Experimental
ChemistryAll solvents and reagents were purchased from commercial suppliers and used without purification, or were prepared according to published procedures. The 1H-NMR and 13C-NMR spectra of compounds synthesized in this study were recorded on a JNM-ECA600, JNM-ECA500 (JEOL Ltd., Tokyo, Japan), or Avance III HD 400 (Bruker Corp., Billerica, MA, U.S.A.) and the chemical shifts were expressed in δ (:) values, with trimethylsilane as the internal standard (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br s = broad singlet). Two sets of NMR signals were observed due to structural variations of the cis- and trans-amide rotamers. Mass spectra were recorded on a Micromass Platform LC (Micromass Ltd., Manchester, U.K.) or Shimadzu LCMS-2010EV (Shimadzu Corp., Kyoto, Japan). High-resolution (HR) mass spectral data were acquired using an LCMS-IT-TOF equipped with an electrospray ionization (ESI)/atmospheric pressure chemical ionization dual ion source (Shimadzu Corp.). Intermediates and final compounds were purified by preparative HPLC using Agilent 1260 Infinity/Agilent 6130 (Agilent Technologies Inc., Santa Clara, CA, U.S.A.) or a GX-281, UV/VIS-155, 331 PUMP, 332 PUMP, and SOFTA Model 300S ELSD (Gilson Inc., Middleton, WI, U.S.A.), under the following conditions: column, Sunfire prep C18 OBD (5.0 µm, 30 × 50 mm) (Waters Corp., Milford, MA, U.S.A.), YMC-Actus Triart C18 (5.0 µm, 30 × 50 mm) (YMC Co., Ltd., Kyoto, Japan), Xbridge Prep C18 OBD (5.0 µm, 30 × 50 mm) (Waters Corp.), or XSelect CSH C18 (5.0 µm, 30× 50 mm) (Waters Corp.); flow, 50 mL/min; linear gradient, 10–95% acetonitrile in water containing 0.1% formic acid for 7.5–11.5 min; detection wavelength, 254 nm.
tert-Butyl [(1S)-1-(5-Amino-1H-pyrazol-3-yl)ethyl]methylcarbamate (5b)To a solution of N-(tert-butoxycarbonyl)-N-methyl-L-alanine (6.0 g, 29.3 mmol) in toluene (18 mL)–methanol (12 mL) was added 2.0 M of trimethylsilyl diazomethane in diethyl ether (22.0 mL, 43.9 mmol) at 0°C, and the mixture was stirred for 1 h at room temperature. Acetic acid was added to the reaction solution until the solution became clear. The reaction mixture was basified with sat. sodium bicarbonate aq. and extracted with chloroform. The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to obtain methyl N-(tert-butoxycarbonyl)-N-methyl-L-alaninate (6.5 g, quant.) as a colorless oil, which was used for the next reaction without further purification.
A 1.9 M of sodium bis(trimethylsilyl)amide in tetrahydrofuran (68 mL, 88.6 mmol) was added to a solution of acetonitrile (4.6 mL, 88.6 mmol) in tetrahydrofuran (75 mL) at −78°C. After stirring at −50°C for 20 min, methyl N-(tert-butoxycarbonyl)-N-methyl-L-alaninate (6.0 g, 29.5 mmol) in tetrahydrofuran (45 mL) at −78°C was added to the mixture. After stirring at −50°C for 1 h, acetic acid (5.2 mL, 91.5 mmol) at −78°C was added to the mixture. The mixture was poured into sat. ammonium chloride aq. and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain tert-butyl (S)-(4-cyano-3-oxobutan-2-yl)methylcarbamate (7.2 g, crude) as a brown oil, which was used for the next reaction without further purification.
To a solution of hydrazine monohydrate (4.1 mL, 84.8 mmol), acetic acid (4.9 mL, 84.8 mmol), and ethanol (20 mL) was added tert-butyl (S)-(4-cyano-3-oxobutan-2-yl)methylcarbamate (6.0 g, 28.3 mmol) in ethanol (20 mL) at 0°C, and the mixture was stirred for 2 d at room temperature. Then, the reaction mixture was concentrated under reduced pressure and diluted with chloroform. The organic layer was washed with sat. sodium bicarbonate aq. and brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (0–10% methanol in chloroform) to obtain 5b (5.8 g, 23.9 mmol, 85%) as a brown gum. 1H-NMR (600 MHz, CDCl3) δ: 1.44 (3H, d, J = 7.0 Hz), 1.48 (9H, s), 2.65 (3H, s), 5.36 (1H, br s), 5.55 (1H, br s); MS (ESI/APCI dual) m/z: 241 [M + H]+.
