2020 Volume 68 Issue 12 Pages 1201-1209
2020 Volume 68 Issue 12 Pages 1201-1209
Regioselectivity for intramolecular Diels–Alder (IMDA) reactions of 6-acetoxy-6-alkenylcyclohexa-2,4-dien-1-ones that were formed by oxidation of 2-alkenylphenols with lead tetraacetate in acetic acid were studied. Bridged regioselectivity was observed in the IMDA reactions of 6-acetoxy-6-alkenylcyclohexa-2,4-dien-1-ones having a dienophile part which could conjugate with an aromatic group. Bridged seven- and eight-membered rings and bicyclo[2.2.2]octane skeletons were constructed by the present IMDA reactions. Density functional theory (DFT) calculations suggested that conjugation of the dienophile with neighboring aromatic groups lowered the highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) energy gap and preceded bridged [4 + 2] adducts.
Intramolecular Diels–Alder (IMDA) reactions1–6) are important reactions for organic synthesis of complex molecules having a six-membered ring with multiple stereogenic centers because of their predictable regiochemistry and stereochemistry. There are two modes in IMDA reactions, which give fused or bridged compounds (Chart 1).

Fused compounds are generally formed by IMDA reactions because of their straight arrangement between diene and dienophile parts.7–10) On the other hand, bridged compounds were obtained selectively in a few cases. Greuter reported that the IMDA reaction of (Z)-1 that was formed by isomerization (E)-1 gave a bridged seven-membered compound 2 in 35% yield as a main product along with a fused isomer 3 in 5% yield11–13) (Chart 2).

As another bridged-selective IMDA reaction, Cook and Danishefsky reported that oxidative dearomatization of a phenol14,15) in the presence of an allenyl alcohol gave a bridged compound 4 instead of a fused one 516) (Chart 3). De Lera and colleagues proposed that stabilization of diradical species in the IMDA reaction was a key factor for the bridged selectivity.17)

Although effects of a Lewis acid18) and effects of electronic factors19) for bridged selectivity have been reported, there has been limited information for favoring bridged products in IMDA reactions.20) Here, we report the effects of the structure of the tether part between an alkene and a diene in 7, which were formed by oxidation of a 2-alkenyl phenol 6, in an IMDA reaction to a fused product 8 or a bridged one 9 (Chart 4).

Yates and Auksi reported that Wessely oxidation21–23) of 6a and 6b with Pb(OAc)4 in acetic acid followed by IMDA reactions of 7a and 7b gave fused adducts 8a24) and 8b18) in low yields (Table 1, entries 1 and 2). We investigated the effects of the length of a tether by using 2-alkenylphenol 6c. Wessely oxidation of 6c was found to give the corresponding 7c in 28% yield (entry 3). However, an IMDA reaction of 7c gave complex mixtures probably due to flexibility of the tether part.
 | |||||
|---|---|---|---|---|---|
| Entry | 6 | Ox cond. | 7 (Yield)a) | DA cond. | 8 or 9 (Yield)a) |
| 1 | 6a | r.t., 12 h | Not isolated | Xylenes, reflux, 24 h | 8a (28%)b,c) |
| 2 | 6b | r.t., 24 h | 7b (30%) | Xylenes, ∆ | 8b (16%)d) |
| 3 | 6c | r.t., 24 h | 7c (28%) | Neat, 170 °C, 20 h | complex |
| 4 | 6d | r.t., 24 h | Not isolated | — | 8d (23%)c) |
| 5 | 6e | r.t., 1 h | 7e (23%) | Xylenes, reflux, 16 h | 9e (73%) |
Next, IMDA reactions of phenols 6d, e, which had a 2-vinylphenyl group, were studied. Wessely oxidation of a phenol 6d at room temperature directly gave a fused Diels–Alder product 8d in 23% yield (entry 4). The corresponding dienone 7d and a bridged [4 + 2] adduct 9d were not obtained. The structure of 8d was determined unambiguously by its X-ray crystallography.25) A one-carbon-homologated phenol 6e was oxidized to 7e and the IMDA reaction of 7e in refluxing xylenes gave a bridged product 9e in 73% yield without formation of a fused product 8e (entry 5). The structure of 9e was determined by heteronuclear multiple bond connectivity (HMBC) correlations.25) Therefore, it was found that one-carbon homologation (7d to 7e) dramatically changed the fused or bridged selectivity in the IMDA reactions.
In order to study further effects of an extended tether, phenols 6f and 6g having a maleimide group and a 2-nitroethynyl group as a strong dienophile part were designed since difficulty of IMDA reactions of dienones 7 having a longer tether was expected (For structures of 6f and 6g, see Charts 5 and 6). Methoxy groups of 6g were introduced for synthesis of a colchicine26)-like structure, 8g or 9g.
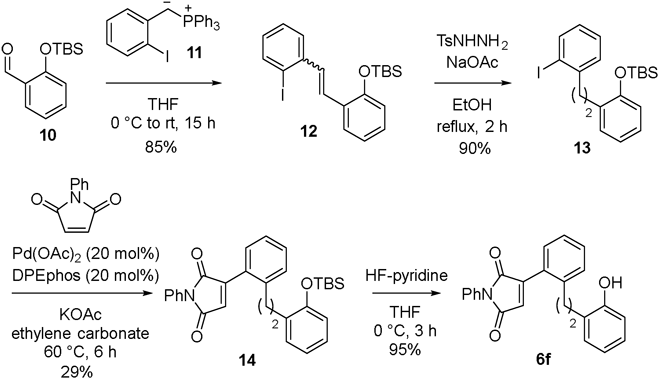

A phenol 6f having a maleimide group was prepared as shown in Chart 5. Wittig reaction of aldehyde 10 with 11 gave a stilbene derivative 12 in 85% yield. Catalytic hydrogenation of 12 by H2 and Pd/C or PtO2 did not give a desired product 13, but hydrogenation with TsNHNH2/NaOAc afforded 13 in 90% yield.27) Heck reaction of aryl iodide 13 with maleimide gave a Heck product 14 in 29% yield by using 20 mol% of Pd(OAc)2 and 20 mol% of DPEphos ((oxydi-2,1-phenylene)bis(diphenylphosphine)) in the presence of KOAc in ethylene carbonate.28) The use of dppe (ethylenebis(diphenylphosphine)) instead of DPEphos did not provide 14. The low yield of 14 might result from a labile maleimide unit under the Heck reaction conditions. Removal of the tert-butyldimethylsilyl (TBS) group of 14 with HF-pyridine gave the phenol 15 in 95% yield.
