2020 Volume 68 Issue 3 Pages 292-297
2020 Volume 68 Issue 3 Pages 292-297
In this study, the adsorption capability of phosphate ion using a novel tri-metals complex hydroxide was evaluated for preventing the eutrophication in water environment. A nickel–aluminum–zirconium complex hydroxide (NAZ) was synthesized using each inorganic sulfate mixing ratio of 0.9 : 1.0 : 0.1 and was calcined at different temperatures. The characteristics of the NAZ were analyzed by scanning electron microscopy images, X-ray diffraction analysis, elemental distribution, and binding energy. Moreover, the amount adsorbed of phosphate ion onto uncalcined and calcined NAZ was measured. That of phosphate ions onto the uncalcined was the largest of all. These results suggested that the adsorption of phosphate ions tends to depend on the physicochemical properties (e.g., amount of hydroxyl groups, pore volumes, and pH) of the adsorbents. Moreover, the adsorption mechanism of phosphate ions was evaluated on the basis of binding energy and elemental analysis. After adsorption, the binding energy of phosphorus P (2s and 2p) peaked and the sulfur peak intensity S(2s) reduced. This result indicated that the adsorption mechanism of phosphate would be exchanged with sulfate ions.
Phosphate ion is an essential nutrient that is known to be responsible for the acceleration of eutrophication in many water environments.1) Eutrophication is the excessive richness of nutrients in a water body, and it causes the abundance of aquatic plants, growth of algae, and depletion of dissolved oxygen, resulting in the red tide frequently observed in lakes and ocean environments.2) Therefore, there is currently an urgent demand for phosphate-ion removal from aqueous solutions. On the other hand, phosphorus is a highly valuable mineral that is necessary for all living organisms on Earth. Phosphate ores account for approximately 80% of fertilizer raw materials.3) Phosphorus fertilizer production is highly dependent on the mining of phosphorus rock, which is a nonrenewable resource. However, Japan depends solely on imports owing to a lack of native rock phosphate. The looming phosphorus scarcity crisis is a serious problem for food supply worldwide.4–6) In recent years, to solve the problems of both eutrophication and unsustainable phosphorus supply, techniques for phosphorus recovery from water environments have been evaluated.7)
The metal complex hydroxides (layered double hydroxides) have received wide attention as an effective adsorbent for the adsorption of phosphate ions because of their structure and high anionic adsorption capacity.8) The general formula of metal complex hydroxides is [M1−x2+Mx3+(OH)2][An−]x/n nH2O, where M2+ and M3+ are divalent and trivalent metal cations, respectively, in the octahedral positions of brucite-like layers. An− is an anion incorporated in the interlayer region for charge neutrality, resulting in the formation of metal complex hydroxides.9,10) Because a part of the divalent metal cations can be isomorphously substituted by trivalent metal cations, the layers acquire positive charges with the compositions ([M1−x2+Mx3+(OH)2]X−). Previous studies reported the adsorption of phosphate ions by calcined Mg–Al–CO3 layered double hydroxides, core–shell bio-ceramic/Zn-layered double hydroxides, Fe–Al–Mn trimetal oxide adsorbents, Zn–Al layered double hydroxides, and layered double-hydroxide nanocomposites.2,4,9,11) Composite adsorbents exhibited a superior phosphate-ion adsorption performance because they possess the inherent advantages of their parent materials.