Compounds 5a, c–f were obtained by the same procedure as that described for 5b.
tert-Butyl [(5-Amino-1H-pyrazol-3-yl)methyl]methylcarbamate (5a)Brown gum; 1H-NMR (600 MHz, CDCl3,) δ: 1.41–1.57 (9H, m), 2.75–3.01 (3H, m), 4.26 (2H, br s), 5.59 (1H, s); MS (ESI/APCI dual) m/z: 227 [M + H]+.
tert-Butyl [(1S)-1-(5-Amino-1H-pyrazol-3-yl)propyl]methylcarbamate (5c)Orange amorphous; 1H-NMR (400 MHz, CDCl3) δ: 0.90–1.02 (4H, m), 1.48 (9H, s), 1.74–1.95 (2H, m), 2.63 (3H, s), 3.62 (2H, br s), 4.96–5.14 (1H, m), 5.54 (1H, s); MS (ESI/APCI dual) m/z: 255 [M + H]+.
tert-Butyl [(1S)-1-(5-Amino-1H-pyrazol-3-yl)-2-methylpropyl]methylcarbamate (5d)Colorless amorphous; 1H-NMR (400 MHz, CDCl3) δ: 0.93 (3H, d, J = 15.4 Hz), 0.94 (3H, d, J = 15.4 Hz), 1.46 (9H, br s), 2.22 (1H, br s), 2.67 (3H, s), 3.43–3.77 (2H, m), 4.52–4.63 (1H, m), 5.55 (1H, s); MS (ESI/APCI dual) m/z: 269 [M + H]+.
tert-Butyl [(1S)-1-(5-Amino-1H-pyrazol-3-yl)propyl]carbamate (5e)Pale yellow amorphous; 1H-NMR (600 MHz, CDCl3) δ: 0.95–1.06 (3H, m), 1.43–1.46 (9H, m), 1.63–1.77 (1H, m), 1.83–1.98 (1H, m), 4.35–4.58 (1H, m), 4.61–4.90 (1H, m), 5.49 (1H, m); MS (ESI/APCI dual) m/z: 241 [M + H]+; chiral HPLC, 99% ee (CHIRALPAK IC-3 5 µm 4.6 × 250 mm; flow, 1 mL/min, 20% ethanol in hexane; detection wavelength, 254 nm), (S)-isomer tR = 6.62 min, (R)-isomer tR = 5.13 min.
tert-Butyl [(1S)-1-(5-Amino-1H-pyrazol-3-yl)ethyl]carbamate (5f)Pale yellow amorphous; 1H-NMR (600 MHz, CDCl3) δ: 1.43–1.49 (12H, m), 4.71–4.81 (1H, m), 4.85 (1H, br s), 5.49 (1H, s), 5.64 (1H, br s); MS (ESI/APCI dual) m/z: 227 [M + H]+.
tert-Butyl Methyl[(1S)-1-(6-methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)ethyl]carbamate (6b)To a solution of 5b (1.7 g, 6.87 mmol) in N,N-dimethylformamide (10 mL) was added ethyl (E)-3-ethoxy-2-methylacrylate (1.6 g, 10.3 mmol) and cesium carbonate (6.7 g, 20.6 mmol). After stirring at 120°C for 8 h, the reaction mixture was poured into water and extracted with chloroform. The organic layer was washed with brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (0–10% methanol in chloroform) to obtain 6b (1.4 g, 4.41 mmol, 64%) as a brown amorphous. 1H-NMR (600 MHz, CDCl3) δ: 1.49 (9H, s), 1.51 (3H, d, J = 7.0 Hz), 2.10 (3H, s), 2.67 (3H, br s), 5.28–5.62 (1H, m), 5.78 (1H, s), 8.01 (1H, s), 10.85 (1H, br s); MS (ESI/APCI dual) m/z: 307 [M + H]+.
