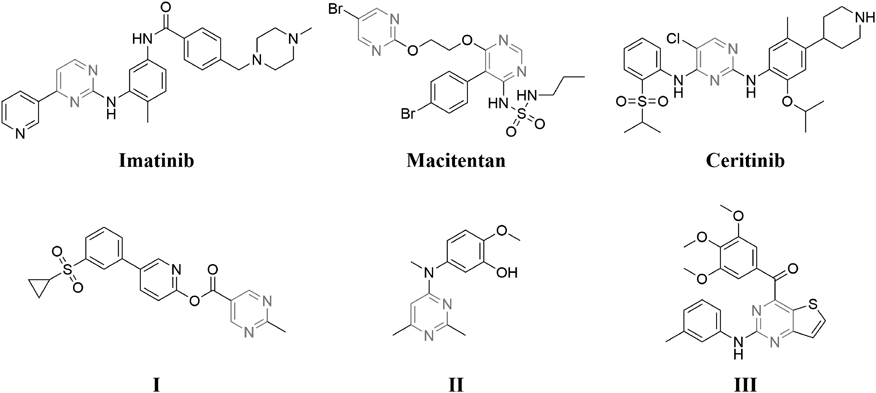
Pyrimidine, a 6-membered heterocyclic ring, containing two nitrogen atoms at 1,3-positions, which is a versatile pharmacophore for designing and development of potent bioactive agents.10) Small molecules with pyrimidine ring have been observed to possess various pharmacological activities, such as anti-inflammatory,11) anti-human immunodeficiency virus (HIV),12) antimicrobial,13) neuroprotective,14) antiviral,15) antimalarial,16) antihypertensive17) and anticancer activity.18–20) Over the last few years, numbers of pyrimidine-containing drugs have been approved for clinical use, such as Imatinib (anticancer), Macitentan (antihypertensive), and Ceritinib (anticancer)21,22) (Fig. 1). Besides, numerous pyrimidine derivatives have been reported to show potent anticancer activity by inhibiting tubulin polymerization. For example, Hansen and colleagues have recently reported that pyridine-pyrimidine amide derivative I (Fig. 1) was a novel microtubule destabilizing agent and can overcome common resistance mechanisms.23) Li and colleagues reported the synthesis of a novel series of 2,6-dimethyl-4-aminopyrimidine derivatives which exhibited potent antiproliferative activities against five human cancer cell lines. Further mechanism studies revealed that compound II (Fig. 1) effectively inhibits tubulin polymerization and disrupt the organization of the cellular microtubule network.24) Tian et al. reported the synthesis of a series of substituted (2-(phenylamino)thieno[3,2-d]pyrimidin-4-yl)(3,4,5-trimethoxy phenyl)methanone analogs and the most active compound III (Fig. 1) displayed potent inhibition against tubulin polymerization with an IC50 value of 4.1 ± 0.1 µM.25)
At present, molecular hybrid-based approach is a useful tool for the design of new tubulin inhibitors.26,27) Based on these findings and in continuation of our search novel tubulin polymerization inhibitors,28,29) we designed the synthesis of a novel series of 4-(4-methoxynaphthalen-1-yl)-5-arylpyrimidin-2-amines (5a–5s) and further evaluated for their antiproliferative and tubulin polymerization inhibitory activities.
The synthesis of compounds 5a–5s was shown in Chart 1. The reaction of commercially available 1-methoxynaphthalene 1 with various substituted phenylacetic acids 2a–2s in the presence of trifluoroacetic anhydride (TFAA) in trifluoroacetic acid (TFA) provided corresponding deoxybenzoins 3a–3s.30) Deoxybenzoins 3a–3s were stirred in N,N-dimethylformamide dimethylacetal (DMF-DMA) at 80 °C for 24 h to provide the key intermediate enamines 4a–4s, which reacted with guanidine hydrochloride in the presence of K2CO3 in acetonitrile to afford the corresponding title compounds 5a–5s. All of the title compounds 5a–5s have not yet been reported in the literature and their structures were elucidated by 1H-NMR, 13C-NMR, and high resolution (HR)MS (see Supplementary materials). The 1H-NMR spectrum of compound 5a shown three singlets at δ 3.39, 3.69, and 3.96 ppm was attributed to the four methoxyl groups in the phenyl moiety and naphthalene nucleus. A two-proton singlet at δ 6.16 ppm was attributed to the aromatic protons of the 3,4,5-trimethoxyphenyl moiety. The six aromatic protons of the naphthalene nucleus appeared as multiplet or doublet in the region of δ 6.69–8.25 ppm. Two singlets signal at δ 5.41 ppm and δ 8.47 ppm were attributed to NH2 and CH of pyrimidin-2-amine ring, respectively. The above analysis clearly confirmed that the 1H-NMR spectrum of compound 5a was in agree with the designed structure. In 13C-NMR spectrum of compound 5a, the number of signals equals the number of different carbons in the molecule.
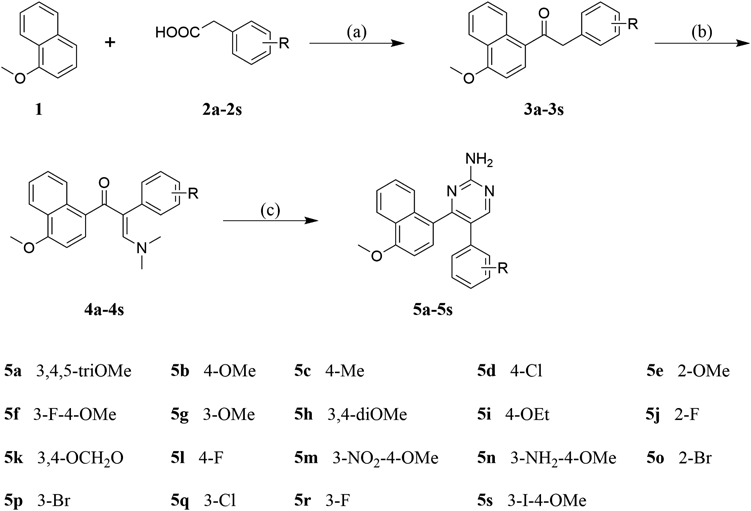
Reagents and conditions: (a) TFAA, TFA, room temperature, 12 h; (b) DMF-DMA, 80 °C, 24 h; (c) guanidine hydrochloride, K2CO3, EtOH, reflux, 24 h.