Preparation of 6g is shown in Chart 6. Wittig reaction of aldehyde 10 with 15 afforded a stilbene derivative 16 in 95% yield. Reduction of 16 using TsNHNH2 and NaOAc gave a 1,2-diphenylethane 17 in 91% yield.27) Halogen-lithium exchange followed by a reaction with N,N-dimethylformamide (DMF) gave an aldehyde 18. Nitro aldol reaction of 18 with nitromethane provided a nitroalkene 19 in 40% in two steps. Removal of the TBS group of 19 with tetrabutylammonium fluoride (TBAF) gave 6g quantitatively.
With phenols 6f,g having strong dienophiles in hand, fused/bridged regioselectivity in their IMDA reactions were investigated. Oxidation of 6f with Pb(OAc)4 in AcOH afforded an acetoxy-2,4-cyclohexadien-1-one 7f in 34% yield (Chart 7). The IMDA reaction of 7f at 170 °C for 3.5 h without using a solvent gave a bridged [4 + 2] adduct 9f in 52% yield, and a fused adduct 8f was not detected. The structure of 9f was determined by HMBC correlations and nuclear Overhauser effect (NOE).25)

Wessely oxidation of 6g with Pb(OAc)4 gave dienone 7g in 43% yield (Table 2). However, the IMDA reaction of 7g did not proceed at 165 °C (entry 1). Attempted activation of the nitro group with a thiourea catalyst 2029,30) was not effective (entry 2). Danishefsky and colleagues reported that the use of 2,2,2-trifluoroethanol as a solvent improved the efficiency of Diels–Alder reactions of nitroalkenes.31) Investigation of the effects of some fluoroalkyl alcohols as solvents revealed that 2,2,2-trifluoroethanol was the most effective for the IMDA reaction of 7g (entry 3). In this case, a bridged IMDA product 9g was formed selectively, and 19% of the starting material 7g was recovered with many unidentified byproducts. It was assumed that 2,2,2-trifluoroethanol activated the nitro group of 7g by its hydrogen bond.
 | ||
|---|---|---|
| Entry | Conditions | 8g or 9g (Yield)a) |
| 1 | Neat, 165 °C, 3 h | 0 |
| 2 | 20b) (20 mol%), toluene, reflux, 3 d | 0 |
| 3 | CF3CH2OH, 130 °C, MW, 18 h | 9g (25%) |
| 4 | CF3CF2CH2OH, 130 °C, MW, 18 h | 9g (21%) |
| 5 | (CF3)2CHOH, 130 °C, MW, 18 h | 9g (12%) |
a) Isolated yield. b) 
Experimental results showed that the IMDA reaction of compound 7d that had no methylene unit between a 2-vinylphenyl group and a dienone group gave the fused compound 8d selectively, while the IMDA reaction of compounds 7e–g that had the one or two methylene units afforded bridged compounds 9e–g. In order to clarify the reason for the change of selectivity, density functional theory (DFT) calculations (B3LYP/6-31(d,p) or B3LYP/6-31G(d))32,33) of IMDA reactions of 7d, e, b, f were performed (Fig. 1). DFT calculation (B3LYP/6-31(d,p)) of the IMDA reaction of 7d to the fused compound 8d or the bridged compound 9d is shown in Fig. 1(a). TS 21, which gives fused 8d, is 6.7 kcal/mol lower than TS 22, which gives bridged 9d. The fused compound 8d is thermodynamically stable in 7.2 kcal/mol compared with the bridged compound 9d. Therefore, the reaction path from 7d to fused 8d is preferred both in kinetic and thermodynamic aspects. In fact, the fused compound 8d was selectively formed from 7d (Table 1, entry 4).

DFT calculation (B3LYP/6-31(d)) of the IMDA reactions of 7e indicated that TS 23, which gives the fused compound 8e, is less stable (2.2 kcal/mol) than TS 24, which gives the bridged product 9e (Fig. 1(b)). It was also pointed out that fused 8e is more stable (5.5 kcal/mol) than bridged 9e. The experimental result of selective formation of the bridged product 9e from 7e (Table 1, entry 5) suggests that the reaction path is kinetically controlled, reflecting the lower energy of TS 24.
Judging from the result of DFT calculation (B3LYP/6-31(d)) of the IMDA reaction of 7b (Fig. 1(c)), it is expected that a fused product 8b would be preferably formed rather than a bridged product 9b since TS 25, which gives 8b, is favored by 3.5 kcal/mol than TS 26. This calculation is supported by the experimental result that the IMDA reaction of 7b gave 8b in 16% yield and 9b was not isolated (Table 1, entry 2).18) Therefore, experimental results and the calculations of IMDA reactions of 7b and 7e suggest that the presence of the phenyl ring in the tether structure of 7e is important for favoring bridged selectivity through TS 24.
As in the case of 7e, DFT calculation (B3LYP/6-31(d)) of the IMDA reaction of 7f (Fig. 1(d)) pointed out that TS 28, which gives the bridged product 9f, is lower in 3.7 kcal/mol than TS 27, which gives a fused compound 8f. The experimental result showing selective formation of 9f (Chart 7) reflects the lower energy of TS 28. In TS 28, the phenyl group and the maleimide group have a highly co-planar conformation (see Supplementary Material, Figure SI2), while these two groups are twisted in TS 27 (see Supplementary Material, Figure SI1). Conjugation between the phenyl and maleimide groups lowers the lowest unoccupied molecular orbital (LUMO) energy of TS 28, and this results in lowering the highest occupied molecular orbital (HOMO)-LUMO energy gap for IMDA reactions. Similar conjugation between the phenyl group and the dienophile is considered in TS 24 from compound 7e. However, in the case of 7d, conjugation of the vinyl group with the phenyl group was difficult both in TS 21 and TS 22 because of their restricted transition states.