Compounds 6a, c–f were obtained by the same procedure as that described for 6b.
tert-Butyl Methyl[(6-methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)methyl]carbamate (6a)Pale yellow amorphous; 1H-NMR (600 MHz, CDCl3) δ: 1.39–1.54 (9H, m), 2.09 (3H, s), 2.78–2.95 (3H, m), 4.31–4.51 (2H, m), 5.72–5.90 (1H, m), 7.96 (1H, s), 10.77 (1H, br s); MS (ESI/APCI dual) m/z: 291 (M – H)-.
tert-Butyl Methyl[(1S)-1-(6-methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)propyl]carbamate (6c)Colorless amorphous; 1H-NMR (400 MHz, CDCl3) δ: 0.92–1.01 (3H, m), 1.49 (9H, s), 1.78–1.93 (1H, m), 1.99–2.13 (4H, m), 2.67 (3H, s), 5.01–5.41 (1H, m), 5.79 (1H, br s), 8.00 (1H, s), 10.81 (1H, br s); MS (ESI/APCI dual) m/z: 321 [M + H]+.
tert-Butyl Methyl[(1S)-2-Methyl-1-(6-methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)propyl]carbamate (6d)Brown powder; 1H-NMR (400 MHz, CDCl3) δ: 0.91 (3H, d, J = 6.5 Hz), 0.97 (3H, d, J = 6.5 Hz), 1.47 (9H, br s), 2.10 (3H, s), 2.31–2.45 (1H, m), 2.71 (3H, s), 4.64–5.03 (1H, m), 5.71–5.90 (1H, m), 8.01 (1H, s), 10.08–10.27 (1H, m); MS (ESI/APCI dual) m/z: 335 [M + H]+.
tert-Butyl [(1S)-1-(6-Methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)propyl]carbamate (6e)Pale yellow powder; 1H-NMR (600 MHz, CDCl3) δ: 0.88–0.97 (3H, m), 1.39–1.51 (9H, m), 1.78 (1H, dt, J = 13.8, 7.1 Hz), 1.90 (1H, dd, J = 13.6, 7.0 Hz), 2.07–2.14 (3H, m), 4.72 (1H, d, J = 6.2 Hz), 5.06 (1H, d, J = 7.0 Hz), 5.82 (1H, s), 7.98 (1H, d, J = 0.8 Hz), 10.81 (1H, br s); MS (ESI/APCI dual) m/z: 307 [M + H]+.
tert-Butyl [(1S)-1-(6-Methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)ethyl]carbamate (6f)Pale yellow powder; 1H-NMR (600 MHz, CDCl3) δ: 1.45 (9H, s), 1.50 (3H, d, J = 6.6 Hz), 2.09 (3H, s), 4.88 (1H, br s), 5.06 (1H, br s), 5.81 (1H, s), 7.96 (1H, s), 10.23 (1H, br s); MS (ESI/APCI dual) m/z: 293 [M + H]+.
2-{(1S)-1-[{5-Chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]ethyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl Trifluoromethanesulfonate (8b)To a solution of 6b (717 mg, 2.34 mmol) and pyridine (0.95 mL, 11.7 mmol) in chloroform (8.0 mL) was added trifluoromethanesulfonic anhydride (0.79 mL, 4.68 mmol) at 0°C, and the mixture was stirred for 3.5 h at room temperature. The reaction mixture was poured into sat. ammonium chloride aq. and extracted with chloroform. The organic layer was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (5–100% ethyl acetate in hexane) to obtain 2-{(1S)-1-[(tert-butoxycarbonyl)(methyl)amino]ethyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl trifluoromethanesulfonate (0.87 g, 1.98 mmol, 84%) as a colorless powder.
To a solution of 2-{(1S)-1-[(tert-butoxycarbonyl)(methyl)amino]ethyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl trifluoromethanesulfonate (0.86 g, 1.96 mmol) in 1,4-dioxane (3.0 mL) was added 4M hydrogen chloride in 1,4-dioxane (10 mL) and the mixture was stirred for 1 h at room temperature. Then, the reaction mixture was concentrated under reduced pressure to obtain 7b (1.1 g, 2.60 mmol, quant.) as a pale yellow oil. This compound was used for the next reaction without further purification.
To a solution of 7b (0.81 g, 1.96 mmol) and 5-chloro-2-(methylsulfonamido)benzoic acid (0.59 g, 2.36 mmol) in N,N-dimethylformamide (10 mL) was added 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (1.1 g, 2.95 mmol) and triethylamine (1.7 mL, 11.8 mmol). After stirring at room temperature for 15 h, the reaction mixture was added to water and extracted with ethyl acetate. The organic layer was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (10–100% ethyl acetate in hexane) to obtain 8b (0.71 g, 1.24 mmol, 63%) as a colorless amorphous. 1H-NMR (600 MHz, CDCl3) δ: 1.68 (1.4H, d, J = 7.4 Hz), 1.72 (1.6H, d, J = 7.4 Hz), 2.39 (1.8H, s), 2.40 (1.2H, s), 2.66 (1.6H, s), 2.80 (1.4H, s), 2.96 (1.8H, s), 3.05 (1.2H, s), 5.00 (0.5H, q, J = 7.4 Hz), 6.31 (0.5H, q, J = 7.4 Hz), 6.45 (0.5H, s), 6.46 (0.5H, s), 7.28–7.34 (1H, m), 7.35–7.42 (1H, m), 7.62 (0.5H, d, J = 8.7 Hz), 7.71 (0.5H, d, J = 8.7 Hz), 8.79 (1H, s), 8.93 (0.5H, s), 9.10 (0.5H, s).