In addition, zirconium (hydr)oxides were reported to be a suitable adsorbent of phosphate ions owing to its high efficiency of ad-desorption for recycling.12,13) The incorporation of more highly charged zirconium ions into brucite-like layers increases the net positive charge. This incorporation also possibly causes unusual anion-exchange selectivity and complex formation by phosphate ions with zirconium ions on the adsorbent surface and in the layers.14) Moreover, we evaluated the adsorption capability and adsorption mechanism of phosphate ions by using metal complex hydroxides (e.g., Fe–Mg-type hydrotalcite, nickel–aluminum complex hydroxides, and nickel–cobalt binary hydroxides).15–17) However, there are few reports on ternary metal complex hydroxides or on zirconium incorporation in binary metals complex hydroxides for the adsorption of phosphate ions from aqueous solutions. Thus, if phosphate-ion adsorption using zirconium incorporation in binary metal complex hydroxides (nickel–aluminum–zirconium) could be developed, its value and applicability would increase drastically. Furthermore, such incorporation could be useful for elucidating the adsorption mechanism of phosphate ions. In this study, a novel nickel–aluminum–zirconium complex hydroxide (NAZ) was prepared. The object of this study was to investigate the adsorption performance of phosphate ions from aqueous solutions with the novel substance NAZ. We focused on the adsorbent surface characteristics before and after adsorption of phosphate ions. The obtained results will provide useful information for phosphate-ion adsorption by NAZ.
NAZ (Ni : Al : Zr molar ratio of 0.9 : 1.0 : 0.1) was prepared and synthesized at the Kansai Catalyst. Co., Ltd., Osaka, Japan. NAZ was obtained the following method. Nickel(II) sulfate hexahydrate (Ni, 0.3 mol), aluminium(III) sulfate octahydrate (Al: 0.334 mol), and zirconium(IV) sulfate tetrahydrate (Zr: 0.03 mol) were dissolved with distilled water (230 g) at heating. This complex solution added to the distilled water (400 g) at pH 9.0 for 800 rpm at 25°C. The solution pH was adjusted by 25% sodium hydroxide solution. After mixing for 2 h, the suspension was filtered, washed, and dried at 110°C for 12 h. Nickel(II) sulfate hexahydrate was prepared by the Kansai Catalyst. Co., Ltd., Osaka, Japan. Aluminium(III) sulfate octahydrate and zirconium(IV) sulfate tetrahydrate were purchased from the Kishida Chemical Co., Ltd., Osaka, Japan. Twenty five percent sodium hydroxide solution was purchased from the Kaname Chemical Co., Ltd., Osaka, Japan. Five kinds of calcined NAZ were gotten by the calcination of uncalcined NAZ in a muffle furnace at temperatures of 200 (NAZ200), 280 (NAZ280), 400 (NAZ400), 470 (NAZ470), and 1000°C (NAZ1000) for 2 h in air.
The characteristics of adsorbents were evaluated by scanning electron microscopy (SEM), SU1510 (Hitachi Ltd., Osaka, Japan) and X-ray diffraction (XRD) analysis, Mini Flex II (Rigaku, Tokyo, Japan). The specific surface area was measured using a NOVA4200e specific surface analyzer (Yuasa Ionic, Osaka, Japan). The number of hydroxyl groups onto uncalcined and calcined NAZ was measured using the adsorption method of fluorine.18) The surface pH was measured using a LAQUA F-73 digital pH meter (HORIBA, Kyoto, Japan). The interaction between phosphorus and NAZ was evaluated using the electron microanalyzer (JXA-8530F, JEOL, Tokyo, Japan) and an X-ray photoelectron spectroscopy system (AXIS-NOVA, Shimadzu Co., Ltd., Kyoto, Japan).
Amount Adsorbed of Phosphate IonsThe adsorbent (0.05 g) was added to a 50 mL aliquot of 300 mg/L phosphate-ion solution (prepared using KH2PO4, FUJIFILM Wako Pure Chemical Corporation, Japan), and then were shaken at 100 rpm for 24 h at 25°C. After equilibrium, the solution was filtered by a 0.45-µm membrane filter, and then the equilibrium concentration of phosphate ion in solutions measured using the ascorbic-acid reduction method19) (DR/890, HACH, U.S.A.).