In conclusion, we found that the presence of a phenyl ring which could conjugate a dienophile part favored bridged-selective IMDA reactions of alkenyl dienones formed by oxidation of 2-alkenylphenols except in the case of a short tether. DFT calculations suggested that a transition state that gave a bridged product had a co-planar conformation between a phenyl ring and a dienophile part, and conjugation of the two co-planar groups lowered the HOMO-LUMO energy gap for IMDA reactions. Bridged-selective construction of an eight-membered ring in 9f, g was performed by IMDA reactions. These results will help in the planning of bridged-selective IMDA reactions for synthesis of complex molecules.
All melting points were determined on Yanagimoto micro melting point apparatus and are uncorrected. IR spectra were recorded on Horiba IR-710. 1H-NMR spectra were recorded on a JEOL JNM ECA600 (600 MHz) or a JEOL JNM ECS400 (400 MHz) spectrometer at room temperature (r.t.); chemical shifts (δ) are reported in parts per million relative to tetramethylsilane. Splitting patterns are designated as s, singlet; d, doublet; t, triplet; q, quartet; sept, septet; m, multiplet; br, broad. 13C-NMR spectra were recorded on a JEOL JNM ECA600 (150 MHz) or a JEOL JNM ECS400 (100 MHz) spectrometer with complete proton decoupling. chemical shifts are reported in parts per million relative to tetramethylsilane with the solvent resonance as the internal standard CDCl3. High resolution (HR)MS were recorded on a JEOL JMS-T100TD. X-ray crystallographic analysis was performed on Rigaku R-AXIS RAPIDII-S. Analytical TLC was performed on Merck precoated TLC plates (silica gel 60 GF254, 0.25 mm). Silica gel column chromatography was carried out on silica gel 60N (Kanto Kagaku Co., Ltd., spherical neutral, 63–210 or 40–50 µm). Preparative thin-layer chromatography (PTLC) was carried out on silica gel Wakogel B-5F. All reactions were carried out under nitrogen in dried glassware with magnetic stirring. Microwave experiments were carried out in sealed vessels in a synthesis reactor (Biotage Initiator 2.5). Compounds 6b,c were prepared by the reported method.34)
Synthesis of 2-(2-Vinylphenyl)phenol (6d)35)To a cooled suspension of methyltriphenylphosphonium iodide (128 mg, 0.318 mmol) in tetrahydrofuran (THF) (3 mL) at 0 °C was added potassium tert-butoxide (56 mg, 0.50 mmol) under nitrogen. After 30 min, 6H-benzo[c]chromen-6-one36) (25 mg, 0.15 mmol) which was prepared from phenyl 2-bromobenzoate was added. The reaction was warmed to r.t. and the mixture was stirred for 15 h. After dropping 1 N HCl until pH below 7, the aqueous phase was separated and extracted with DCM. The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (Hexane/EtOAc) to give the product 6d (20 mg, 0.12 mmol, 81%). Compound 6d: 1H-NMR (CDCl3, 400 MHz) δ: 7.72 (1H, d, J = 7.2 Hz), 7.49–7.20 (4H, m), 7.13 (1H, d, J = 6.8 Hz), 6.99 (2H, d, J = 6.8 Hz), 6.56 (1H, dd, J = 17.6, 11.2 Hz), 5.75 (1H, d, J = 17.6 Hz), 5.22 (1H, d, J = 11.2 Hz), 4.80 (1H, s); 13C-NMR (CDCl3, 100 MHz) δ: 152.6, 136.7, 134.9, 134.4, 130.9, 130.7, 129.4, 128.6, 128.4, 126.7, 125.6, 120.5, 115.9, 115.5.
Synthesis of 2-(2-Vinylbenzyl)-1-(tert-butyldimethylsilyloxy)benzene (6e)A mixture of 1-bromomethyl-2-tert-butyldimethylsilyloxybenzene (1.38 g, 4.59 mmol), 2-formylphenylboronic acid (688 mg, 4.59 mmol), Pd(PPh3)4 (133 mg, 0.115 mmol), K2CO3 (1.90 g, 13.8 mmol) in THF (15 mL) was heated at 95 °C for 12 h. After cooling to room temperature, he reaction mixture was diluted by adding H2O and ethyl acetate. The mixture was extracted with ethyl acetate (three times), and the combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated. The crude product was purified by column chromatography on silica gel (hexanes/ethyl acetate = 10 : 1) to afford 2-(2-((tert-butyldimethylsilyl)oxy)benzyl)benzaldehyde (29) (1.20 g, 3.68 mmol, 80%). Compound 29: 1H-NMR (CDCl3, 600 MHz) δ: 10.26 (1H, s), 7.88 (1H, d, J = 7.6 Hz), 7.49 (1H, t, J = 6.8 Hz), 7.39 (1H, t, J = 7.2 Hz), 7.15–7.10 (2H, m), 6.85–6.83 (3H, m), 4.00 (2H, s), 0.92 (9H, s), 0.23 (6H, s); 13C-NMR (CDCl3, 150 MHz) δ: 192.4, 153.3, 143.2, 134.0, 133.8, 131.1, 131.0, 130.7, 130.5, 127.4, 126.7, 121.2, 118.3, 32.6, 25.7, 18.2, −4.2; IR (CHCl3, cm−1): 2958, 2931, 2860, 1693; HRMS (DART+): m/z [M + H]+ Calcd for C20H26O2Si: 327.17803. Found: 327.17867.
To a stirred mixture of methyltriphenylphosphonium iodide (1.95 g, 4.82 mmol), t-BuOK (800 mg, 7.13 mmol) in THF (50 mL) was added a solution of 29 (750 mg, 2.30 mmol) in THF (30 mL) at 0 °C for 4 h. The reaction was quenched by addition of a solution of hydrochloric acid (<pH 7.0), and the mixture was extracted with ethyl acetate. The combined organic extracts were dried over anhydrous Na2SO4, filtered, and concentrated. The crude product was purified by column chromatography on silica gel (hexanes) to afford the corresponding Wittig product (528 mg, 1.63 mmol, 71%).
