2020 Volume 68 Issue 3 Pages 288-291
2020 Volume 68 Issue 3 Pages 288-291
We report a Pd-catalyzed β-arylation of cyclic α,β-unsaturated O-methyl oximes with aryl iodides. This reaction shows complete regioselectivity and excellent functional group tolerance. β-Arylation of 2-cyclohexen-1-one O-methyl oxime (existing as 2 : 1 E/Z mixture) with certain aryl iodides such as 4-iodoanisole affords only β-arylated (E)-O-methyl oximes.
Carbonyl compounds are very important in organic and organometallic chemistry. Functionalization of carbonyl compounds using metal catalysts has been widely and energetically researched.1–5) Recently, numerous reports on the direct β-functionalization of carbonyl compounds have been published, using simple saturated and α,β-unsaturated carbonyl compounds6–15) (Chart 1A). Similarly, acyclic oxime species have also been investigated.16–23) Oxime species are easily prepared from carbonyl compounds, and play an important and versatile role in organic synthesis.16–30) In metal-promoted direct β-functionalization of acyclic oxime species, the oxime group usually behaves as a directing N-coordinating functionality16–23) (Chart 1B). On the other hand, metal-promoted direct β-functionalization of cyclic oxime species such as 2-cyclohexen-1-one oxime, whose oxime group is not effective as a directing N-coordinating functionality, has not been reported yet (Chart 1C). Thus, we focused on the β-functionalization of cyclic α,β-unsaturated oxime species. This paper describes the Pd-catalyzed β-arylation of cyclic α,β-unsaturated O-methyl oximes with aryl iodides.

As a model study, we first examined the β-arylation of 2-cyclohexen-1-one (1) with aryl iodide according to the procedures described by Huang and Dong.10) This was followed by oximation of the resulting β-arylated ketone to yield the desired β-arylated oxime species (Chart 2). Pd-catalyzed β-arylation of 2-cyclohexen-1-one (1) with 4-iodoanisole afforded 3-(4-methoxyphenyl)cyclohexanone. Subsequent oximation of the resulting ketone gave the desired 3-(4-methoxyphenyl)cyclohexanone O-methyl oxime (2) in 54% yield (2 steps). The obtained product (2) was a 1 : 1 mixture of E- and Z-isomers.

Next, we attempted the β-arylation of O-methyl oxime (3)31) (existing as a 2 : 1 E/Z mixture), prepared from 2-cyclohexen-1-one (1), with 4-iodoanisole. This reaction proceeded to afford 3-(4-methoxyphenyl)-2-cyclohexen-1-one O-methyl oxime (4a) instead of 3-(4-methoxyphenyl)cyclohexanone O-methyl oxime (2). Conjugate addition to the α,β-unsaturated ketone and subsequent protonation of the resulting Pd(II) enolate typically occurs.10,14,15) However, in the O-methyl oxime, it is thought that β-hydrogen elimination occurred (Chart 3). The reaction yield was low (11% yield), but this series of reactions, oximation of ketone (1) followed by β-arylation of O-methyl oxime (3), had the synthetic advantage of giving α,β-unsaturated O-methyl oxime (4a) which could not be obtained by β-arylation of the ketone (1) and subsequent oximation.