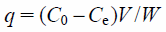 | (1) |
where q is the amount adsorbed of phosphate ion (mg/g), C0 and Ce is the initial and equilibrium concentration (mg/L), respectively, V is the solvent volume (L), and W is the adsorbent weight (g). Moreover, the concentration of sulfate ion in the filtrate after adsorption was measured using ion chromatography (DIONEX ICS-900, Thermo Fisher Scientific Inc., Japan).16) The adsorption data (n = 3) are expressed as the mean ± standard deviation (S.D.). The student’s t-test was used for analysis of adsorption capability of each adsorbent. A minimum p value of 0.05 (p < 0.05) was chosen as the significant level.
Firstly, the physicochemical properties of metal complex hydroxides are drastically changed by calcination. The obtained absorbent characteristics strongly affect the amount adsorbed for phosphate ions from aqueous solutions. We reported that the crystal structure characteristics of nickel hydroxide and aluminum hydroxide were changed by calcination at 280 or 400°C and at 200, 470, or 1000°C, respectively, and the effects of calcination treatment on the removal of anions such as phosphate.20–22) Therefore, it is very important to understand the behavior in the physicochemical properties of NAZ by calcination treatment in this study.
Recently, the adsorption properties of phosphate-ion onto novel Zr-modified metal complex hydroxides were evaluated. That adsorbates have the larger net positive charge by Zr modification, and it may also cause an unusual anion-exchange selectivity with complex formation by phosphate ions and Zr ions in the layers.14) In this study, many kinds of NAZ were synthesized and evaluated their adsorption capability for phosphate ions in liquid phase.
Figure 1 shows SEM images of uncalcined and five kinds of calcined NAZ. These adsorbents showed a rounded morphology that lacks a perfect crystal boundary. This result showed a poor degree of crystallinity and the typical hydrotalcite-like layered double hydroxides, which was formed by co-precipitation methods. This result was similar to previous studies.23–25) Figure 2 was shown the XRD patterns of the adsorbents. Those of NAZ, NAZ200, and NAZ280 are same, they have the structure of nickel hydroxide, and showed several diffraction peaks that could be indexed to the crystal structure of hydrotalcite. Next, the physicochemical characteristics of adsorbents are listed in Table 1. The specific surface area and amount of hydroxyl groups onto uncalcined NAZ 51.9 m2/g and 1.08 mmol/g, respectively. While those of NAZ400 or NAZ470 were 177.0 m2/g and 1.46 mmol/g or 180.3 m2/g and 2.13 mmol/g, respectively. The specific surface area and polarity of NAZ400 or NAZ470 were higher than those of NAZ. The calcination induced the increase of specific surface area and number of hydroxyl groups of aluminum oxyhydroxide or nickel hydroxide, and these results were same to previous studies.20,21) Moreover, the pore volumes were classified to three types, micropore (d ≦ 20 Å), mesopore (20 < d ≦ 500 Å), and macropore (d > 500 Å), and in this study the micropore and mesopore volumes were developed by calcination.

Nickel–aluminum–zirconium complex hydroxide (NAZ) was calcined at 200°C (NAZ200), 280°C (NAZ280), 400°C (NAZ400), 470°C (NAZ470), and 1000°C (NAZ1000). Measurement condition is 5 kV.

Nickel–aluminum–zirconium complex hydroxide (NAZ) was calcined at 200°C (NAZ200), 280°C (NAZ280), 400°C (NAZ400), 470°C (NAZ470), and 1000°C (NAZ1000). Measurement condition is CuKα.