To a stirred solution of the Wittig product (500 mg, 1.54 mmol) in THF (10 mL) was added a solution of TBAF in THF (1.0 M, 2.3 mL, 2.3 mmol) at 0 °C, and the reaction mixture was stirred at 0 °C for 25 min. The reaction was quenched by adding a solution of saturated aqueous NH4Cl. The resulting mixture was extracted with ethyl acetate (three times), and the combined organic extracts were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated. The crude product was purified by column chromatography on silica gel (hexanes/ethyl acetate = 10 : 1) to afford 6e (364 mg, 1.17 mmol, 76%). Compound 6e: 1H-NMR (CDCl3, 600 MHz) δ: 7.42 (1H, d, J = 7.2 Hz), 7.15–7.07 (2H, m), 7.01–6.95 (2H, m), 6.86 (1H, dd, J = 17.4, 11.4 Hz), 6.78 (1H, d, J = 7.2 Hz), 6.73 (1H, t, J = 7.8 Hz), 6.65 (1H, d, J = 8.4 Hz), 5.53 (1H, d, J = 17.4 Hz), 5.15 (1H, d, J = 11.4 Hz), 4.73 (1H, s), 3.92 (2H, s); 13C-NMR (CDCl3, 150 MHz) δ: 153.4, 137.1, 136.6, 134.5, 130.5, 129.8, 128.0, 127.6, 126.8, 126.5, 126.0, 120.9, 116.0, 115.3, 33.0; IR (CHCl3, cm−1): 3597, 3010; HRMS (DART+): m/z [M + H]+ Calcd for C14H13O1: 197.09664. Found: 197.09670.
The IMDA Reaction of 6d to 8d (Table 1, Entry 4)To a solution of 6d (68 mg, 0.35 mmol) in AcOH (3 mL) was added Pb(OAc)4 (922 mg, 2.08 mmol) at 0 °C, and the reaction temperature was allowed to reach room temperature. After 72 h, the precipitate was filtered off with EtOAc, and the filtrate was washed with saturated aqueous solution of NaHCO3. The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (EtOAc/Hexane) to yield 8d (21 mg, 0.08 mmol, 23%) as a yellow solid. Compound 8d: mp: 128.0–129.0 °C (recryst. from CH2Cl2); 1H-NMR (CDCl3, 600 MHz) δ: 7.26–7.18 (4H, m), 6.59 (1H, t, J = 6.0 Hz), 6.39 (1H, t, J = 6.6 Hz), 4.38–4.35 (1H, m), 3.42–4.39 (1H, m), 3.27–3.22 (1H, m), 2.16 (3H, s), 1.89–1.88 (2H, m); 13C-NMR (CDCl3, 150 MHz) δ: 202.3, 169.2, 146.2, 139.3, 132.3, 131.5, 129.0, 127.2, 123.6, 122.6, 85.5, 52.1, 51.0, 38.4, 37.6, 21.5; IR (CHCl3, cm−1): 2951, 1738; HRMS (DART+): m/z [M + H]+ Calcd for C16H15O3: 255.10212. Found: 255.10187.
6-Acetoxy-6-(2-vinylbenzyl)cyclohexa-2,4-dien-1-one (7e)To a solution of 6e (54 mg, 0.24 mmol) in AcOH (1 mL) was added Pb(OAc)4 (317 mg, 0.715 mmol) at 0 °C, and the reaction temperature was allowed to reach room temperature. After 1 h, the precipitate was filtered off with EtOAc, and the filtrate was washed with saturated aqueous solution of NaHCO3. The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (EtOAc/Hexane/1% triethylammonium acetate (TEA)) to yield 7e (15 mg, 0.055 mmol, 23%) as a yellow oil. Compound 7e: 1H-NMR (CDCl3, 600 MHz) δ: 7.52 (1H, d, J = 7.8 Hz), 7.19 (2H, d, J = 4.2 Hz), 7.11–7.06 (2H, m), 6.97–6.94 (1H, m), 6.27–6.25 (1H, m), 6.18 (1H, d, J = 9.6 Hz), 6.07 (1H, d, J = 9.0 Hz), 5.63 (1H, d, J = 16.8 Hz), 5.23 (1H, d, J = 10.8 Hz), 3.15 (1H, d, J = 13.8 Hz), 3.06 (1H, d, J = 13.8 Hz), 2.08 (3H, s); 13C-NMR (CDCl3, 150 MHz) δ: 198.4, 169.3, 141.2, 140.5, 138.3, 135.3, 135.2, 132.3, 131.3, 127.7, 127.1, 125.9, 123.0, 115.8, 81.3, 40.1, 20.5; IR (CHCl3, cm−1): 2927, 2856, 1739, 1676; HRMS (DART+): m/z [M + H]+ Calcd for C17H17O3: 269.11777. Found: 269.11855.
The IMDA Reaction of 7e to 9e (Table 1, Entry 5)A solution of 7e (59 mg, 0.22 mmol) in xylenes (1 mL) was refluxed for 16 h. After cooling to room temperature, the crude products were purified by column chromatography on silica gel (EtOAc/Hexane) to provide the product 9e (43 mg, 0.16 mmol, 52%). Compound 9e: mp: 97.5–98.5 °C (recryst. from CH2Cl2); 1H-NMR (CDCl3, 600 MHz) δ: 7.14–7.06 (4H, m), 6.66 (1H, t, J = 7.2 Hz), 6.21 (1H, t, J = 7.2 Hz), 4.28 (1H, d, J = 15.6), 3.86 (1H, t, J = 5.4 Hz), 3.56 (1H, t, J = 6.0 Hz), 3.15 (1H, d, J = 16.2 H), 2.99 (1H, dd, J = 10.2, 4.8 Hz), 2.29 (1H, dd, J = 13.2, 4.2 Hz), 2.12 (3H, s), 1.84 (1H, dd, J = 11.4, 12.6 Hz); 13C-NMR (CDCl3, 150 MHz) δ: 203.1, 170.4, 144.3, 139.7, 134.9, 130.4, 128.1, 126.8, 126.6, 126.0, 80.8, 57.0, 43.7, 38.9, 36.3, 32.4, 22.0; IR (CHCl3, cm−1): 3024, 1736; HRMS (DART+): m/z [M + H]+ Calcd for C17H17O3: 269.11777. Found: 269.11805.
