2020 年 68 巻 3 号 p. 220-226
2020 年 68 巻 3 号 p. 220-226
This study demonstrates the structure–activity relationship of Col-003, a potent collagen–heat-shock protein 47 (Hsp47) interaction inhibitor. Col-003 analogues were successfully synthesized by Pd(0)-catalyzed cross-coupling reactions of 5-bromosalicylaldehyde derivatives with alkyl–metal species, and the inhibitory activities of the synthetic analogues were evaluated using surface plasmon resonance analysis (BIAcore). We succeeded in discovering two potent inhibitors that showed 85 and 81% inhibition at a concentration of 1.9 µM against the collagen–Hsp47 interaction. This indicates that elongation of an alkyl linker between two aromatic rings could considerably improve inhibitory activity due to the adjustment of a pendant phenyl moiety to an appropriate position, in addition to the hydrophobic interaction with an alkyl linker moiety.
Protein–protein interactions (PPIs) are known to be important triggers in biological processes, and therefore they are being addressed as novel therapeutic targets in drug discovery.1,2) PPIs commonly act on interfaces that are broad, uneven protein surfaces3–5); therefore, the development of PPI inhibitors remains a difficult and challenging issue. Heat-shock protein 47 (Hsp47) is a collagen-specific molecular chaperone expressed in the endoplasmic reticulum and plays an important role in the correct folding of procollagen for the secretion of triple-helix collagen.6–8) Abnormal secretion and accumulation of collagen cause fibrotic diseases, such as liver and lung fibrosis; therefore, the inhibition of collagen secretion is expected to be one of the therapeutic alternatives for the above diseases, and the collagen–Hsp47 interaction is a challenging target for developing novel inhibitors of collagen secretion.9–12)
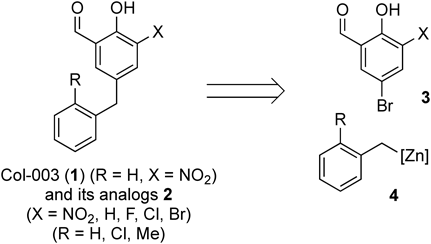
We have recently reported a small-molecule inhibitor of the collagen–Hsp47 interaction, 5-benzyl-3-nitrosalicylaldehyde, called Col-003 (1). It was discovered by screening chemical libraries using surface plasmon resonance (SPR) analysis (BIAcore), which revealed that 1 strongly binds to Hsp47 but not to collagen.13) In addition, the binding site of 1 on Hsp47 was analyzed not only by druggable pocket analysis using the crystal structure of canine Hsp47 but also by two-dimensional NMR (2D-NMR) analysis of the interactions between Hsp47 and collagen model peptide (GPP)10 or 1. The results indicated that 1 would competitively bind near the collagen-binding interface on Hsp47 via interaction of a pendant benzyl moiety in 1 with the aromatic rings of Tyr-355 and His-245 in Hsp47. To obtain further information regarding the mode of action, we planned a structure–activity relationship study on Col-003 (1) based on the synthesis of its analogues and evaluation of their inhibitory activities.
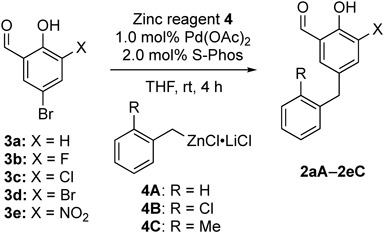
To elucidate the substituent effects of Col-003 (1), we designed analogues 2 that possess a halogen atom instead of a nitro group and a 2-methyl- or 2-chlorobenzyl group as a pendant moiety for inducing steric and electronic effects with an ortho-substituted aromatic ring. Analogues 2 can be prepared through the Pd(0)-catalyzed cross-coupling reaction of 5-bromosalicylaldehyde 3 with benzylzinc reagents 4, as shown in Chart 1.
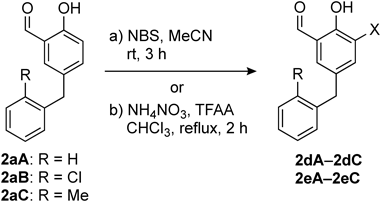
One-step synthesis of analogues 2a–2e with the Pd(0)-catalyzed Negishi cross-coupling reaction was attempted, and the results are presented in Table 1. The reaction of 5-bromosalicylaldehyde (3a)14) (X = H) with benzylzinc reagents15)4A–C (R = H, Cl or Me) was performed in the presence of a catalytic amount of Pd(OAc)2/S-Phos at room temperature to afford the desired 2aA–2aC (entries 1–3). Similarly, reactions of 5-bromo-3-fluorosalicylaldehyde (3b)16) and 5-bromo-3-chlorosalicylaldehyde (3c)17) with benzylzinc reagents 4A–C successfully provided the corresponding analogues 2bA–2bC and 2cA–2cC in moderate to good yields (entries 4–9). Alternatively, all the reactions of 3,5-dibromosalicylaldehyde (3d)18) and 5-bromo-3-nitrosalicylaldehyde (3e)19) with 4A–4C resulted in a complex mixture (entries 10–15), probably because 3d has two reactive bromide atoms, which might have made the regioselective reaction difficult, and 3e would undergo nucleophilic addition of the organozinc reagents to an aldehyde moiety activated by an adjacent highly electron-withdrawing NO2 group.