| Adsorbents | Specific surface area (m2/g) | Number of hydroxyl groups (mmol/g) | Surface pH | Pore volume (µL/g) | |||
|---|---|---|---|---|---|---|---|
| d ≦ 20 Å | 20<d ≦ 500 Å | d > 500 Å | Total | ||||
| NAZ | 51.9 | 1.08 | 6.18 | 1.45 × 10−4 | 0.20 | 0.06 | 0.27 |
| NAZ200 | 55.2 | 1.37 | 6.07 | 8.5 × 10−6 | 0.20 | 0.07 | 0.26 |
| NAZ280 | 58.7 | 1.01 | 6.07 | 1.7 × 10−3 | 0.21 | 0.09 | 0.30 |
| NAZ400 | 177.0 | 1.46 | 6.10 | 6.5 × 10−3 | 0.32 | 0.07 | 0.40 |
| NAZ470 | 180.3 | 2.13 | 6.12 | 5.5 × 10−3 | 0.31 | 0.05 | 0.37 |
| NAZ1000 | 31.5 | 0.42 | 7.12 | 4.0 × 10−4 | 0.14 | 0.04 | 0.18 |
Figure 3 showed the saturated amount adsorbed of phosphate ions onto uncalcined and calcined NAZ at different temperatures. The amount adsorbed was in the order NAZ (157.3 mg/g) > NAZ400 (105.9 mg/g) ≒ NAZ470 (106.7 mg/g) > NAZ200 (88.1 mg/g) > NAZ280 (65.8 mg/g) > NAZ1000 (19.3 mg/g). The amount adsorbed onto uncalcined NAZ was higher than that onto calcined NAZs. This result indicated that calcination treatment is not suitable for increasing the amount adsorbed for phosphate ions in aqueous phase. Table 2 presents a comparison of the amount adsorbed of phosphate ion onto NAZs with those of other reported adsorbents. The amount adsorbed of phosphate ion onto NAZ are the highest of the other adsorbents reported in the literature. These results indicate that NAZ can serve as a potential adsorbent for phosphate-ion removal from aqueous solutions.1,11,14,26–28)

Experimental condition; Initial concentration: 300 mg/L, solvent volume: 50 mL, adsorbent: 0.05 g, contact time: 24 h, temperature: 25°C, agitation speed: 100 rpm, the data are expressed as the mean ± S.D., n = 3, * p < 0.05 vs. virgin NAZ for each adsorbent.
| Samples | Adsorption capability (mg/g) | pH | Temperature (°C) | Initial concentration (mg/L) | Contact time (h) | Adsorbent (g/L) | References |
|---|---|---|---|---|---|---|---|
| Fe–Zr oxide | 13.65 | 4 | 25 | 100 | 24 | 1 | 26) |
| Magnetic Fe–Zr binary oxide | 40.95 | 4 | 25 | 100 | 24 | 1 | 26) |
| Iron–Zirconium binary oxide sorbent | 100.2 | 5.5 ± 0.1 | 25 | 200 | 24 | 0.2 | 27) |
| Calcined Mg–Al–CO3 layered double hydroxides | 153.6 | Around 6.0 | 25 ± 0.2 | 148.8 | 24 | 0.5 | 28) |
| Zr4+ incorporated MgAl-layered double hydroxide | 90 | 8.7 | Room temperature | 6.0 | 72 | 0.05 | 14) |
| Nanostructured Fe–Al–Mn oxide | 48.3 | 6.8 | 25 | — | 24 | 0.2 | 11) |
| Mg/Al-LDHs biochar | 81.83 | 3.0 | 23 ± 0.2 | 500 | < 2 | 2.5 | 1) |
| NAZ | 157.2 | — | 25 | 300 | 24 | 1 | This study |
The adsorption mechanism of phosphate ions onto metal complex hydroxides is reported the electrostatic attraction, ligand exchange, and ion exchange.29–32) The presence of surface hydroxyl groups onto metal complex hydroxides contributes to the adsorption, and these surface hydroxyl groups could get exchanged with the adsorbed phosphate ion. Simultaneously, electrostatic attraction exists between the phosphate ion and electropositive adsorbent surface. At low pH, the surface hydroxyl groups onto metal complex hydroxides are protonized and facilitate the ligand exchange process because –OH2+ is easier to displace from the metal binding sites than hydroxyl groups. The ion exchange also occurs between the phosphate ion and the interlayer sulfate ion in metal complex hydroxides33) (Fig. 4). Firstly, the relationship between the amount adsorbed of phosphate ions and the physicochemical characteristics of the adsorbent was evaluated. The result shows that the amount adsorbed of phosphate ions tends to depend upon the number of hydroxyl groups (r = 0.542), mesopore volume (r = 0.470), and pH (r = −0.691). On the other hand, the specific surface area (r = 