1-(tert-Butyldimethylsilyloxy)-2-(2-iodostyryl)benzene (12)To a cooled suspension of triphenyl(2-vinylbenzyl)phosphonium bromide (5.19 g, 9.28 mmol) in THF (46 mL) at 0 °C was added potassium tert-butoxide (1.21 g, 10.8 mmol) under argon. After 30 min, 2-tert-butyldimethylsiloxybenzaldehyde 10 (1.83 g, 7.73 mmol) was added over 10 min. The reaction temperature was warmed to r.t. and the mixture was stirred for 12 h. After adding water (30 mL) slowly, the aqueous phase was separated and extracted with EtOAc. The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (Hexane) to give the product 12 (2.77 g, 6.34 mmol, 82%, a mixture of (E)- and (Z)-12) as a yellow oil. Compound 12: 1H-NMR (CDCl3, 600 MHz, E/Z = 5 : 1, data of (E)-12 is shown here) δ: 7.87 (1H, d, J = 9.0 Hz), 7.69 (1H, d, J = 7.2 Hz), 7.60 (1H, d, J = 7.8 Hz), 7.37 (1H, d, J = 10.8 Hz), 7.34 (1H, dd, J = 7.8, 7.8 Hz), 7.24 (1H, d, J = 16.2 Hz), 7.17 (1H, dd, J = 6.6, 6.6 Hz), 7.00 (1H, dd, J = 7.8, 7.8 Hz), 6.94 (1H, dd, J = 6.6, 6.6 Hz), 6.84 (1H, d, J = 8.4 Hz), 1.04 (9H, s), 0.24 (6H, s); 13C-NMR (CDCl3, 150 MHz, data of (E)-12 is shown here) δ: 153.3, 140.7, 139.6, 131.9, 128.9, 128.7, 128.4, 126.7, 126.4, 126.0, 121.6, 119.7, 100.5, 25.8, 18.3, −4.1; IR (neat, cm−1):1597, 1252, 1011; HRMS (DART+): m/z [M + H]+ Calcd for C20H26IOSi: 437.07976. Found: 437.08039.
1-(tert-Butyldimethylsilyloxy)-2-(2-iodophenethyl)benzene (13)Tosylhydrazide (50 mg, 0.12 mmol) and sodium acetate (47 mg, 0.57 mmol) were added to a solution of 12 (50 mg, 0.12 mmol) in EtOH (0.5 mL). The mixture was refluxed for 2 h with stirring. When the reaction was complete, the mixture was cooled to room temperature. The mixture was diluted with Et2O (5 mL) and washed with saturated solution of NaHCO3 and brine. The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (EtOAc/hexane) to give 13 (45.6 mg, 0.104 mmol, 90%) as a yellow oil. Compound 13: 1H-NMR (CDCl3, 600 MHz) δ: 7.82 (1H, d, J = 7.8 Hz), 7.23 (1H, dd, J = 7.2, 7.2 Hz), 7.14 (1H, d, J = 6.0 Hz), 7.13 (1H, d, J = 7.8 Hz), 6.89–6.84 (2H, m), 6.81 (1H, d, J = 7.8 Hz), 2.98 (2H, dd, J = 10.8, 9.0 Hz), 2.87 (2H, dd, J = 10.8, 9.0 Hz), 1.03 (9H, s), 0.26 (6H, s); 13C-NMR (CDCl3, 150 MHz) δ: 153.6, 144.5, 139.4, 131.7, 130.4, 129.4, 128.4, 128.2, 127.7, 127.0, 120.1, 118.4, 100.8, 41.2, 30.9, 25.9, 18.3, −4.1; IR (neat, cm−1): 1252, 1011; HRMS (DART+): m/z [M + H]+ Calcd for C20H28IOSi: 439.09541. Found: 439.09442.
3-(2-(2-((tert-Butyldimethylsilyl)oxy)phenethyl)phenyl)-1-phenyl-1H-pyrrole-2,5-dione (14)A dry 30 mL flask containing a magnetic stirring bar was charged with 13 (900 mg, 2.05 mmol), Pd(OAc)2 (92 mg, 0.41 mmol), DPEphos (221 mg, 0.410 mmol), N-phenylmaleimide (1.78 g, 2.05 mmol), KOAc (604 mg, 6.15 mmol) and dry ethylene carbonate (5.4 g). The mixture was stirred at 60 °C for 6 h, and the mixture was cooled to room temperature. The crude product was purified by column chromatography on silica gel (EtOAc/Hexane) to give 14 (259 mg, 0.59 mmol, 29%) as a yellow oil. Compound 14: 1H-NMR (CDCl3, 600 MHz) δ: 7.48 (2H, dd, J = 9.0, 9.0 Hz), 7.41–7.28 (7H, m), 7.07–7.04 (1H, m), 6.82–6.75 (3H, m), 3.03 (2H, t, J = 7.2 Hz), 2.90 (2H, t, J = 7.2 Hz), 0.99 (9H, s), 0.21 (6H, s); 13C-NMR (CDCl3, 150 MHz) δ: 169.2, 169.1, 153.7, 145.7, 141.3, 131.7, 131.3, 130.6, 130.40, 130.35, 130.12, 130.09, 129.6, 129.0, 128.4, 128.2, 128.0, 127.6, 127.55, 127.46, 126.03, 125.91, 33.4, 32.6, 29.7, 25.8, 18.2, −4.1; IR (CHCl3, cm−1): 3473, 1768, 1714, 1623, 1598, 1502, 1257; HRMS (DART+): m/z [M + H]+ Calcd for C30H34NO3Si: 484.23079. Found: 484.23058.