 | ||||
|---|---|---|---|---|
| Entry | X | R | Product | Yield (%)a) |
| 1 | H | H | 2aA | 64 |
| 2 | H | Cl | 2aB | 57 |
| 3 | H | Me | 2aC | 97 |
| 4 | F | H | 2bA | 95 |
| 5 | F | Cl | 2bB | 70 |
| 6 | F | Me | 2bC | 64 |
| 7 | Cl | H | 2cA | 93 |
| 8 | Cl | Cl | 2cB | 90 |
| 9 | Cl | Me | 2cC | 43 |
| 10 | Br | H | 2dA | —b) |
| 11 | Br | Cl | 2 dB | —b) |
| 12 | Br | Me | 2dC | —b) |
| 13 | NO2 | H | 1 (2eA) | —b) |
| 14 | NO2 | Cl | 2eB | —b) |
| 15 | NO2 | Me | 2eC | —b) |
a) Isolated yield. b) Complex mixture.
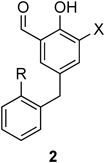
Next, we employed ortho-bromination and ortho-nitration of phenols in the coupling products 2aA–2aC. Treatment of 2aA–2cA with NBS/MeCN or NH4NO3/trifluoroacetic anhydride (TFAA)20) provided the brominated analogues 2dA–2dC or nitrated analogues 2eA–2eC, respectively (Table 2).
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | X | R | Product | Yield (%)c) |
| 1a) | 2aA | Br | H | 2dA | 75 |
| 2a) | 2aB | Br | Cl | 2dB | 90 |
| 3a) | 2aC | Br | Me | 2dC | 33 |
| 4b) | 2aA | NO2 | H | 2eA | 70 |
| 5b) | 2aB | NO2 | Cl | 2eB | 12 |
| 6b) | 2aC | NO2 | Me | 2eC | 41 |
a) Condition a). b) Condition b). c) Isolated yield.
With the availability of the desired analogues, we evaluated the inhibitory effect of the synthetic analogues against the collagen–Hsp47 interaction in SPR analysis using an Hsp47-immobilized sensor tip,21) and the results are summarized in Table 3. The parent Col-003 (1) exhibited 80% inhibition of the collagen–Hsp47 interaction at a concentration of 16.7 µM (entry 1); however, the inhibitory rate of analogue 2aA lacking the nitro group decreased considerably to 34% (entry 2). Interestingly, replacement of the benzyl group in 2aA with 2-chloro and 2-methylbenzyl groups maintained inhibitory activity (entries 7 and 12). Substitution of the nitro group in Col-003 (1) with a halogen atom markedly affected the inhibition of the collagen–Hsp47 interaction. Analogues 2b, with a fluorine atom in the salicylaldehyde moiety (entries 3, 8, and 13), exhibited more potent inhibition than the other halogenated analogues 2c (X = Cl) (entries 4, 9, and 14) and 2d (X = Br) (entries 5, 10, and 15). The results indicated that a nitro group or a fluorine atom at position 3 of the salicylaldehyde moiety, which exhibits strong electronegativity, is required to induce potent inhibitory activity against the collagen–Hsp47 interaction. In addition, the introduction of substituents to the pendant benzyl moiety affected the inhibitory activity. Interestingly, the introduction of an electron-donating methyl group at position 2 of the benzyl group maintained the inhibition of the collagen–Hsp47 interaction (entries 11–15), although the analogues possessing a 2-chlorobenzyl group were less potent than the parent compound (entries 6–10). Thus, π-electron density in the analogues could also play an important role in the potency of inhibitory activity against the collagen–Hsp47 interaction. To this end, it should be noted that the fluorinated analogue 2bC is slightly superior to Col-003 (1) at a concentration of 1.9 µM (entry 13 vs. entry 1).
 | ||||
|---|---|---|---|---|
| Entry | X | R | Compound | Inhibition rate (%)a) |
| 1 | NO2 | H | 2eAb) | 80 (18)c) |
| 2 | H | H | 2aA | 34 |
| 3 | F | H | 2bA | 73 |
| 4 | Cl | H | 2cA | 55 |
| 5 | Br | H | 2dA | 49 |
| 6 | NO2 | Cl | 2eB | 55 |
| 7 | H | Cl | 2aB | 63 |
| 8 | F | Cl | 2bB | 67 |
| 9 | Cl | Cl | 2cB | 43 |
| 10 | Br | Cl | 2dB | 10 |
| 11 | NO2 | Me | 2eC | 66 |
| 12 | H | Me | 2aC | 64 |
| 13 | F | Me | 2bC | 81 (35)c) |
| 14 | Cl | Me | 2cC | 59 |
| 15 | Br | Me | 2dC | 39 |
a) 16.7 µM concentration of the compound. b) Col-003 (1). c) 1.9 µM concentration of the compound.