All of the target compounds 5a–5s were screened for their anti-proliferative activities against human breast cancer cell line (MCF-7) and human liver cancer cell line (HepG2) by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Cisplatin was used as positive control. The antiproliferative results were listed in Table 1. Most of the synthesized compounds (5b–5d, 5f, 5g, 5i–5k, and 5n–5r) exhibited moderate to high antiproliferative activity in comparison to the standard drug cisplatin. Among them, compound 5i bearing ethoxy at the 4-position of the phenyl was found to be the most active compound in this series of compounds. The replacement of the ethoxy group (5i) with methoxy (5b), methyl (5c), chlorine (5d) or fluorine (5l) resulted in a decrease of antiproliferative activity. These results indicated the 4-ethoxy substitution at phenyl ring seems to be the optimal group of this class of compounds. Interestingly, this pharmacophore (4-ethoxyphenyl) was also found in the other tubulin inhibitors.31,32)
| Compd | R | IC50 (µM) | |
|---|---|---|---|
| MCF-7 | HepG2 | ||
| 5a | 3,4,5-triCH3O | >10.0 | >10.0 |
| 5b | 4-CH3O | 7.31 ± 0.32 | 6.49 ± 0.49 |
| 5c | 4-CH3 | 8.12 ± 0.64 | 7.03 ± 0.52 |
| 5d | 4-Cl | 7.62 ± 0.27 | 6.50 ± 0.45 |
| 5e | 2-CH3O | >10.0 | 7.20 ± 0.39 |
| 5f | 3-F-4-CH3O | 7.11 ± 0.48 | 7.33 ± 0.41 |
| 5g | 3-CH3O | 4.97 ± 0.39 | 4.49 ± 0.43 |
| 5h | 3,4-diCH3O | >10.0 | >10.0 |
| 5i | 4-C2H5O | 3.77 ± 0.36 | 3.83 ± 0.26 |
| 5j | 2-F | 8.01 ± 0.64 | 6.22 ± 0.27 |
| 5k | 3,4-OCH2O | 7.65 ± 0.49 | 6.01 ± 0.34 |
| 5l | 4-F | >10.0 | >10.0 |
| 5m | 3-NO2-4-CH3O | >10.0 | >10.0 |
| 5n | 3-NH2-4-CH3O | 8.36 ± 0.53 | 7.11 ± 0.49 |
| 5o | 2-Br | 7.63 ± 0.26 | 7.21 ± 0.35 |
| 5p | 3-Br | 7.11 ± 0.55 | 7.36 ± 0.46 |
| 5q | 3-Cl | 8.05 ± 0.58 | 6.57 ± 0.28 |
| 5r | 3-F | 8.66 ± 0.42 | 7.64 ± 0.52 |
| 5s | 3-I-4-CH3O | >10.0 | >10.0 |
| Cisplatin | 15.24 ± 1.27 | 6.83 ± 0.59 | |
| Colchicine | 0.074 ± 0.013 | 0.058 ± 0.009 | |
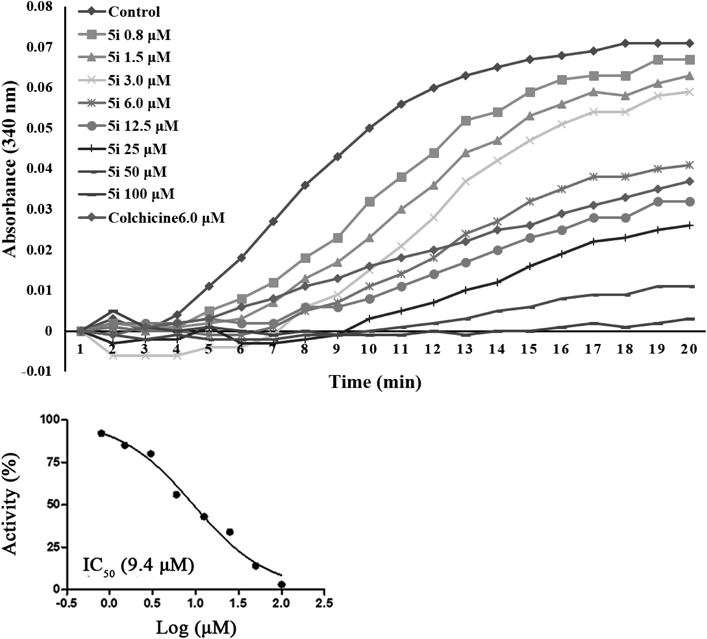
To verify whether these newly synthesized target molecules interacted with microtubule systems, 5i, the most potent compound of this series, was selected and examined for its inhibitory activity on microtubule dynamics. Typical microtubule-destabilizing agent colchicine was chosen as the positive control. As shown in Fig. 2, after incubation with purified pig brain tubulin, the fluorescence emission of compound 5i was less than the control group and similar to the colchicine group, indicating that compound 5i was a microtubule-destabilizing agent. Compound 5i exhibited a dose-dependent inhibition of tubulin polymerization, with an IC50 value of 9.4 µM.