0.339), micropore (r = 0.193), and macropore (r = 0.160) were not related to the adsorption capability of phosphate ion. As previously mentioned, the NAZ surface characteristics are very important for the removal of phosphate ions in aqueous phase. Therefore, Fig. 5 showed the elemental distribution onto the NAZ surface before and after adsorption of phosphate ion (a warm or cold color indicates a high or low concentration, respectively). After adsorption, the intensity of phosphorus atoms increased, indicating that the phosphate ions were adsorbed onto the NAZ surface. These phenomena indicate that the adsorption mechanism is related to the electrostatic attraction and complex formation on the surface inner sphere. Subsequently, Fig. 6 showed the binding energies of phosphorus or sulfur before and after adsorption of phosphate ions onto NAZs surface. Phosphorous (P) peaks (179 eV for 2s and 130 eV for 2p), which were not detected before adsorption, were clearly detected after adsorption of phosphate ion. On the other hand, the intensity of the S peak (165 eV for 2p) decreased after adsorption in this study. We could confirm that ion exchange occurred between the phosphate ion and the interlayer sulfate ion in NAZ under this experimental condition. In addition, Fig. 7 showed the concentration of sulfate ions released from NAZ after adsorption. We confirmed that the amount adsorbed of phosphate ions onto NAZ and the amount released of sulfate ions were positively correlated in this study (r = 0.960), supporting the theory that sulfate ions in the interlayer of NAZ are exchanged with phosphate ions. However, further studies are needed to understand the adsorption mechanism in detail. Therefore, we must evaluate the effect of temperature, solution pH, contact time, and other properties on the adsorption of phosphate ions from aqueous solutions using NAZ.

M2+ and M3+ shows divalent cation and trivalent cation.

Experimental condition; Initial concentration: 300 mg/L, solvent volume: 50 mL, adsorbent: 0.05 g, contact time: 24 h, temperature: 25°C, agitation speed: 100 rpm. A warm or cold color indicates a high or low concentration, respectively. (Color figure can be accessed in the online version.)

Experimental condition; Initial concentration: 300 mg/L, solvent volume: 50 mL, adsorbent: 0.05 g, contact time: 24 h, temperature: 25°C, agitation speed: 100 rpm. Phosphorous (P) peaks are 179 eV for 2s and 130 eV for 2p, respectively, and the S peak is 165 eV for 2p.

Experimental condition; Initial concentration 300 mg/L, solvent volume 50 mL, adsorbent 0.05 g, contact time 24 h, temperature 25°C, agitation speed 100 rpm.
We synthesized a novel nickel–aluminum–zirconium complex hydroxide (NAZ), and adsorption characterization of phosphate ions from aqueous phase was evaluated. The specific surface area and the number of hydroxyl groups of calcined NAZ 400 and NAZ470 were higher than those of uncalcined NAZ. Thus, calcination affects the physicochemical characteristics of NAZ. The amount adsorbed of phosphate ions onto uncalcined NAZ was higher than that adsorbed onto calcined NAZ. The removal of phosphate ion may be suitable for uncalcined NAZ. In addition, the amount adsorbed of phosphate ions may depend upon the number of hydroxyl groups, the pore volumes, and pH of adsorbents. Moreover, the elemental distribution and binding energy of NAZs surfaces before and after adsorption was evaluated. Phosphorous (P) peaks were detected after the adsorption, which clearly shows that the NAZ surface characteristics are very important for the removal of phosphate ions.
The Research Foundation for Pharmaceutical Sciences.
The authors declare no conflict of interest.