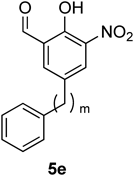
Because the pendant benzyl moiety in 1 was found to be important in potent inhibitory activity, we next investigated the synthesis and inhibitory activity of the nitrated analogues possessing alkyl linkers of different lengths between two aromatic rings in 1, and their syntheses are summarized in Chart 2. Suzuki–Miyaura cross-coupling of aryl bromide 3e with phenylboronic acid (6D) was smoothly performed in the presence of Pd(PPh3)4/K3PO4 to provide the corresponding biphenyl analogue 5eD with 56% yield. In addition, the analogues 5eE–5eG possessing alkyl linkers of different lengths were prepared using Suzuki–Miyaura cross-coupling. However, the reactions of 3e with (phenylalkyl)boranes22) 8E–8G were problematic because a complex mixture was afforded in the presence of a 3-nitrosalicylaldehyde moiety, and the reaction of the simpler aryl bromide 3a also failed to afford the corresponding products. Thus, we utilized a phenol-protected aryl bromide 7 to promote Suzuki–Miyaura cross-coupling leading to the formation of Col-003 analogues 5. Eventually, the methoxymethyl (MOM)-protected aryl bromide 7 was readily coupled with 8E–8G under ambient conditions (45°C, 12 h) to provide 9E–9G in moderate to good yields. After removal of the MOM group under acidic conditions, ortho-nitration of the resulting phenols using NH4NO3/TFAA provided the desired analogues 5eE–5eG in moderate yields.
Inhibitory activities of the synthetic analogues 5eD–5eG were evaluated using SPR analysis in the same manner, and the results are presented in Table 4. The inhibitory activity of biphenyl analogue 5eD was similar to that of the parent Col-003 (1) in the range of concentrations between 1.9 and 5.6 µM (entries 1 and 2). Therefore, a 3-nitrosalicylaldehyde moiety and a pendant phenyl group seem to be a requisite scaffold to induce the desired activity. Notably, elongation of the alkyl linker markedly improved the inhibitory activity against the collagen–Hsp47 interaction at lower concentrations. For example, analogue 5eE, having a phenylethyl moiety as a pendant, effectively inhibited the interaction, with 89% inhibition at 5.6 µM and 65% at 1.9 µM (entry 3). Moreover, analogue 5eF containing a phenylpropyl moiety and analogue 5eG possessing a phenylbutyl moiety exhibited potent activity with 85% and 81% inhibition at 1.9 µM and 59% and 52% inhibition at 0.6 µM, respectively, although bell-shaped concentration–response curves were observed (entries 4 and 5). In our previously proposed binding model of Col-003 (1) with Hsp47, a pendant benzyl moiety would interact with the aromatic rings of Tyr-355 and His-245 in Hsp47.13) Therefore, elongation of the alkyl linker in Col-003 (1) might adjust to an appropriate position in the pendant phenylpropyl or phenylbutyl moiety in 5eF or 5eG to interact with the aromatic rings of the Tyr-355 and His-245 residues. This should induce potent inhibition of the collagen–Hsp47 interaction in addition to a hydrophobic interaction with an alkyl linker moiety.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | m | Product | Inhibition rate (%) | |||
| 0.2 µM | 0.6 µM | 1.9 µM | 5.6 µM | |||
| 1 | 1 | 1 | 0.8 | 6.2 | 22 | 53 |
| 2 | 0 | 5eD | N.D. | N.D. | 34 | 71 |
| 3 | 2 | 5eE | 9.0 | 26 | 65 | 89 |
| 4 | 3 | 5eF | 25 | 59 | 85 | 74 |
| 5 | 4 | 5eG | 23 | 52 | 81 | 41 |
We demonstrated the synthesis of analogues of the collagen–Hsp47 interaction inhibitor Col-003 and evaluated their inhibitory activities using SPR analysis. The Pd(0)-catalyzed Negishi cross-coupling reaction was found to be effective for the preparation of the Col-003 analogues 2a, 2b, and 2c in moderate to good yields. Although brominated or nitrated analogues 2d and 2e were hardly synthesized by the abovementioned Pd(0)-catalyzed reaction, ortho-bromination and ortho-nitration of 2a were performed to prepare the corresponding analogues 2d and 2e. In addition, the analogues possessing alkyl linkers of different lengths were synthesized by Pd(0)-catalyzed Suzuki–Miyaura cross-coupling in acceptable yields. The inhibitory activity of the synthetic analogues evaluated using SPR analysis indicated that a nitro group could be replaced with a halogen atom. Particularly, the fluorinated analogue 2bC was found to be as potent as the parent 1. In addition, the inhibitory activity decreased with the introduction of an electron-withdrawing chlorine atom to the pendant benzyl moiety, whereas substitution with an electron-donating methyl group was tolerated. Notably, two- and three-carbon elongation of the alkyl linker between two aromatic rings in 1 markedly increased the inhibitory potency. Thus, the above results indicated that potent inhibition of the collagen–Hsp47 interaction required a pendant phenylpropyl or phenybutyl group, which would interact with the aromatic rings of Tyr-355 and His-245 in Hsp47. Elucidation of the mechanism of action of Col-003 is underway based on the information obtained from the structure–activity relationship in our laboratories.