3-(2-(2-Hydroxyphenethyl)phenyl)-1-phenyl-1H-pyrrole-2,5-dione (6f)To a solution of 14 (50 mg, 0.10 mmol) in THF (1 mL) was added pyridine (0.15 mL) and HF-pyridine (0.15 mL) at 0 °C. The solution was stirred at 0 °C for 3 h. The reaction mixture was diluted with EtOAc and H2O. The organic phase was washed with a saturated aqueous solution of NH4Cl, a saturated aqueous solution of NaHCO3, and brine. After the organic phase was concentrated under reduced pressure, the crude product was purified by column chromatography on silica gel (EtOAc/hexane) to yield 6f (35 mg, 0.095 mmol, 95%) as a yellow oil. Compound 6f: 1H-NMR (CDCl3, 600 MHz) δ: 7.49 (2H, dd, J = 9.0, 9.0 Hz), 7.45–7.37 (6H, m), 7.33–7.30 (1H, m), 7.07 (1H, dd, J = 9.0, 9.0 Hz), 7.00 (1H, dd, J = 7.2, 1.8 Hz), 6.82 (1H, dd, J = 6.6, 6.6 Hz), 6.69 (1H, s), 6.69 (1H, d, J = 6.6 Hz), 5.00 (1H, s), 3.00 (2H, dd, J = 10.2, 9.0 Hz), 2.90 (2H, dd, J = 10.8, 9.0 Hz); 13C-NMR (CDCl3, 150 MHz) δ: 169.7, 169.6, 153.7, 145.5, 141.3, 131.6, 130.7, 130.45, 130.41, 130.3, 129.1, 128.6, 128.0, 127.8, 127.7, 127.1, 126.2, 126.0, 120.8, 115.3, 34.2, 33.0; IR (CHCl3, cm−1): 3588, 3473, 3286, 1770, 1712, 1596, 1502; HRMS (DART+): m/z [M + H]+ Calcd for C24H20NO3: 370.14432. Found: 370.14448.
The Wessely Oxidation of 6f to 7f (Chart 7)To a solution of 6f (98 mg, 0.26 mmol) in AcOH (4 mL) was added Pb(OAc)4 (235 mg, 0.529 mmol) at 0 °C, and the reaction temperature was allowed to reach room temperature. After 30 min, the precipitate was filtered off with EtOAc, and the filtrate was washed with saturated aqueous solution of NaHCO3. The organic phase was dried over anhydrous MgSO4, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (EtOAc/Hexane) to yield 7f (39 mg, 0.090 mmol, 34%) as a yellow oil. Compound 7f: 1H-NMR (CDCl3, 600 MHz) δ: 7.52–7.50 (2H, m), 7.47–7.45 (2H, m), 7.41–7.39 (3H, m), 7.33–7.29 (2H, m), 6.77 (1H, s), 6.31–6.26 (2H, m), 6.15 (1H, d, J = 10.2 Hz), 2.82 (1H, ddd, J = 13.2, 13.2, 4.8 Hz), 2.73 (1H, ddd, J = 13.2, 13.2, 4.8 Hz), 2.14–2.07 (1H, m), 2.11 (3H, s); 13C-NMR (CDCl3, 150 MHz) δ: 169.5, 169.4, 169.0, 145.6, 141.0, 140.5, 140.0, 130.6, 130.5, 130.0, 129.1, 128.6, 128.0, 127.9, 126.8, 126.6, 126.1, 123.0, 81.1, 39.0, 26.5, 20.4; IR (CHCl3, cm−1): 3473, 1770, 1737, 1716, 1675, 1502, 1390; HRMS (DART+): m/z [M + H]+ Calcd for C26H22NO5: 428.14980. Found: 428.15020.
The IMDA Reaction of 7f to 9f (Chart 7)Compound 7f (38 mg, 0.089 mmol) was heated at 170 °C in a vial without any solvent for 3.5 h. After cooling to room temperature, the crude products were purified by column chromatography on silica gel (EtOAc/Hexane) to provide the product 9f (20 mg, 0.047 mmol, 52%). Compound 9f: mp: 284.0–285.0 °C (recryst. from CH2Cl2); 1H-NMR (CDCl3, 600 MHz) δ: 8.61 (1H, d, J = 10.8 Hz), 7.49–7.47 (2H, m), 7.44–7.41 (1H, m), 7.34 (1H, dd, J = 7.2, 7.2 Hz), 7.27–7.20 (3H, m), 7.13 (1H, d, J = 7.8 Hz), 6.56 (1H, dd, J = 6.6, 6.6 Hz), 6.34 (1H, ddd, J = 7.8, 7.8, 1.2 Hz), 5.06 (1H, ddd, J = 5.1, 5.1, 1.8 Hz), 4.76 (1H, d, J = 4.2 Hz), 3.79 (1H, d, J = 6.0 Hz), 3.70–3.64 (1H, m), 3.17 (1H, ddd, J = 12.0, 6.6, 2.4 Hz), 2.92 (1H, ddd, J = 20.8, 6.6, 2.4 Hz), 2.73 (1H, ddd, J = 13.2, 13.2, 4.8 Hz), 2.36 (1H, ddd, J = 13.2, 13.2, 6.0 Hz), 2.00 (3H, s); 13C-NMR (CDCl3, 150 MHz) δ: 201.0, 176.0, 174.0, 169.7, 136.5, 136.3, 134.6, 133.9, 131.5, 129.3, 129.1, 128.9, 128.5, 128.1, 126.5, 126.3, 79.6, 60.3, 51.4, 43.6, 39.2, 34.3, 33.9, 22.1,; IR (CHCl3, cm−1):3477, 1779, 1737, 1712, 1498, 1386; HRMS (DART+): m/z [M + H]+ Calcd for C26H22NO5: 428.14980. Found: 428.14995.
tert-Butyl(2-(2-iodo-3,4,5-trimethoxystyryl)phenoxy)dimethylsilane (16)To triphenylphosphine (2.1 g, 11 mmol) in a flask under a nitrogen atmosphere and heated to 100 °C in oil bath was added 1-(bromomethyl)-2-iodo-3,4,5-trimethoxybenzene (1.9 g, 4.9 mmol). After 10 min, to this was added CH2Cl2, and the mixture was concentrated, then filtered and washed extensively with EtOAc and CHCl3 to yield the phosphonium salt (2.8 g, 4.8 mmol, 97%) as a white solid. mp: 224.0–225.0 °C (recryst. from CH2Cl2); 1H-NMR (CDCl3, 600 MHz) δ: 7.56–7.89 (15H, m), 7.16 (1H, br s), 5.54–5.79 (2H, br m), 3.84 (3H, br d, J = 1.4 Hz), 3.72 (3H, br d, J = 1.4 Hz), 3.58 (3H, brd, J = 2.1 Hz); 13C-NMR (CDCl3, 150 MHz) δ: 142.2, 135.2, 134.5, 130.2, 125.6, 117.5, 117.0, 112.3, 92.8, 61.1, 60.6, 56.4, 35.6; IR (neat, cm−1):2939, 2854, 1482, 1483; HRMS (DART+): m/z [M-C10H12O3I]+ Calcd for C18H16P: 263.0990. Found: 263.0980.