Compound 8a was obtained by the same procedure as that described for 8b.
2-{[{5-Chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]methyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl Trifluoromethanesulfonate (8a)Pale yellow oil; 1H-NMR (600 MHz, CDCl3) δ: 2.36–2.42 (3H, m), 2.83–3.14 (6H, m), 4.58 (0.6H, br s), 5.02 (1.4H, br s), 6.37–6.51 (1H, m), 7.15–7.78 (3H, m), 8.72–8.87 (1H, m), 9.08 (0.7H, s), 10.09 (0.3H, s).
5-Chloro-N-[(1S)-1-(5-chloro-6-methylpyrazolo[1,5-a]pyrimidin-2-yl)propyl]-2-[(methanesulfonyl)amino]-N-methylbenzamide (8c)To a solution of 6c (1.6 g, 5.12 mmol) in 1,4-dioxane (15 mL) was added 4M hydrogen chloride in 1,4-dioxane (15 mL) and the mixture was stirred for 0.5 h at room temperature. Then, the reaction mixture was concentrated under reduced pressure to obtain (S)-6-methyl-2-[1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride (1.3 g, 5.90 mmol, quant.) as a colorless powder, which was used for the next reaction without further purification.
The mixture of (S)-6-methyl-2-[1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride (1.3 g, 5.90 mmol) and phosphorus oxychloride (30 mL) was stirred at 100°C for 3 h. Then, the reaction mixture was concentrated under reduced pressure to obtain 7c (1.4 g, 5.91 mmol, quant.) as black oil, which was used for the next reaction without further purification.
To a solution of 7c (1.4 g, 5.91 mmol) and 5-chloro-2-(methylsulfonamido)benzoic acid (1.8 g, 7.08 mmol) in N,N-dimethylformamide (15 mL) was added 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (2.9 g, 7.67 mmol) and triethylamine (6.0 mL, 59.0 mmol). After stirring at room temperature for 2 h, the reaction mixture was added to water and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure. The residue was purified by silica gel column chromatography (20–65% ethyl acetate in hexane) to obtain 8c (0.88 g, 1.88 mmol, 32%) as a colorless amorphous. 1H-NMR (400 MHz, CDCl3) δ : 1.07–1.20 (3H, m), 1.86–2.35 (4H, m), 2.36–2.44 (3H, m), 2.60 (1H, s), 2.78 (2H, s), 2.96 (1H, s), 3.04 (2H, s), 4.69–4.78 (0.5H, m), 6.03–6.13 (0.5H, m), 6.40–6.55 (1H, m), 7.24–7.32 (2H, m), 7.33–7.43 (1H, m), 7.56–7.77 (1H, m), 8.81 (0.5H, s), 8.92 (0.5H, s), 9.08 (0.5H, s), 10.52 (0.5H, br s); MS (ESI/APCI dual) m/z: 470 [M + H]+.
Compound 8d was obtained by the same procedure as that described for 8c.
5-Chloro-N-[(1S)-1-(5-chloro-6-methylpyrazolo[1,5-a]pyrimidin-2-yl)-2-methylpropyl]-2-[(methanesulfonyl)amino]-N-methylbenzamide (8d)Colorless amorphous; 1H-NMR (400 MHz, CDCl3) δ: 0.95–1.34 (6H, m), 2.35–2.53 (4H, m), 2.55 (1H, s), 2.73 (2H, s), 2.94–3.03 (3H, m), 4.32–4.44 (0.7H, m), 5.78–5.90 (0.3H, m), 6.52–6.63 (1H, m), 7.17–7.50 (2H, m), 7.59–7.74 (1H, m), 8.36–8.49 (0.3H, m), 8.83 (0.7H, br s), 9.00 (0.3H, br s), 10.51–10.81 (0.7H, m); MS (ESI/APCI dual) m/z: 484 [M + H]+.