Tubulin polymerization inhibitors usually arrest cell cycle at G2/M phase and lead to cell apoptosis. Effects of compound 5i on MCF-7 cell cycle progress was determined by a flow cytometry analysis to understand whether the antiproliferative effect this class of compounds is on account of cell cycle arrest. As illustrated in Fig. 3, compound 5i could induce cell cycle arrest at G2/M phase in MCF-7 cells. Compared to the control cells incubated with dimethyl sulfoxide (DMSO), the percentage of cells in the G2/M phase increased significantly from 10.82 to 40.79% after treatment with compound 5i for 24 h. The results showed that compound 5i could arrest cell cycle at G2/M phase by targeting tubulin.
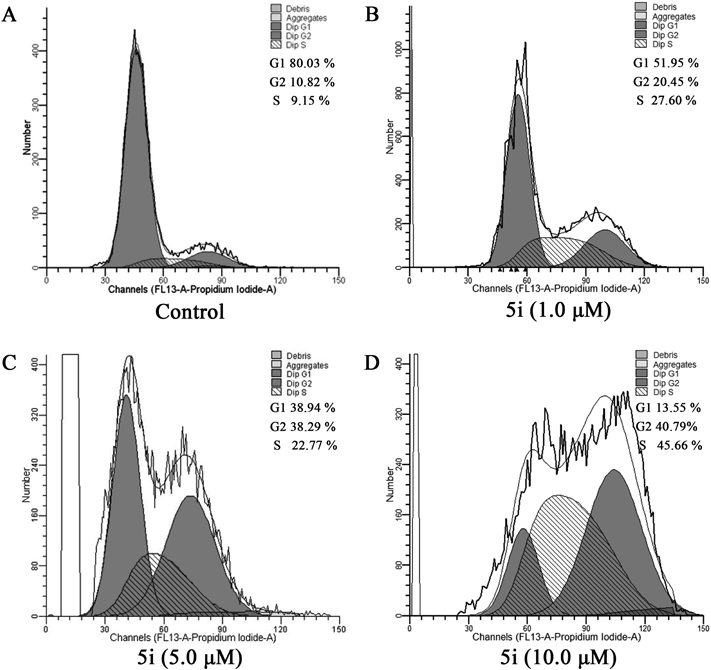
(A) Untreated control cells (DMSO); (B) compound 5i (1.0 µM); (C) compound 5i (5.0 µM); (D) compound 5i (10.0 µM).
Numerous studies have demonstrated that tubulin polymerization inhibitors can induce cancer cell apoptosis.33) The apoptotic effect of compound 5i was further evaluated by Annexin V fluorescein isothiocyanate/propidium iodide (FITC/PI) dual staining assay to understand whether this series of compounds induced cell death in MCF-7 cell line occurred via induction of apoptosis. As shown in Fig. 4, the percentage of apoptotic cells was only 5.8% in the control group, but the total numbers of early (the lower right quadrant) and late (the upper right quadrant) apoptotic cells increased to 19.11, 18.05 and 24.11% after treatment with compound 5i at 1.0, 5.0 and 10.0 µM for 24 h, respectively. These results demonstrated that compound 5i can effectively induce cell apoptosis in MCF-7 cells in a dose-dependent manner.
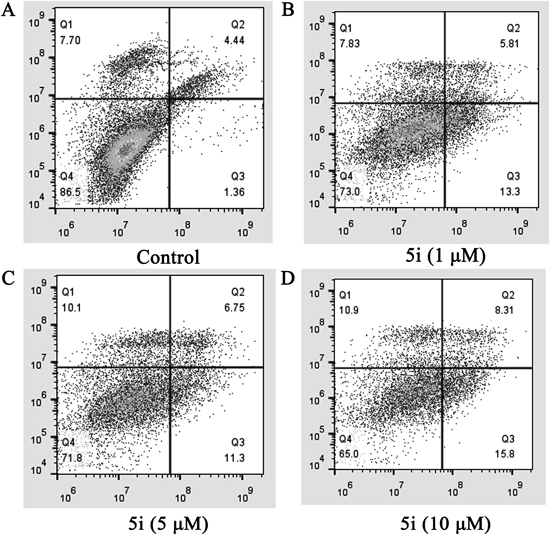
(A) Untreated control cells (DMSO); (B) compound 5i (1.0 µM); (C) compound 5i (5.0 µM); (D) compound 5i (10.0 µM).
There are four unique binding sites in tubulin to which taxanes, vinca alkaloids, laulimalide, and colchicine bind, respectively.34) Based on our experiment results (Fig. 2), compound 5i displayed good tubulin polymerization inhibition activity and showed a similar trend with colchicine. The results indicating that compound 5i was a microtubule-destabilizing agent. On the other hand, numerous pyrimidine derivatives have been reported to show potent anticancer activity by targeting tubulin colchicine binding site.24,25,35) But to the best of our knowledge, there are are no reports of pyrimidine derivatives acting on the vinca alkaloids site of tubulin. Hence, we assume that compound 5i was a tubulin polymerization inhibitor by targeting colchicine binding site.