All commercially available reagents were used as received. Dry THF was obtained by passing commercially available predried, oxygen-free formulations through an activated alumina column. Column chromatography was carried out with silica gel 60N (Kanto Chemical Co., 100–210 µm). All reactions were monitored by TLC carried out on 0.2-mm E. Merck silica gel plates (60F-254) with UV light. 1H-NMR spectra (400 MHz) and 13C-NMR spectra (100 MHz) were recorded on a JEOL JNM-AL400 spectrometer. Chemical shifts (δ) are given from TMS (0 ppm) as an internal standard for 1H-NMR and 13CDCl3 (77.0 ppm) for 13C-NMR. The following abbreviations are used: s (singlet); d (doublet); t (triplet); q (quartet); quin (quintet); m (multiplet); and dd (double doublet). Mass spectra and high-resolution mass spectra (HRMS) were measured on JEOL JMS-700 and MS-AX500 instruments, respectively. IR spectra were recorded on a JASCO FT/IR-4100. Only the strongest and/or structurally important absorption is reported as the IR data given in cm−1. Melting points were determined with Yazawa Micro Melting Point BY-2 and are not corrected.
General Procedure: Synthesis of Col-003 Analogues by Negishi Cross-CouplingTo the solution of 5-bromosalicylaldehyde derivative 3 (1.0 equiv), Pd(OAc)2 (0.01 equiv) and S-Phos (0.02 equiv) in THF (1.0 mL/mmol) was added dropwise the benzylzinc reagent 415) (1.3 equiv) in THF (0.70 mL/mmol) at room temperature under an argon atmosphere. After being stirred at the same temperature for 4 h, the reaction mixture was quenched with saturated aqueous NH4Cl. The organic layer was separated, and the water layer was extracted twice with Et2O. The organic layer was washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the resulting residue was purified by column chromatography on silica gel (eluted with hexane/EtOAc = 50 : 1) to afford Col-003 analogues 2.
5-Benzylsalicylaldehyde (2aA)Colorless oil; 64% yield (682 mg, 3.2 mmol); 1H-NMR (400 MHz, CDCl3) δ: 10.9 (1H, s), 9.82 (1H, s), 7.37 (1H, dd, J = 8.0, 2.4 Hz), 7.31 (3H, t, J = 8.0 Hz), 7.24–7.20 (1H, m), 7.17 (2H, d, J = 7.2 Hz), 6.92 (1H, d, J = 8.8 Hz), 3.96 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.5, 160.1, 140.4, 137.8, 133.3, 132.7, 128.8, 128.6, 126.4, 120.4, 117.7, 40.6; IR (neat) 3164, 3027, 2845, 1624, 1483, 1279, 1146, 735, 700 cm−1; HR electron ionspray (EI)MS m/z: 212.0852 ([M]+ calcd for C14H12O2: 212.0837).
5-(2-Chlorobenzyl)salicylaldehyde (2aB)White solid; 57% yield (140 mg, 0.57 mmol); mp 39.5–40.6°C; 1H-NMR (400 MHz, CDCl3) δ: 10.9 (1H, s), 9.83 (1H, s, a), 7.40–7.36 (2H, m), 7.33 (1H, d, J = 2.4 Hz), 7.22–7.16 (3H, m), 6.93 (1H, d, J = 8.8 Hz), 4.08 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.5, 160.2, 138.0, 137.7, 134.2, 133.4, 131.0, 130.9, 129.8, 128.1, 127.0, 120.5, 117.8, 38.1; IR (neat) 3194, 3064, 2848, 1658, 1483, 1281, 1146, 770, 752 cm−1; HREIMS m/z: 246.0449 ([M]+ calcd for C14H11ClO2: 246.0448).
5-(2-Methylbenzyl)salicylaldehyde (2aC)Colorless oil; 97% yield (1.5 g, 6.6 mmol); 1H-NMR (400 MHz, CDCl3) δ: 10.9 (1H, s), 9.76 (1H, s), 7.29 (1H, dd, J = 8.0, 2.0 Hz), 7.22 (1H, d, J = 0.8 Hz), 7.17–7.15 (3H, m), 7.10–7.06 (1H, m), 6.89 (1H, m), 3.94 (2H, s), 2.22 (3H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.5, 159.8, 138.1, 137.5, 136.4, 132.9, 131.8, 130.4, 129.7, 126.7, 126.1, 120.3, 117.5, 38.1, 19.5; IR (neat) 3103, 3018, 2850, 1653, 1483, 1279, 1144, 770, 743 cm−1; HREIMS m/z: 226.0994 ([M]+ calcd for C15H14O2: 226.0994).
5-Benzyl-3-fluorosalicylaldehyde (2bA)White solid; 95% yield (436 mg, 1.9 mmol); mp 64.9–66.2°C; 1H-NMR (400 MHz, CDCl3) δ: 10.8 (1H, s), 9.85 (1H, d, J = 1.6 Hz), 7.32 (2H, t, J = 8.0 Hz), 7.26–7.22 (1H, m), 7.19–7.16 (4H, m), 3.95 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.2 (d, J = 2.5 Hz), 152.0, 148.1 (d, J = 12 Hz), 139.6, 133.1 (d, J = 4.9 Hz), 128.8 (d, J = 3.3 Hz), 128.0 (d, J = 3.3 Hz), 126.7, 123.5, 123.3, 122.0 (d, J = 4.2 Hz), 40.6; IR (neat) 3165, 3028, 2853, 1663, 1480, 1388, 1276, 718, 701 cm−1; HREIMS m/z: 230.0757 ([M]+ calcd for C14H11FO2: 230.0743).