To improve the yield of the resulting O-methyl oxime (4a), we examined the reaction conditions (Table 1). Using Pd(OAc)2 and AgOAc, the yield was twice as much as that using the trifluoroacetate counterion (entry 2). Cu(OAc)2, AgTFA, Cs2CO3 and NaOtBu were less effective as additives (entries 3–6). It was thought that the less electron-rich PPh3 would be more suitable as the phosphine ligand (entries 8 and 9), but the presence or absence of PPh3 was not crucial in this reaction (entry 9 vs. entry 10). In contrast to the phosphine ligands, AgOAc was essential (entry 11). Solvent effects were also surveyed. Only 1,4-dioxane without 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) resulted in a decreased yield (entry 7), thus HFIP was important. Toluene also proved to be efficient (entries 12 and 13). However, toluene afforded not only the desired 3-(4-methoxyphenyl)-2-cyclohexen-1-one O-methyl oxime (4a) but also a mixture of undesired β-arylated O-methyl oximes, 3-tolyl-2-cyclohexen-1-one O-methyl oximes (4e–g),32) resulting from starting O-methyl oxime (3) and toluene instead of 4-iodoanisole. Finally, Pd catalysts were investigated (entries 14 and 15). As a result, replacing Pd(OAc)2 with Pd(PPh3)2Cl2 afforded the desired product (4a) in 48% yield (entry 15). The resulting product (4a) was only the E-isomer, which was determined by a 2D nuclear Overhauser effect spectroscopy (NOESY) NMR experiment. Along with the product (4a), only the starting O-methyl oxime (3) was recovered (46% recovery). O-Methyl oxime (2) was not observed. From the ratio of E- and Z-isomers of recovered starting O-methyl oxime (3) (existing as approx. 2 : 3 E/Z mixture), it was considered that there was no possibility of isomerization of the O-methyl oxime under this reaction conditions.33)
 | ||||
|---|---|---|---|---|
| Entry | Pd catalyst (10 mol%) | Ligand (20 mol%) | Additive (2 equiv.) | Yield (%) |
| 1 | Pd(TFA)2 | PCy3 | AgTFA | 11 |
| 2 | Pd(OAc)2 | PCy3 | AgOAc | 22 |
| 3 | Pd(OAc)2 | PCy3 | Cu(OAc)2 | 6 |
| 4 | Pd(OAc)2 | PCy3 | AgTFA | 2 |
| 5b) | Pd(OAc)2 | PCy3 | Cs2CO3 | 8 |
| 6b) | Pd(OAc)2 | PCy3 | NaOtBu | 7 |
| 7b) | Pd(OAc)2 | PCy3 | AgOAc | 9 |
| 8 | Pd(OAc)2 | P(iPr)3 | AgOAc | 28 |
| 9 | Pd(OAc)2 | PPh3 | AgOAc | 34 |
| 10 | Pd(OAc)2 | None | AgOAc | 30 |
| 11 | Pd(OAc)2 | PPh3 | None | 0 |
| 12c) | Pd(OAc)2 | PPh3 | AgOAc | 37 |
| 13c) | Pd(OAc)2 | None | AgOAc | 32 |
| 14 | Pd(dppf)Cl2 | None | AgOAc | 36 |
| 15 | Pd(PPh3)2Cl2 | None | AgOAc | 48 |
a) All the reactions were run with 3 (0.2 mmol) and 4-iodoanisole (0.2 mmol) in 1.0 mL solvent for 18 h. b) HFIP was not added. c) Toluene was used instead of 1,4-dioxane.
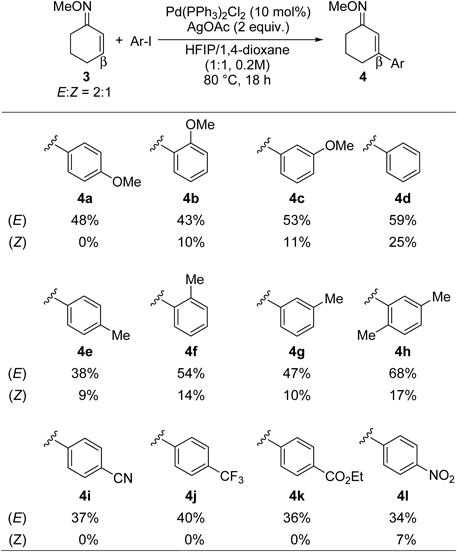
With the optimized conditions in hand, the substrate scope of the aryl iodides was investigated (Table 2). Substitutions on the aryl group at the ortho, meta, or para positions were all tolerated (4a–c, 4e–h). Aryl iodides with both electron-donating and electron-withdrawing groups participated to give the corresponding β-arylated O-methyl oximes (4i–l). Under this reaction conditions, a variety of functional groups were tolerated, including nitriles (4i), esters (4k), and nitro groups (4l). In all cases, E-isomers were obtained preferentially over Z-isomers.34) The E- and Z-isomers were easily separable by silica gel column chromatography. The exact reason for the different ratios of resulting E- and Z-isomers based on the starting aryl iodides is unclear.
 |
a) All the reactions were run with 3 (0.2 mmol) and aryl iodide (0.2 mmol) in 1.0 mL solvent for 18 h.
The scope of the O-methyl oxime component with different ring sizes was also examined (Table 3). The 5- and 7-membered ring O-methyl oximes such as 2-cyclopenten-1-one O-methyl oxime35,36) and 2-cyclohepten-1-one O-methyl oxime37) afforded the desired products (5i, 6i).34,38)
 |
a) All the reactions were run with O-methyl oxime (0.2 mmol) and 4-iodobenzonitrile (0.2 mmol) in 1.0 mL solvent for 18 h.
Inspired by the side reaction of O-methyl oxime (3) and toluene in Table 1 (entries 12 and 13), we also examined Fujiwara-Moritani-type arylation.39–42) Using p-xylene as both the aryl source and solvent resulted in the β-arylated product (4h) in 49% total yield43) (Chart 4).

In summary, we have developed a novel method for the Pd-catalyzed β-arylation of O-methyl oximes, which can be prepared from cyclic α,β-unsaturated ketones such as 2-cyclohexen-1-one. This reaction can stand further improvement with respect to the yield, but it shows complete site-selectivity and extensive functional group tolerance. Using certain aryl iodides, only the E-isomer was obtained from the starting O-methyl oxime which existed as a mixture of E- and Z-isomers. In addition, not only aryl iodides but also arenes (e.g., p-xylene and toluene) can be used as the aryl sources. Efforts on direct β-arylation of cyclic saturated O-methyl oximes with aryl halides and arenes are ongoing.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.