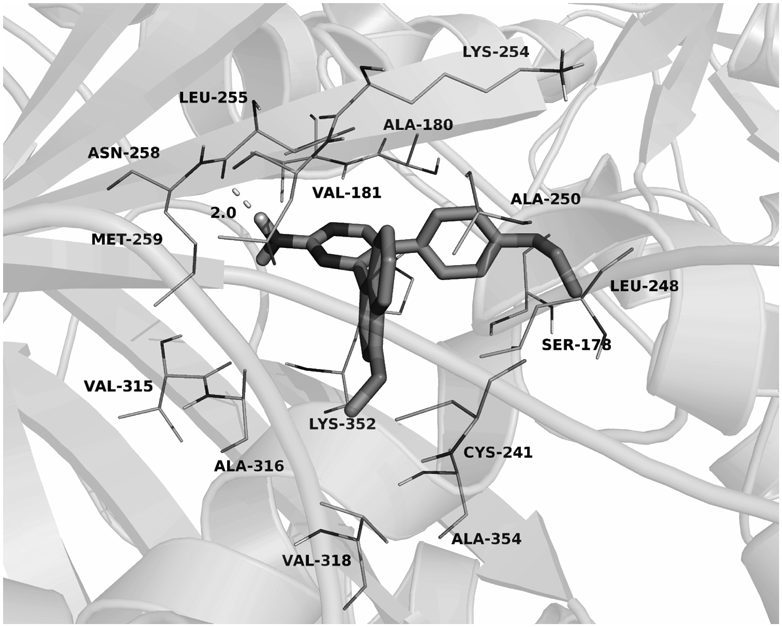
To investigate the binding mechanism of compound 5i with the colchicine binding site of tubulin, molecular docking study was performed. The result was shown in Fig. 5, compound 5i adopted an “L-shaped” conformation in the pocket of the tubulin. The estimated binding energy was −9.5 kcal·mol−1. The naphthyl group of 5i located at the hydrophobic pocket, surrounded by the residues Cys-241, Leu-248, Ala-250, Leu-255, Met-259, Val-315, Ala-316, Val-318, and Ala-354, forming a strong hydrophobic binding. Detailed analysis showed that the phenyl group of 5i formed cation–π interactions with the residues Lys-254 and Lys-352. It was shown that the residue Asn-258 (bond length: 2.0 Å) formed a hydrogen bond with 5i, which was the main interaction between 5i and tubulin.
In conclusion, we designed and synthesized a novel series of 4-(4-methoxynaphthalen-1-yl)-5-arylpyrimidin-2-amines 5a–5s as novel tubulin polymerization inhibitors that interact with colchicine binding site. These newly synthesized compounds were evaluated for their in vitro antiproliferative activity against MCF-7 and HepG2 cancer cell lines. Most of the synthesized compounds exhibited moderate to high antiproliferative activity in comparison to the standard drug cisplatin. Particularly, compound 5i was found to be the most active on MCF-7 and HepG2 cancer cell lines, with IC50 values of 3.77 ± 0.36 and 3.83 ± 0.26 µM, respectively. In vitro tubulin polymerization assay displayed that compound 5i exhibited potent anti-tubulin activity with IC50 value of 9.4 µM. In addition, cellular mechanism studies elucidated that compound 5i induced cell cycle arrest at G2/M phase and cell apoptosis in MCF-7 cell line. Molecular docking study indicated that this class of compounds may bind to colchicine binding site of tubulin. These results indicated that these compounds may have potential for further development as anticancer agents.
All starting materials and reagents were purchased from commercial suppliers. TLC was performed on 0.20 mm Silica Gel 60 F254 plates (Qingdao Ocean Chemical Factory, Shandong, China). NMR spectra were recorded on a JNM spectrometer (JEOL Ltd., Japan) with tetramethylsilane (TMS) as an external reference and reported in parts per million. HPLC-grade acetonitrile and methanol were purchased from Sinopharm Chemical Reagent (Shanghai, China). Water was purified using a water purifier (Voter, Sichuan, China). An HPLC system (Thermo Fisher, UltiMate 3000, U.S.A.) comprised of a system controller, a low pressure gradient module, an infusion pump, a degassing unit, an automatic sample injector, an oven column compartment and a diode array detector. Instrument control was performed using the Chromeleon ChemStation software (7.0, Themo Fisher Scientific).
General Procedure for the Synthesis of 3a–3sA mixture of 1 (12 mmol), phenylacetic acids 2a–2s (8 mmol), and trifluoroacetic anhydride (16 mmol) in trifluoroacetic acid (5 mL) was stirred at room temperature for overnight. After the completion of the reaction, the content was poured into water (100 mL) and extracted with ethyl acetate (100 mL × 3). The combined organic extracts were dried over Na2SO4, filtered, and concentrated. The residue was purified by chromatography to give the product 3a–3s, which containing some impurities. These compounds were used in the next step without further purification.
General Procedure for the Synthesis of 4a–4sA solution of 3a–3s (5 mmol) in DMF-DMA (12 mL) was stirred at 80 °C for 24 h. After cooling to room temperature, the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography to provide product 4a–4s. These compounds were used in the next step without further purification.
General Procedure for the Synthesis of 5a–5sTo a solution of compound 4 (0.5 mmol) in 10 mL ethanol was added K2CO3 (1.5 mmol) and guanidine hydrochloride (0.5 mmol), and the mixture was stirred at reflux for 24 h. After the completion of the reaction, the solvent was concentrated in vacuo and the crude product was purified by chromatography on silica gel (EtOAc/petroleum ether) to give the title compounds 5a–5s, respectively.
4-(4-Methoxynaphthalen-1-yl)-5-(3,4,5-trimethoxyphenyl)pyrimidin-2-amine (5a)Yellow solid, yield 73%; 1H-NMR (400 MHz, CDCl3) δ: 3.39 (s, 6H), 3.69 (s, 3H), 3.96 (s, 3H), 5.41 (s, 2H), 6.16 (s, 2H), 6.69 (d, 1H, J = 8.0 Hz), 7.13 (d, 1H, J = 8.0 Hz), 7.40–7.42 (m, 2H), 7.68–7.70 (m, 1H), 8.22–8.25 (m, 1H), 8.47 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.7, 55.8, 60.9, 103.2, 105.9, 122.3, 125.1, 125.4, 125.5, 126.0, 127.2, 127.4, 128.4, 131.9, 132.1, 136.8, 152.8, 156.0, 158.8, 161.7, 166.2; HRMS (electrospray ionization (ESI)) Calcd for [M + H]+ C24H24N3O4+: 418.1761 found 418.1751; HPLC purity = 97.44%.