5-(2-Chlorobenzyl)-3-fluorosalicylaldehyde (2bB)White solid; 70% yield (183 mg, 0.69 mmol); mp 93.5–95.3°C; 1H-NMR (400 MHz, CDCl3) δ: 10.8 (1H, s), 9.85 (1H, m), 3.90 (1H, dd, J = 12.0, 2.4 Hz), 7.24–7.16 (5H, m), 4.07 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.2 (d, J = 2.5 Hz), 152.0, 148.2 (d, J = 13 Hz), 137.3, 131.5, 131.4 (d, J = 4.9 Hz), 130.9, 129.9, 128.4, 128.1 (d, J = 3.3 Hz), 127.2, 123.4, 123.2, 122.1 (d, J = 3.3 Hz), 38.1; IR (neat) 3420, 2940, 2865, 1665, 1476, 1266, 1142, 739, 716 cm−1; HREIMS m/z: 264.0341 ([M]+ calcd for C14H10ClFO2: 264.0353).
3-Fluoro-5-(2-methylbenzyl)salicylaldehyde (2bC)Yellow solid; 64% yield (157 mg, 0.64 mmol); mp 79.0–80.4°C; 1H-NMR (400 MHz, CDCl3) δ: 10.8 (1H, s), 9.82 (1H, s), 7.19–7.07 (6H, m), 3.95 (2H, s), 2.22 (3H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.3 (d, J = 3.3 Hz), 147.9 (d, J = 3.4 Hz), 137.4, 136.5, 132.4 (d, J = 5.4 Hz), 130.6, 129.8, 127.8 (d, J = 2.4 Hz), 127.1, 126.3, 123.3, 123.1, 122.0 (d, J = 3.3 Hz), 38.2 (d, J = 1.6 Hz), 19.5; IR (neat) 3052, 3015, 2849, 1662, 1477, 1384, 1276, 725, 713 cm−1; HREIMS m/z: 244.0900 ([M]+ calcd for C15H13FO2: 244.0900).
5-Benzyl-3-chlorosalicylaldehyde (2cA)Yellow solid; 93% yield (459 mg, 1.86 mmol); mp 94.0–95.0°C; 1H-NMR (400 MHz, CDCl3) δ: 11.3 (1H, s), 9.83 (1H, s), 7.46 (1H, d, J = 2.0 Hz), 7.32 (2H, t, J = 7.2 Hz), 7.28–7.25 (2H, m), 7.17 (2H, d, J = 7.2 Hz), 3.95 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.0, 155.5, 139.6, 137.4, 134.1, 133.5, 131.8, 128.8, 126.7, 122.1, 121.2, 40.5; IR (neat) 3257, 3033, 2892, 1647, 1453, 1436, 1222, 1140, 752, 701 cm−1; HREIMS m/z: 246.0443 ([M]+ calcd for C14H11ClO2: 246.0448).
3-Chloro-5-(2-chlorobenzyl)salicylaldehyde (2cB)Yellow solid; 90% yield (254 mg, 0.90 mmol); mp 111.0–112.0°C; 1H-NMR (400 MHz, CDCl3) δ: 11.3 (1H, s), 9.83 (1H, s), 7.47 (1H, d, J = 2.0 Hz), 7.41–7.39 (1H, m), 7.28 (1H, d, J = 2.0 Hz), 7.25–7.22 (2H, m), 7.20–7.18 (1H, m), 4.07 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.0, 155.6, 137.24, 137.21, 134.2, 131.9, 131.8, 130.9, 129.9, 128.3, 127.2, 122.1, 121.1, 37.9; IR (neat) 3060, 2924, 2868, 1652, 1445, 1220, 1140, 759, 704 cm−1; HREIMS m/z: 280.0049 ([M]+ calcd for C14H10Cl2O2: 280.0058).
3-Chloro-5-(2-methylbenzyl)salicylaldehyde (2cC)Yellow solid; 43% yield (112 mg, 0.43 mmol); mp 96.8–98.0°C; 1H-NMR (400 MHz, CDCl3) δ: 11.3 (1H, s), 9.79 (1H, s), 7.41 (1H, d, J = 2.0 Hz), 7.20–7.17 (4H, m), 7.10–7.08 (1H, m), 3.95 (2H, s), 2.23 (3H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.1, 155.4, 137.4, 137.1, 136.4, 132.8, 131.5, 130.6, 129.8, 127.1, 126.3, 122.0, 121.1, 38.0, 19.6; IR (neat) 3071, 3021, 2921, 1652, 1458, 1217, 1140, 746, 700 cm−1; HREIMS m/z: 260.0604 ([M]+ calcd for C15H13ClO2: 260.0604).
General Procedure: Synthesis of Col-003 Analogues 2d by ortho-BrominationTo a solution of 2a (1.0 equiv) in MeCN (10 mL/mmol) was added N-bromosuccinimide (NBS) (1.3 equiv) at room temperature under an argon atmosphere. After being stirred at the same temperature for 3 h, the reaction mixture was quenched with saturated aqueous Na2S2O3. The organic layer was separated, and the water layer was extracted once with Et2O and twice with EtOAc. The organic layer was washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the resulting residue was purified by column chromatography on silica gel (eluted with hexane/EtOAc = 60 : 1) to afford the 3-bromosalicylaldehyde derivative 2d.