To a cooled suspension of a phosphonium bromide (2.0 g, 3.1 mmol) in THF (15 mL) was added potassium tert-butoxide (404 mg, 3.60 mmol) under nitrogen at 0 °C. After stirring the mixture for 30 min, 2-tert-butyldimethylsiloxybenzaldehyde 10 (607 mg, 2.57 mmol) was added over 10 min. The reaction temperature was warmed to room temperature and the mixture was stirred for 12 h. After adding water (30 mL), the aqueous phase was separated and extracted with EtOAc. The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (Hexane) to give 16 (1.29 g, 2.44 mmol, 95%, a mixture of (E)-16 and (Z)-16) as a colourless oil. Compound 16: 1H-NMR (CDCl3, 400 MHz) δ: 7.71 (0.4H, d, J = 8.0 Hz), 7.32 (0.8H, s), 7.17 (0.4H, t, J = 8.0 Hz), 6.96–7.11 (2.0H, m), 6.66–6.86 (2.2H, m), 6.51–6.60 (1.2H, m), 3.90 (5.4H, s), 3.87 (1.8H, s), 3.42 (1.8H, s), 1.06 (3.6H, s), 1.02 (5.4H, s), 0.25 (2.4H, s), 0.24 (s, 3.6H); 13C-NMR (CDCl3, 150 MHz) δ: 153.8, 153.6, 153.1, 153.0, 141.6, 141.0, 136.5, 133.1, 131.9, 130.5, 128.9, 128.5, 128.2, 127.9, 127.1, 126.1, 125.6, 121.6, 120.9, 119.5, 109.6, 104.9, 88.9, 87.8, 61.1, 61.0, 60.8, 60.7, 55.9, 55.6, 25.9, 25.8, 18.3, 4.08, −4.08, −4.22; IR (neat, cm−1): 2932, 2856, 1596, 1255; HRMS (DART+): m/z [M + H]+ Calcd for C23H32IO4Si: 527.1115. Found: 527.1106.
tert-Butyl(2-(2-iodo-3,4,5-trimethoxyphenethyl)phenoxy)dimethylsilane (17)Tosylhydrazide (1.04 g, 5.58 mmol) and sodium acetate (1.21 g, 14.0 mmol) were added to a solution of 16 (1.47 g, 2.79 mmol) in EtOH (13 mL), and the mixture was refluxed for 4 h. The reaction temperature was cooled to room temperature, and the mixture was diluted with Et2O. The mixture was washed with saturated aqueous solution of NaHCO3 and brine. The organic phase was dried over anhydrous Na2SO4, and concentrated under reduced pressure. The products were purified by column chromatography on silica gel (EtOAc/Hexane) to give 17 (1.34 g, 2.54 mmol, 91%) as a colourless oil. Compound 17: 1H-NMR (CDCl3, 600 MHz) δ: 7.13–7.18 (1H, m), 7.06–7.11 (m, 1H), 6.85–6.90 (m, 1H), 6.79–6.83 (1H, m), 6.51 (1H, s), 3.88 (3H, s), 3.86 (3H, s), 3.74 (3H, s), 2.95–3.02 (2H, m), 2.82–2.89 (2H, m), 1.05 (9H, s), 0.27 (6H, s); 13C-NMR (CDCl3, 150 MHz) δ: 153.6, 153.3, 153.0, 140.3, 131.7, 130.6, 127.0, 121.1, 118.4, 108.8, 88.1, 61.0, 60.7, 55.9, 41.5, 31.0, 25.9, 18.3, −4.05; IR (CHCl3, cm−1): 2932, 2856, 1252; HRMS (DART+): m/z [M + H]+ Calcd for C23H34IO4Si: 529.1271. Found: 529.1270.
6-(2-((tert-Butyldimethylsilyl)oxy)phenethyl)-2,3,4-trimethoxybenzaldehyde (18)To a solution of 17 (500 mg, 0.95 mmol) in THF (10 mL) was added a solution of n-butyllithium (1.62 M in hexane, 1.46 mL, 2.37 mmol) dropwise at –78 °C. After stirring for 30 min at –78 °C, anhydrous DMF (0.73 mL, 9.5 mmol) was added dropwise. The reaction mixture was stirred for another 1 h at –78 °C. The reaction was warmed to room temperature and stirred for another 2 h, followed by the addition of 3M HCl (3 mL). The resulting mixture was extracted with Et2O, and the organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (EtOAc/Hexane) to afford compound 18 which was not completely pure. The crude product 18 was used without further purification in the next step.
(E)-tert-Butyldimethyl(2-(3,4,5-trimethoxy-2-(2-nitrovinyl)phenethyl)phenoxy)silane (19)The mixture of the crude 18, nitromethane (3.62 g, 59.4 mmol) and ammonium acetate (15 mg, 0.19 mmol) was stirred at 100 °C for 3 h. After cooling the reaction mixture to room temperature, the mixture was concentrated, and then H2O and dichloromethane were added. The mixture was washed with H2O, 1 N hydrochloric acid and brine, dried over Na2SO4, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (CH2Cl2/hexane) to yield 19 as a yellow solid (179 mg, 0.38 mmol, 40% yield for two steps). Compound 19: mp: 73.5–74.5 °C (recryst. from CH2Cl2); 1H-NMR (CDCl3, 600 MHz) δ: 8.11 (1H, d, J = 13.4 Hz), 7.92 (1H, d, J = 13.4 Hz), 7.07 (1H, t, J = 6.9 Hz), 6.97 (1H, d, J = 6.5 Hz), 6.83 (1H, t, J = 7.4 Hz), 6.78 (1H, d, J = 7.9 Hz), 6.45 (1H, s), 3.91 (3H, s), 3.85 (3H, s), 3.80 (3H, s), 3.03 (3H, t, J = 7.6 Hz), 2.86 (2H, t, J = 7.6 Hz), 1.03 (9H, s), 0.26 (6H, s); 13C-NMR (CDCl3, 150 MHz) δ: 156.0, 154.6, 153.6, 141.1, 140.6, 138.0, 132.2, 130.7, 130.6, 127.4, 121.2, 118.4, 115.3, 109.3, 60.9, 60.4, 55.8, 34.4, 32.9, 25.8, 18.2, −4.13 ; IR (CHCl3, cm−1): 2932, 2856, 1589, 1261; HRMS (DART+): m/z [M + H]+ Calcd for C24H36NO6Si: 474.23119. Found: 474.2310.