4-(4-Methoxynaphthalen-1-yl)-5-(4-methoxyphenyl)pyrimidin-2-amine (5b)Yellow solid, yield 93%; 1H-NMR (400 MHz, CDCl3) δ: 3.66 (s, 3H), 3.96 (s, 3H), 5.35 (s, 2H), 6.59–6.63 (m, 2H), 6.66 (d, 1H, J = 8.0 Hz), 6.88–6.92 (m, 2H), 7.13 (d, 1H, J = 8.4 Hz), 7.38–7.44 (m, 2H), 7.72–7.75 (m, 1H), 8.23–8.25 (m, 1H), 8.41 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.2, 55.6, 103.1, 113.8, 122.2, 125.3, 125.4, 125.6, 127.0, 128.1, 128.2, 128.9, 129.9, 131.9, 156.0, 158.5, 159.3, 161.6, 165.8; HRMS (ESI) Calcd for [M + H]+ C22H20N3O2+: 358.1550 found 358.1544; HPLC purity = 97.15%.
4-(4-Methoxynaphthalen-1-yl)-5-(p-tolyl)pyrimidin-2-amine (5c)Yellow solid, yield 92%; 1H-NMR (400 MHz, d6-DMSO) δ: 2.08 (s, 3H), 3.90 (s, 3H), 6.79 (s, 2H), 6.84–6.86 (m, 5H), 7.14 (d, 1H, J = 8.0 Hz), 7.37–7.42 (m, 2H), 7.58–7.61 (m, 1H), 8.09–8.11 (m, 1H), 8.31 (s, 1H); 13C-NMR (100 MHz, d6-DMSO) δ: 21.0, 56.1, 104.0, 122.1, 124.1, 125.0, 125.7, 125.9, 127.2, 128.2, 128.8, 129.2, 129.3, 131.9, 134.5, 136.0, 155.2, 159.4, 162.8, 165.0; HRMS (ESI) Calcd for [M + H]+ C22H20N3O+: 342.1601 found 342.1595; HPLC purity = 99.96%.
5-(4-Chlorophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5d)Yellow solid, yield 77%; 1H-NMR (400 MHz, d6-DMSO) δ: 3.91 (s, 3H), 6.86 (d, 1H, J = 8.0 Hz), 6.90 (s, 2H), 6.96 (d, 2H, J = 8.4 Hz), 7.09 (d, 2H, J = 8.4 Hz), 7.17 (d, 1H, J = 8.0 Hz), 7.36–7.43 (m, 2H), 7.59–7.61 (m, 1H), 8.09–8.12 (m, 1H), 8.35 (s, 1H); 13C-NMR (100 MHz, d6-DMSO) δ: 56.1, 104.1, 122.2, 123.0, 125.0, 125.8, 127.3, 128.5, 128.6, 128.8, 130.7, 131.8, 136.4, 155.5, 159.4, 163.0, 165.1; HRMS (ESI) Calcd for [M + H]+ C21H17ClN3O+: 362.1055 found 362.1030; HPLC purity = 97.27%.
4-(4-Methoxynaphthalen-1-yl)-5-(2-methoxyphenyl)pyrimidin-2-amine (5e)Yellow solid, yield 44%; 1H-NMR (400 MHz, CDCl3) δ: 3.13 (s, 3H), 3.92 (s, 3H), 5.34 (s, 2H), 6.55–6.60 (m, 2H), 6.76 (t, 1H, J = 7.6 Hz), 7.00–7.04 (m, 2H), 7.07–7.12 (m, 11H), 7.39–7.46 (m, 2H), 7.93–7.96 (m, 1H), 8.19–8.21 (m, 1H), 8.38 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 54.7, 55.5, 102.6, 110.5, 120.5, 121.9, 122.6, 125.2, 125.6, 125.7, 125.7, 126.7, 127.3, 128.6, 128.9, 131.0, 131.9, 155.8, 156.5, 160.0, 161.6, 167.0; HRMS (ESI) Calcd for [M + H]+ C22H20N3O2+: 358.1550 found 358.1536; HPLC purity = 98.79%.
5-(3-Fluoro-4-methoxyphenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5f)Yellow solid, yield 69%; 1H-NMR (400 MHz, CDCl3) δ: 3.75 (s, 3H), 3.98 (s, 3H), 5.38 (s, 2H), 6.62–6.75 (m, 4H), 7.15 (d, 1H, J = 8.0 Hz), 7.39–7.44 (m, 2H), 7.68–7.70 (m, 1H), 8.23–8.26 (m, 1H), 8.38 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.6, 56.1, 103.1, 113.0, 116.3 (d, 1C, J = 18.6 Hz), 122.3, 124.7 (d, 1C, J = 3.6 Hz), 125.1, 125.5, 125.7, 127.1, 127.9, 128.1, 129.6 (d, 1C, J = 7.1 Hz), 131.7, 146.5 (d, 1C, J = 10.6 Hz), 150.7 (d, 1C, J = 244.1 Hz), 156.2, 159.2, 161.8, 165.9; HRMS (ESI) Calcd for [M + H]+ C22H19FN3O2+: 376.1456 found 376.1446; HPLC purity = 96.05%.