5-Benzyl-3-bromosalicylaldehyde (2dA)Yellow solid; 75% yield (108 mg, 0.37 mmol); mp 109.0–110.3°C; 1H-NMR (400 MHz, CDCl3) δ: 11.4 (1H, s), 9.78 (1H, s), 7.63 (1H, d, J = 2.0 Hz), 7.34–7.32 (3H, m), 7.26–7.23 (1H, m), 7.17 (2H, d, J = 7.2 Hz), 3.95 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.0, 156.5, 140.4, 139.6, 138.1, 134.1, 132.7, 128.8, 126.7, 121.0, 111.2, 40.4; IR (neat) 3173, 3027, 2851, 1657, 1453, 1239, 1136, 737, 698 cm−1; HREIMS m/z: 289.9934 ([M]+ calcd for C14H11BrO2: 289.9942).
5-(2-Chlorobenzyl)-3-bromosalicylaldehyde (2 dB)White solid; 90% yield (108 mg, 0.37 mmol); mp 122.3–123.6°C; 1H-NMR (400 MHz, CDCl3) δ: 11.5 (1H, s), 9.79 (1H, s), 7.64 (1H, d, J = 2.4 Hz), 7.41–7.39 (1H, m), 7.32 (1H, d, J = 2.4 Hz), 7.24–7.22 (2H, m), 7.20–7.18 (1H, m), 4.09 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 195.9, 170.2, 156.6, 140.3, 137.3, 134.2, 133.6, 132.7, 130.9, 129.9, 128.4, 127.2, 111.2, 37.9; IR (neat) 3422, 3067, 2851, 1654, 1444, 1277, 1136, 752, 696 cm−1; HREIMS m/z: 323.9549 ([M]+ calcd for C14H10BrClO2: 323.9553).
3-Bromo-5-(2-methylbenzyl)salicylaldehyde (2dC)White solid; 33% yield (101 mg, 0.33 mmol); mp 99.2–100.9°C; 1H-NMR (400 MHz, CDCl3) δ: 11.4 (1H, s), 9.75 (1H, s), 7.58 (1H, d, J = 2.0 Hz), 7.22–7.17 (4H, m), 7.10 (1H, m), 3.95 (2H, s), 2.23 (3H, m); 13C-NMR (100 MHz, CDCl3) δ: 196.0, 156.4, 140.2, 137.4, 136.5, 133.4, 132.4, 130.7, 129.8, 127.1, 126.3, 121.0, 111.2, 38.0, 19.6; IR (neat) 3063, 3025, 2965, 1652, 1455, 1217, 1140, 745, 691 cm−1; HREIMS m/z: 304.0086 ([M]+ calcd for C15H13BrO2: 304.0099).
General Procedure: Synthesis of Col-003 Analogues 2e by ortho-NitrationTo a suspension of 2a (1.0 equiv) and NH4NO3 (2.0 equiv) in chloroform (7 mL/mmol) was added trifluoroacetic anhydride (TFAA) (8.0 equiv) at 0°C under an argon atmosphere. After being stirred in reflux for 2 h, the reaction mixture was cooled to room temperature and diluted with H2O. The organic layer was separated, and the water layer was extracted three times with chloroform. The combined organic layers were washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the resulting residue was purified by column chromatography on silica gel (eluted with hexane/EtOAc = 8 : 1) to afford the 3-nitrosalicylaldehyde derivative 2e.
5-Benzyl-3-nitrosalicylaldehyde (2eA) (Col-003 (1))Orange solid; 70% yield (715 mg, 2.8 mmol); mp 101.8–103.5°C; 1H-NMR (400 MHz, CDCl3) δ: 11.3 (1H, s), 10.4 (1H, s), 8.15 (1H, d, J = 2.4 Hz), 7.93 (1H, d, J = 2.4 Hz), 7.32 (2H, t, J = 8.0 Hz), 7.27–7.23 (1H, m), 7.17 (2H, d, J = 7.2 Hz), 4.00 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 189.3, 155.0, 138.8, 137.3, 133.5, 131.0, 128.9, 128.7, 126.9, 125.3, 116.8, 40.4; IR (neat) 3173, 3027, 2851, 1657, 1453, 1239, 1136, 737, 698 cm−1; HREIMS m/z: 257.0675 ([M]+ calcd for C14H11NO4: 257.0688).
5-(2-Chlorobenzyl)-3-nitrosalicylaldehyde (2eB)Orange solid; 12% yield (29 mg, 0.10 mmol); mp 86.5–88.0°C; 1H-NMR (400 MHz, CDCl3) δ: 11.3 (1H, s), 10.4 (1H, s), 8.15 (1H, d, J = 2.4 Hz), 7.93 (1H, d, J = 2.4 Hz), 7.41–7.39 (1H, m), 7.26–7.22 (3H, m), 4.11 (2H, s); 13C-NMR (100 MHz, CDCl3) δ: 189.2, 155.1, 137.2, 136.4, 135.1, 134.2, 131.9, 131.03, 130.99, 130.1, 128.8, 127.4, 125.3, 38.1; IR (neat) 3224, 3072, 2854, 1665, 1530, 1260, 1094, 1038, 799 cm−1; HREIMS m/z: 291.0287 ([M]+ calcd for C14H10ClNO4: 291.0298).