(E)-2-(3,4,5-Trimethoxy-2-(2-nitrovinyl)phenethyl)phenol (6g)To a solution of 19 (140 mg, 0.30 mmol) in THF (6 mL) was added TBAF (1.0 M solution in THF, 0.36 mL, 0.36 mmol) dropwise at 0 °C. The solution was stirred at 0 °C for 30 min. Water was added to the reaction mixture, and the resulting mixture was extracted with Et2O. The organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (EtOAc/Hexane) to yield 6g (104 mg, 0.029 mmol, 98%) as a yellow oil. Compound 6g: 1H-NMR (CDCl3, 600 MHz) δ: 8.27 (1H, d, J = 13.1 Hz), 7.94 (1H, d, J = 13.4 Hz), 7.05–7.10 (1H, m), 6.94 (1H, d, J = 7.6 Hz), 6.80 (1H, t, J = 7.4 Hz), 6.73 (1H, d, J = 7.9 Hz), 6.59 (1H, s), 5.18 (1H, brs), 3.90 (6H, s), 3.86 (3H, s), 3.06 (2H, t, J = 7.9 Hz), 2.86 (2H, t, J = 7.9 Hz); 13C-NMR (CDCl3, 150 MHz) δ: 156.2, 154.7, 153.7, 141.5, 140.7, 138.0, 132.9, 130.6, 127.7, 126.8, 120.8, 115.7, 115.1, 109.3, 60.9, 60.4, 56.0, 34.3, 33.7; IR (CHCl3, cm−1): 3444, 2938, 2850, 1589; HRMS (DART+): m/z [M + H]+ Calcd for C19H22NO6Si: 360.14471. Found: 360.14454.
The Wessely Oxidation of 6g to 7g (Table 2, Entry 1)Compound 6g (300 mg, 0.84 mmol) in AcOH (5 mL) was added to a solution of Pb(OAc)4 (556 mg, 1.25 mmol) in AcOH (5 mL) at 0 °C. The reaction temperature was warmed to room temperature, and the mixture was stirred for 2 h. The precipitate was filtered off with AcOEt and the filtrate was wash with saturated aqueous solution of NaHCO3. The organic phase was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (EtOAc/hexane) to yield 7g (151 mg, 0.36 mmol, 43%) as a yellow amorphous. Compound 7g: 1H-NMR (CDCl3, 600 MHz) δ: 8.11 (1H, d, J = 13.2 Hz), 7.98 (1H, d, J = 12.6 Hz), 7.04–7.09 (1H, m), 6.51 (1H, s), 6.44–6.48 (1H, m), 6.38–6.42 (1H, m), 6.24 (1H, d, J = 9.6 Hz), 3.94 (3H, s), 3.89 (3H, s), 3.84 (3H, s), 2.83–2.93 (1H, m), 2.74–2.82 (1H, m), 2.19 (3H, s), 1.99–2.01 (1H, m), 1.82–1.92 (1H, m); 13C-NMR (CDCl3, 150 MHz) δ: 198.1, 169.6, 156.4, 155.0, 140.9, 140.8, 139.8, 138.5, 131.8, 126.8, 123.5, 114.9, 109.2, 80.9, 60.9, 60.5, 56.1, 39.6, 27.5, 20.4; IR (CHCl3, cm−1): 2927, 2854, 1749, 1716, 1595; HRMS (DART+): m/z [M + H]+ Calcd for C21H24NO8: 418.1502. Found: 418.1507.
The IMDA Reaction of 7g to 9g (Table 2, Entry 3)A solution of 7g (39 mg, 0.092 mmol) in 2,2,2-trifluoroethanol (1 mL) was heated in a vial at 130 °C under microwave irradiation for 18 h. After concentration, the crude product was purified by column chromatography on silica gel (EtOAc/CH2Cl2) to provide 9g (9.6 mg, 0.023 mmol, 25%) as a brown solid. Compound 9g: mp: 84.0–85.5 °C (recryst. from CH2Cl2); 1H-NMR (CDCl3, 600 MHz) δ: 6.38 (1H, t, J = 7.2 Hz), 6.35 (1H, s), 6.31(1H, t, J = 6.9 Hz), 5.81 (1H, d, J = 3.6 Hz), 5.21 (1H, s), 4.89 (1H, d, J = 4.2 Hz), 3.97 (3H, s), 3.86 (3H, s), 3.83–3.89 (1H, m), 3.82 (3H, s), 3.39 (1H, t, J = 5.2 Hz), 3.23–3.35 (1H, m), 3.00–3.09 (1H, m), 2.71–2.81 (1H, m), 2.24–2.33 (1H, m), 2.02 (3H, s); 13C-NMR (CDCl3, 150 MHz) δ: 202.3, 170.2, 152.8, 152.0, 141.8, 133.1, 131.9, 129.0, 121.8, 112.3, 82.9, 79.9, 61.8, 61.1, 56.1, 53.7, 42.5, 36.1, 33.3, 33.0, 22.5; IR (CHCl3, cm−1):2940, 2838, 1736, 1552; HRMS (DART+): m/z [M + H]+ Calcd for C21H24NO8: 418.1502. Found: 418.1505.
Computation DetailsGeometry optimization was performed with Spartan’1837) and the Gaussian 09 packages.38) The ground-state geometries of all compounds were determined by means of the following successive steps: Conformational search with MMFF94,39) then DFT calculation with B3LYP functionals.40) The transition-state geometries of all compounds were determined by the DFT calculation with B3LYP functionals followed by IRC calculations.41,42) The basis set employed for DFT geometry optimization was the native 6–31G(d, p) for Fig. 1(a) or the native 6–31G(d) for Fig. 1(b)–(d).
We thank Ms. Mizuki Yamazaki, Mr. Daichi Arai, and Ms. Akari Nakamura for assistance. This work was supported by JSPS KAKENHI Grant Number JP19K05473 and Kanazawa University SAKIGAKE project.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.