4-(4-Methoxynaphthalen-1-yl)-5-(3-methoxyphenyl)pyrimidin-2-amine (5g)Yellow solid, yield 81%; 1H-NMR (400 MHz, CDCl3) δ: 3.41 (s, 3H), 3.95 (s, 3H), 5.42 (s, 2H), 6.51–6.52 (m, 1H), 6.86–6.62 (m, 2H), 6.67 (d, 1H, J = 8.0 Hz), 6.99 (t, 1H, J = 8.0 Hz), 7.14 (d, 1H, J = 8.0 Hz), 7.40–7.42 (m, 2H), 7.73–7.76 (m, 1H), 8.22–8.25 (m, 1H), 8.45 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.0, 55.6, 103.1, 112.8, 114.0, 121.2, 122.2, 125.2, 125.4, 125.6, 125.8, 127.1, 127.9, 128.3, 129.3, 132.0, 138.0, 156.0, 159.2, 159.4, 161.9, 166.0; HRMS (ESI) Calcd for [M + H]+ C22H20N3O2+: 358.1550 found 358.1535; HPLC purity = 97.12%.
5-(3,4-Dimethoxyphenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5h)Yellow solid, yield 57%; 1H-NMR (400 MHz, CDCl3) δ: 3.24 (s, 3H), 3.75 (s, 3H), 3.96 (s, 3H), 5.34 (s, 2H), 6.32 (d, 1H, J = 1.6 Hz), 6.63–6.70 (m, 3H), 7.13 (d, 1H, J = 8.0 Hz), 7.38–7.44 (m, 2H), 7.70–7.72 (m, 1H), 8.22–8.25 (m, 1H), 8.46 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.3, 55.7, 55.8, 103.2, 110.8, 112.1, 120.7, 122.3, 125.1, 125.5, 125.6, 125.8, 127.2, 127.7, 128.3, 129.0, 131.9, 147.9, 148.2, 156.0, 158.9, 161.4, 166.0; HRMS (ESI) Calcd for [M + H]+ C23H22N3O3+: 388.1656 found 358.1641; HPLC purity = 97.38%.
5-(4-Ethoxyphenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5i)Yellow solid, yield 40%; 1H-NMR (400 MHz, d6-DMSO) δ: 1.18 (t, 3H, J = 6.8 Hz), 3.79 (q, 2H, J = 6.8 Hz), 3.92 (s, 3H), 6.60 (d, 2H, J = 8.8 Hz), 6.87–6.90 (m, 3H), 7.21 (d, 1H, J = 8.0 Hz), 7.39–7.44 (m, 2H), 7.63–7.65 (m, 1H), 8.10–8.12 (m, 1H), 8.41 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 14.8, 55.8, 63.5, 103.0, 114.8, 122.6, 124.6, 125.0, 125.3, 125.8, 125.9, 127.7, 129.7, 129.8, 131.1, 156.3, 157.7, 158.8; HRMS (ESI) Calcd for [M + H]+ C22H22N3O2+: 372.1707 found 372.1691; HPLC purity = 97.35%.
5-(2-Fluorophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5j)Yellow solid, yield 60%; 1H-NMR (400 MHz, CDCl3) δ: 3.94 (s, 3H), 5.54 (s, 2H), 6.63 (d, 1H, J = 8.0 Hz), 6.80–6.92 (m, 3H), 7.04–7.07 (m, 1H), 7.15 (d, 1H, J = 8.0 Hz), 7.39–7.42 (m, 2H), 7.79–7.81 (m, 1H), 8.20–8.22 (m, 1H), 8.41 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.6, 102.9, 115.5 (d, 1C, J = 22.3 Hz), 119.9, 122.1, 123.9, 124.3 (d, 1C, J = 15.0 Hz), 125.2, 125.4, 125.6, 127.0, 127.8, 127.9 (d, 1C, J = 7.8 Hz), 129.3, 131.6, 131.7, 156.1, 158.5 (d, 1C, J = 245.2), 160.0 162.1, 166.8; HRMS (ESI) Calcd for [M + H]+ C21H17FN3O+: 346.1350 found 358.1328; HPLC purity = 96.87%.
5-(Benzo[d][1,3]dioxol-5-yl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5k)Yellow solid, yield 59%; 1H-NMR (400 MHz, CDCl3) δ: 3.97 (s, 3H), 5.41 (s, 2H), 5.81 (s, 2H), 6.43–6.56 (m, 3H), 6.69 (d, 1H, J = 8.0 Hz), 7.16 (d, 1H, J = 8.0 Hz), 7.38–7.44 (m, 2H), 7.70 (d, 1H, J = 8.8 Hz), 8.22–8.25 (m, 1H), 8.38 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.6, 101.0, 103.1, 108.3, 109.3, 122.2, 122.5, 125.2, 125.4, 125.6, 125.7, 127.1, 128.0, 128.2, 130.5, 131.7, 146.6, 147.4, 156.1, 159.2, 161.6, 165.8; HRMS (ESI) Calcd for [M + H]+ C22H18N3O3+: 372.1343 found 372.1347; HPLC purity = 96.44%.
5-(4-Fluorophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5l)White solid, yield 67%; 1H-NMR (400 MHz, CDCl3) δ: 4.04 (s, 3H), 6.81–6.87 (m, 2H), 6.96 (dt, 1H, J = 8.0 Hz, 1.6 Hz), 7.05–7.10 (m, 1H), 7.16–7.21 (m, 1H), 7.41–7.45 (m, 1H), 7.47–7.51 (m, 2H), 7.69 (d, 1H, J = 8.0 Hz), 8.32 (d, 1H, J = 8.4 Hz), 8.68 (d, 1H, J = 2.8 Hz); 13C-NMR (100 MHz, CDCl3) δ: 55.8, 103.4, 112.0, 116.2, 117.3, 117.7 (d, 1C, J = 14.1 Hz), 122.5, 124.4, 125.1, 125.8, 125.9, 127.7, 129.4, 129.5, 130.1, 132.0, 151.5, 157.5, 158.6 (d, 1C, J = 247.2 Hz), 166.1; HRMS (ESI) Calcd for [M + H]+ C21H17FN3O+: 346.1350 found 346.1339; HPLC purity = 97.66%.