3-Nitro-5-(2-methylbenzyl)salicylaldehyde (2eC)Orange solid; 41% yield (121 mg, 0.45 mmol, 41%); mp 93.3–94.9°C; 1H-NMR (400 MHz, CDCl3) δ: 10.9 (1H, s), 9.83 (1H, d, J = 2.0 Hz), 8.06–7.97 (2H, m), 7.36–7.23 (3H, m), 6.96 (1H, d, J = 8.4 Hz), 4.05 (2H, s), 2.35 (3H, s); 13C-NMR (100 MHz, CDCl3) δ: 196.4, 160.3, 133.8, 131.3, 130.3, 130.0, 126.5, 125.2, 124.4, 122.5, 121.9, 121.3, 120.5, 38.1, 19.9; IR (neat) 3161, 3068, 2853, 1657, 1520, 1345, 1280, 771, 740 cm−1; HREIMS m/z: 271.0745 ([M]+ calcd for C15H13NO4: 271.0845).
Synthesis of 3-Nitro-5-phenylsalicylaldehyde (5eD)To a solution of 3-nitro-5-bromosalicylaldehyde (3e) (48 mg, 0.196 mmol, 1.0 equiv) in 1,4-dioxane (1.6 mL, 8.3 mL/mmol) and H2O (1.6 mL, 0.83 mL/mmol) was added phenyl boronic acid (6D) (48 mg, 0.392 mmol, 2.0 equiv), K3PO4 (83 mg, 0.392 mmol, 2.0 equiv) and Pd(PPh3)4 (23 mg, 0.0196 mmol, 0.1 equiv) at room temperature under an argon atmosphere. After being stirred at 95°C for 18 h, the reaction mixture was cooled to room temperature and diluted with H2O. The organic layer was separated, and the water layer was extracted three times with EtOAc. The organic layer was washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the resulting residue was purified by column chromatography on silica gel (eluted with hexane/EtOAc = 40 : 1) to afford 3-nitro-5-phenylsalicylaldehyde (5eD) (27 mg, 0.11 mmol, 56%) as an orange solid; mp 115.3–116.4°C; 1H-NMR (400 MHz, CDCl3) δ: 11.4 (1H, s), 10.5 (1H, s), 8.57 (1H, d, J = 2.4 Hz), 8.35 (1H, d, J = 2.4 Hz), 7.60–7.58 (2H, m), 7.51–7.48 (2H, m), 7.45–7.41 (1H, m); 13C-NMR (100 MHz, CDCl3) δ: 189.1, 155.6, 137.0, 135.4, 135.2, 133.6, 129.3, 129.0, 128.6, 126.7, 125.7; IR (neat) 3237, 3067, 2926, 1693, 1462, 1261, 1139, 761, 696 cm−1; HREIMS m/z: 243.0529 ([M]+ calcd for C13H9NO4: 243.0532).
General Procedure: Synthesis of Compounds 9 by Suzuki–Miyaura Cross-CouplingTo a solution of alkylborane 822) (1.5 equiv) in 1,4-dioxane (9.0 mL/mmol) was added 5-bromo-2-(methoxymethoxy)benzaldehyde 723) (1.0 equiv), K3PO4 (2.0 equiv), and Pd(PPh3)4 (0.01 equiv) at room temperature. After being stirred at 45°C for 12 h, the reaction mixture was diluted with H2O. The organic layer was separated, and the water layer was extracted three times with EtOAc. The organic layer was washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the resulting residue was purified by column chromatography on silica gel (eluted with hexane/EtOAc = 30 : 1) to afford coupling products 9.
2-(Methoxymethoxy)-5-phenethylbenzaldehyde (9E)Brown oil; 69% yield (230 mg, 0.85 mmol); 1H-NMR (400 MHz, CDCl3) δ: 10.5 (1H, s), 7.69 (1H, d, J = 2.4 Hz), 7.32–7.10 (7H, m), 5.28 (2H, s), 3.52 (3H, s), 2.89 (4H, s); 13C-NMR (100 MHz, CDCl3) δ: 190.1, 158.1, 141.2, 136.1, 128.44, 128.39, 128.36, 128.3, 127.8, 126.0, 115.1, 94.7, 56.4, 37.7, 36.8; IR (neat) 2929, 2853, 1683, 1495, 1151, 987, 700 cm−1; HREIMS m/z: 270.1234 ([M]+ calcd for C17H18O3: 270.1256).
2-(Methoxymethoxy)-5-(3-phenylpropyl)benzaldehyde (9F)Orange oil; 73% yield (255 mg, 0.89 mmol); 1H-NMR (400 MHz, CDCl3) δ: 10.5 (1H, s), 7.66 (1H, d, J = 2.4 Hz), 7.34 (1H, dd, J = 8.6, 2.4 Hz), 7.28 (2H, t, J = 7.6 Hz), 7.20–7.12 (4H, m), 5.27 (2H, s), 3.51 (3H, s), 2.63 (4H, q, J = 6.8 Hz), 1.97–1.89 (2H, quin); 13C-NMR (100 MHz, CDCl3) δ: 189.9, 158.0, 141.9, 136.0, 135.8, 128.4, 128.3, 127.7, 125.8, 125.2, 115.1, 94.7, 56.4, 35.3, 34.3, 32.8; IR (neat) 2935, 2858, 1684, 1582, 1149, 988 cm−1; HREIMS m/z: 284.1411 ([M]+ calcd for C18H20O3: 284.1412).