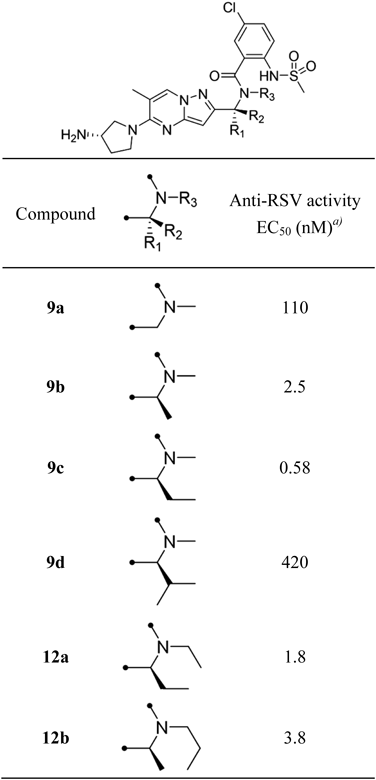
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}ethyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (9b)To a solution of 8b (0.70 g, 1.23 mmol) and (S)-tert-butyl pyrrolidin-3-ylcarbamate (0.46 g, 2.46 mmol) in tetrahydrofuran (10 mL) was added trimethylamine (0.87 mL, 6.16 mmol). After stirring at 80°C for 1 h, the reaction mixture was added to water and extracted with chloroform. The organic layer was dried over ISOLUTE® Phase Separator and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (5–100% ethyl acetate in hexane) to obtain tert-butyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]ethyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (0.73 g, 1.21 mmol, 98%) as a colorless amorphous. 1H-NMR (600 MHz, CDCl3) δ: 1.45 (9H, s), 1.60 (2H, d, J = 7.0 Hz), 1.64 (1H, d, J = 7.0 Hz), 1.86–1.97 (1H, m), 2.16–2.27 (1H, m), 2.35–2.39 (3H, m), 2.63 (1H, s), 2.81 (2H, s), 2.95 (1H, s), 3.03 (2H, s), 3.50–3.58 (1H, m), 3.69–3.76 (1H, m), 3.77–3.85 (1H, m), 3.90–3.97 (1H, m), 4.24–4.34 (1H, m), 4.67 (1H, br s), 4.88 (0.5H, q, J = 7.0 Hz), 5.97–6.04 (1H, m), 6.23 (0.5H, q, J = 7.0 Hz), 7.27–7.31 (1H, m), 7.33–7.40 (1H, m), 7.61 (0.6H, d, J = 8.7 Hz), 7.69 (0.4H, d, J = 8.7 Hz), 8.36 (1H, s), 8.64 (0.5H, s), 9.40 (0.5H, s); MS (ESI/APCI dual) m/z: 606 [M + H]+.
To a solution of tert-butyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]ethyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (0.69 g, 1.14 mmol) in 1,4-dioxane (6.9 mL) was added 4M hydrogen chloride in 1,4-dioxane (6.9 mL) and the mixture was stirred for 5 h at room temperature. Then, the reaction mixture was concentrated under reduced pressure to obtain 9b (0.62 g, 1.14 mmol, 100%) as a colorless powder. 1H-NMR (600 MHz, CDCl3) δ: 1.59 (1.8H, d, J = 7.0 Hz), 1.64 (1.2H, d, J = 7.0 Hz), 1.76–1.86 (1H, m), 2.11–2.22 (1H, m), 2.37 (1.2H, s), 2.38 (1.8H, s), 2.62 (1.2H, s), 2.81 (1.8H, s), 2.95 (1.2H, s), 3.03 (1.8H, s), 3.46 (1H, dd, J = 10.9, 4.3 Hz), 3.66–3.78 (2H, m), 3.88 (2H, dd, J = 10.3, 4.5 Hz), 4.88 (0.6H, q, J = 6.6 Hz), 5.98 (0.6H, s), 5.99 (0.4H, s), 6.22 (0.4H, d, J = 7.0 Hz), 7.22–7.32 (1H, m), 7.32–7.40 (1H, m), 7.61 (0.6H, d, J = 8.7 Hz), 7.68 (0.4H, d, J = 8.7 Hz), 8.34 (0.6H, s), 8.61 (0.4H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 15.63, 17.66, 29.22, 31.35, 40.05, 40.54, 46.73, 48.73, 52.61, 89.81, 107.96, 126.69, 126.77, 129.67, 129.99, 132.40, 133.49, 134.61, 147.03, 155.29, 155.73, 166.67; HR-MS ESI/APCI dual m/z: 506.1711 [M + H]+ (Calcd for C22H28ClN7O3S: 506.1736).