5-(4-Methoxy-3-nitrophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5m)Yellow oil, yield 86%; 1H-NMR (400 MHz, CDCl3) δ: 3.79 (s, 3H), 3.99 (s, 3H), 6.06 (s, 2H), 6.68 (d, 1H, J = 8.8 Hz), 6.72 (d, 1H, J = 8.0 Hz), 6.96 (dd, 1H, J = 8.8 Hz, 2.4 Hz), 7.18 (d, 1H, J = 8.0 Hz), 7.37–7.44 (m, 2H), 7.60–7.63 (m, 2H), 8.23–8.25 (m, 1H), 8.36 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.7, 56.5, 103.2, 113.4, 122.5, 123.1, 124.8, 125.5, 125.6, 125.7, 126.9, 127.4, 128.4, 128.9, 131.4, 134.6, 139.2, 152.0, 156.5, 158.2, 161.9, 166.6; HRMS (ESI) calcd for [M + H]+ C22H19N4O4+: 403.1401 found 403.1375; HPLC purity = 98.80%.
5-(3-Amino-4-methoxyphenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5n)Yellow oil, yield 94%; 1H-NMR (400 MHz, CDCl3) δ: 3.71 (s, 3H), 3.97 (s, 3H), 6.26 (dd, 1H, J = 8.4 Hz, 2.0 Hz), 6.31 (d, 1H, J = 2.4 Hz), 6.47 (d, 1H, J = 8.0 Hz), 6.68 (d, 1H, J = 8.0 Hz), 7.22–7.25 (m, 2H), 7.42–7.45 (m, 2H), 7.71–7.74 (m, 1H), 8.18 (s, 1H), 8.24–8.27 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.5, 55.8, 103.0, 110.4, 115.0, 119.3, 122.6, 124.6, 125.1, 125.5, 125.6, 125.9, 126.3, 127.7, 129.8, 131.2, 135.8, 147.4, 156.0, 157.6; HRMS (ESI) Calcd for [M + H]+ C22H21N4O2+: 373.1659 found 373.1648; HPLC purity = 96.13%.
5-(2-Bromophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5o)Yellow oil, yield 51%; 1H-NMR (400 MHz, CDCl3) δ: 3.91 (s, 3H), 6.40 (s, 2H), 6.60 (d, 1H, J = 8.0 Hz), 6.85–6.88 (m, 1H), 6.94–6.96 (m, 2H), 7.15 (d, 1H, J = 8.0 Hz), 7.39–7.47 (m, 3H), 7.87 (d, 1H, J = 8.0 Hz), 8.19 (d, 1H, J = 8.0 Hz), 8.31 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.6, 102.8, 122.2, 124.3, 124.6, 125.3, 125.5, 126.8, 127.1, 127.2, 128.4, 129.2, 131.8, 132.0, 132.9, 136.7, 156.5, 158.1, 160.8, 167.6; HRMS (ESI) Calcd for [M + H]+ C21H17BrN3O+: 406.0550 found 406.0526; HPLC purity = 96.78%.
5-(3-Bromophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5p)Yellow solid, yield 68%; 1H-NMR (400 MHz, CDCl3) δ: 3.97 (s, 3H), 5.44 (s, 2H), 6.69 (d, 1H, J = 8.0 Hz), 6.76 (d, 1H, J = 8.0 Hz), 6.86 (t, 1H, J = 8.0 Hz), 7.14 (d, 1H, J = 8.0 Hz), 7.19 (d, 1H, J = 8.0 Hz), 7.27 (s, 1H, J = 2.0 Hz), 7.41–7.45 (m, 2H), 7.70–7.72 (m, 1H), 8.24–8.26 (m, 1H), 8.41 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.7, 103.1, 122.3, 122.4, 124.6, 125.0, 125.5, 125.7, 127.2, 127.4, 127.7, 128.5, 129.7, 130.0, 131.4, 131.7, 138.8, 156.3, 159.2, 161.8, 166.1; HRMS (ESI) Calcd for [M + H]+ C21H17BrN3O+: 406.0550 found 406.0515; HPLC purity = 99.06%.
5-(3-Chlorophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5q)Yellow solid, yield 48%; 1H-NMR (400 MHz, CDCl3) δ: 3.97 (s, 3H), 5.35 (s, 2H), 6.68 (d, 1H, J = 8.0 Hz), 6.74 (d, 1H, J = 8.0 Hz), 6.93 (t, 1H, J = 8.0 Hz), 7.04–7.06 (m, 1H), 7.10 (t, 1H, J = 2.0 Hz), 7.14 (d, 1H, J = 8.0 Hz), 7.41–7.44 (m, 2H), 7.71–7.74 (m, 1H), 8.24–8.26 (m, 1H), 8.42 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.7, 103.1, 122.3, 124.8, 125.1, 125.5, 125.7, 127.0, 127.2, 127.5, 128.4, 128.6, 129.4, 131.7, 134.1, 138.6, 156.3, 159.3, 161.9, 166.1; HRMS (ESI) Calcd for [M + H]+ C21H17ClN3O+: 362.1055 found 362.1038; HPLC purity = 99.56%.