2-(Methoxymethoxy)-5-(4-phenylbutyl)benzaldehyde (9G)Brown oil; 65% yield (238 mg, 0.80 mmol); 1H-NMR (400 MHz, CDCl3) δ: 10.5 (1H, s), 7.64 (d, J = 2.4 Hz), 7.32 (1H, dd, J = 8.6, 2.4 Hz), 7.28–7.25 (2H, m), 7.18–7.11 (4H, m), 5.23 (2H, s), 3.51 (3H, s), 2.64–2.59 (4H, m), 1.66–1.62 (4H, m); 13C-NMR (100 MHz, CDCl3) δ: 189.9, 157.9, 142.3, 136.1, 135.9, 128.33, 128.27, 128.2, 127.6, 125.7, 125.2, 115.0, 94.7, 56.4, 35.7, 34.7, 30.9; IR (neat) 2933, 2857, 1685, 1495, 1149, 988, 700 cm−1; HREIMS m/z: 298.1550 ([M]+ calcd for C19H22O3: 298.1569).
General Procedure: Synthesis of Col-003 Analogues 5eE–5eG by Regioselective NitrationTo a solution of MOM ether 9 (1.0 equiv) in 1,4-dioxane (70 mL/mmol) was added 4 M HCl in 1,4-dioxane (70 mL/mmol) slowly at 10°C under an argon atmosphere. After being stirred at the same temperature for 1 h, the reaction mixture was concentrated in vacuo, and the resulting residue was used for the next reaction without further purification.
To a suspension of crude product and NH4NO3 (2.0 equiv) in chloroform (7 mL/mmol) was added TFAA (8.0 equiv) at 0°C under an argon atmosphere. After being stirred in reflux for 1.5 h, the reaction mixture was cooled to room temperature and diluted with H2O. The organic layer was separated, and the water layer was extracted three times with chloroform. The organic layer was washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the resulting residue was purified by column chromatography on silica gel (eluted with hexane/EtOAc = 25 : 1) to afford nitrated analogues 5eE–5eG.
3-Nitro-5-(2-phenethyl)salicylaldehyde (5eE)Orange solid; 38% yield (64 mg, 0.24 mmol); mp 107.1–108.2°C; 1H-NMR (400 MHz, CDCl3) δ: 11.3 (1H, s), 10.4 (1H, s), 8.08 (1H, d, J = 2.4 Hz), 7.87 (1H, d, J = 2.4 Hz), 7.31–7.20 (3H, m), 7.13 (2H, d, J = 6.8 Hz), 3.01–2.91 (4H, m); 13C-NMR (100 MHz, CDCl3) δ: 189.2, 154.9, 139.9, 137.1, 134.9, 133.6, 130.8, 128.6, 128.4, 126.5, 125.2, 37.1, 36.3; IR (neat) 3055, 2921, 2861, 1691, 1532, 1305, 768, 700 cm−1; HREIMS m/z: 271.0835 ([M]+ calcd for C15H13NO4: 271.0845).
3-Nitro-5-(3-phenylpropyl)benzaldehyde (5eF)Orange oil; 36% yield (65 mg, 0.23 mmol); 1H-NMR (400 MHz, CDCl3) δ: 11.2 (1H, s), 10.4 (1H, s), 8.14 (1H, d, J = 2.0 Hz), 7.92 (1H, d, J = 2.4 Hz), 7.30 (2H, t, J = 7.6 Hz), 7.22–7.16 (3H, m), 2.70–2.65 (4H, m), 1.98 (2H, quin, J = 7.6 Hz); 13C-NMR (100 MHz, CDCl3) δ: 189.2, 154.9, 141.2, 136.9, 134.9, 134.4, 130.6, 128.5, 128.4, 126.1, 125.3, 35.1, 33.9, 32.3; IR (neat) 3219, 2934, 2860, 1694, 1538, 1463, 1308, 751, 700 cm−1; HREIMS m/z: 285.0987 ([M]+ calcd for C16H15NO4: 285.1001).
3-Nitro-5-(4-phenylbutyl)salicylaldehyde (5eG)Orange solid; 21% yield (40 mg, 0.13 mmol); mp 52.4–53.1°C; 1H-NMR (400 MHz, CDCl3) δ: 11.2 (1H, s), 10.4 (1H, s), 8.12 (1H, d, J = 2.0 Hz), 7.90 (1H, d, J = 2.0 Hz), 7.28 (2H, t, J = 6.8 Hz), 7.20–7.15 (3H, m), 2.69–2.63 (4H, m), 1.67 (4H, quin, J = 4.0 Hz); 13C-NMR (100 MHz, CDCl3) δ: 189.2, 154.8, 141.9, 136.9, 134.9, 134.6, 130.6, 128.4, 128.3, 125.9, 125.2, 35.6, 34.3, 30.7, 30.4; IR (neat) 3218, 2934, 2858, 1693, 1537, 1309, 749, 701 cm−1; HREIMS m/z: 299.1146 ([M]+ calcd for C17H17NO4 299.1158).
This study was supported by a Grant from the New Energy and Industrial Technology Development Organization (NEDO) of Japan. This work was partially supported by Japan Society for the Promotion of Science KAKENHI Grant No. JP15H05837 (Grant-in-Aid for Scientific Research on Innovative Areas: Middle Molecular Strategy) and the Platform Project for Supporting Drug Discovery and Life Science Research from AMED under Grant Nos. JP19am0101095 and JP19am0101100.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.