5-(3-Fluorophenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5r)Yellow solid, yield 67%; 1H-NMR (400 MHz, CDCl3) δ: 3.97 (s, 3H), 5.52 (s, 2H), 6.69–6.77 (m, 4H), 7.00–7.05 (m, 1H), 7.17 (d, 1H, J = 8.0 Hz), 7.38–7.45 (m, 2H), 7.70–7.72 (m, 1H), 8.24–8.26 (m, 1H), 8.42 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.7, 103.1, 113.8 (d, 1C, J = 20.5 Hz), 115.5 (d, 1C, J = 21.9 Hz), 122.4, 124.6, 124.8, 125.0, 125.5, 125.7, 127.2, 127.4, 128.4, 129.8, 131.7, 138.8, 156.4, 159.1, 161.3 (d, 1C, J = 244.5 Hz), 161.8, 166.1; HRMS (ESI) Calcd for [M + H]+ C21H17FN3O+: 346.1350 found 346.1340; HPLC purity = 99.31%.
5-(3-Iodo-4-methoxyphenyl)-4-(4-methoxynaphthalen-1-yl)pyrimidin-2-amine (5s)Yellow oil, yield 79%; 1H-NMR (400 MHz, CDCl3) δ: 3.70 (s, 3H), 3.96 (s, 3H), 5.51 (s, 2H), 6.38 (d, 1H, J = 8.8 Hz), 6.68 (d, 1H, J = 8.0 Hz), 6.72 (dd, 1H, J = 8.8 Hz, 2.0 Hz), 7.13 (d, 1H, J = 8.0 Hz), 7.40–7.43 (m, 2H), 7.54 (d, 1H, J = 2.0 Hz), 7.68–7.70 (m, 1H), 8.23–8.25 (m, 1H), 8.37 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 55.7, 56.3, 85.6 103.1, 110.4, 122.3, 124.1, 125.2, 125.5, 125.6, 127.2, 127.8, 128.2, 130.2, 131.0, 131.7, 139.2, 156.1, 157.0, 159.2, 161.9, 165.8; HRMS (ESI) Calcd for [M + H]+ C22H19IN3O2+: 484.0517 found 484.0485; HPLC purity = 98.74%.
HPLCMethods 1: Separation was achieved on ACE Excel-5-C18 columns (250 × 4.6 mm, Phenomenex, Guangzhou, China) in series at a flow rate of 1 mL·min−1. The mobile phase was consisted of eluent A (acetonitrile) and eluent B (water) with a gradient elution as follows: 0–15 min, 5–95% A. The column temperature was maintained at 35 °C. The injection volume was 10 µL, and the UV detector was set at 190 to 325 nm. Compounds 5a, 5b, 5e, 5f, 5g, 5h, 5k, 5m, 5n, and 5r were used in methods 1.
Methods 2: Separation was achieved on ACE Excel-5-C18 columns (250 × 4.6 mm, Phenomenex, Guangzhou, China) in series at a flow rate of 1 mL·min−1. The mobile phase was consisted of eluent A (methanol) and eluent B (water) with a gradient elution as follows: 0–30 min, 5–95% A. The column temperature was maintained at 35 °C. The injection volume was 10 µL, and the UV detector was set at 190 to 325 nm. Compounds 5c, 5d, 5i, 5j, 5l, 5o, 5p, 5q, and 5s were used in methods 2. As shown in Fig. S12, there are solvent peaks for water and methanol.
Biological ScreeningIn Vitro Anticancer AssayHuman cancer cells (MCF-7 or HepG2) were seeded in 96 well plates and cultured for 24 h. Then, the cells were treated with different concentrations of the compounds for 48 h. Finally, cell viability was determined by the MTT method.
In Vitro Tubulin Polymerization AssayThe effects of compounds 5i and colchicine on the polymerization of tubulin were determined by using in vitro tubulin polymerization assay. In brief, different concentrations of test compounds were incubated with purified pig tubulin and buffer containing 100 mM PIPES, 1 mM MgCl2, 1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM guanosine 5′-triphosphate (GTP) and 5% glycerol at 37 °C. Then, the assembly of tubulin was monitored by SPECTRA MAX 190 (Molecular Device) spectrophotometer.
Cell Cycle AnalysisHuman breast cancer cells (MCF-7) were seeded into a 6-well plate and incubated for 24 h at 37 °C, and then were exposed to tested compound (1.0, 5.0 and 10.0 µM) or DMSO for 24 h. Cells were harvest, washed with phosphate buffered saline (PBS), fixed and stained with propidium iodide dye. The samples were then analysed by flow cytometry (TASC240, U.S.A.).
Cell Apoptosis AnalysisHuman breast cancer cells (MCF-7) were seeded into a 6-well plate and incubated for 24 h at 37 °C. After exposed to tested compound (1.0, 5.0 and 10.0 µM) or DMSO for 24 h, cells were harvest, washed with PBS, and stained with Annexin-V and PI. Then cells were subjected to flow cytometry analysis.
Molecular Docking StudiesMolecular docking studies were performed to investigate the binding mode between compound 5i and tubulin using Autodock Vina 1.1.2 (http://vina.scripps.edu/). The three-dimensional (3D) coordinate of the β-tubulin (PDB ID: 1SA0) was downloaded from Protein Data Bank. The search grid of tubulin was identified as center_x: 118.921, center_y: 89.718, and center_z: 5.932 with dimensions size_x: 15, size_y: 15, and size_z: 15. The value of exhaustiveness was set to 20. The best-scoring pose was visually analyzed using PyMoL 1.7.6 software (www.pymol.org).
This work was supported by Guizhou Science and Technology Department ([2016]5613/5677), Local Science and Technology Project Guided by Central Administration ([2018]4006).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.