Compounds 9a, c, d were obtained by the same procedure as that described for 9b.
N-({5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}methyl)-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (9a)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 2.02–2.13 (1H, m), 2.19–2.30 (1H, m), 2.35 (3H, s), 2.82 (1.5H, s), 2.93 (1.5H, s), 3.00–3.06 (3H, m), 3.71–3.80 (2H, m), 3.82–3.90 (2H, m), 3.90–3.99 (1H, m), 4.37 (1H, s), 4.75 (1H, s), 6.05 (0.5H, s), 6.10 (0.5H, s), 7.42–7.56 (3H, m), 8.33–8.39 (3H, m), 8.45 (0.5H, s), 8.50 (0.5H, s), 9.27 (0.5H, s), 9.65 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 18.05, 29.64, 37.12, 40.47, 45.00, 47.19, 49.12, 53.04, 90.16, 108.93, 126.41, 127.18, 127.54, 130.17, 133.06, 133.59, 135.07, 147.41, 152.54, 155.67, 167.05; HR-MS ESI/APCI dual m/z: 492.1564 [M + H]+ (Calcd for C21H26ClN7O3S: 492.1579).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (9c)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.91 (1.5H, br s), 1.03 (1.5H, t, J = 7.4 Hz), 1.82–2.11 (3H, m), 2.12–2.30 (2H, m), 2.35 (1.5H, s), 2.37 (1.5H, s), 2.58 (1.5H, s), 2.72 (1.5H, br s), 2.97–3.06 (3H, m), 3.71–3.82 (2H, m), 3.82–3.90 (2H, m), 3.90–3.98 (1H, m), 4.54 (0.5H, dd, J = 9.9, 5.0 Hz), 5.73 (0.5H, dd, J = 10.3, 5.4 Hz), 6.13 (1H, s), 7.36–7.57 (3H, m), 8.34 (3H, br s), 8.51 (0.5H, s), 9.21 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.27, 17.24, 21.98, 28.8, 30.83, 39.63, 40.15, 46.32, 48.31, 52.17, 89.59, 107.62, 126.15, 126.63, 129.23, 129.81, 131.78, 133.55, 134.17, 146.53, 154.51, 154.89, 167.01; HR-MS ESI/APCI dual m/z: 520.1873 [M + H]+ (Calcd for C23H30ClN7O3S: 520.1892).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}-2-methylpropyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (9d)Colorless powder; 1H-NMR (400 MHz, DMSO-d6) δ: 0.73–1.10 (6H, m), 2.00–2.13 (1H, m), 2.19–2.31 (1H, m), 2.31–2.40 (3H, m), 2.60–2.81 (3H, m), 2.92–3.05 (3H, m), 3.69–3.99 (5H, m), 4.09–4.21 (0.5H, m), 5.42–5.52 (0.5H, m), 6.13 (0.5H, s), 6.26 (0.5H, br s), 7.21–7.61 (3H, m), 8.27–8.40 (3H, m), 8.50 (0.5H, s), 8.99 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 17.53, 19.36, 20.55, 27.56, 29.21, 31.74, 40.75, 46.76, 48.72, 52.59, 57.27, 90.95, 108.27, 126.52, 128.73, 129.58, 130.68, 132.17, 134.60, 135.35, 146.34, 153.92, 155.28, 167.06; HR-MS ESI/APCI dual m/z: 534.2023 [M + H]+ (Calcd for C24H32ClN7O3S: 534.2049).
tert-Butyl [(3S)-1-{2-[(1S)-1-Aminopropyl]-6-methylpyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl]carbamate (10a)To a solution of 6e (5.4 g, 17.6 mmol) in methanol (30 mL) was added 4M hydrogen chloride in 1,4-dioxane (30 mL) and the mixture was stirred for 2 h at room temperature. Then, the reaction mixture was concentrated under reduced pressure to obtain 2-[(1S)-1-aminopropyl]-6-methylpyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride (4.7 g) as a brown powder, which was used for the next reaction without further purification.
The mixture of 2-[(1S)-1-aminopropyl]-6-methylpyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride (4.5 g, 18.5 mmol) and phosphorus oxychloride (85 g, 556 mmol) was stirred at 100°C for 2 h. Then, the reaction mixture was concentrated under reduced pressure to obtain (1S)-1-(5-chloro-6-methylpyrazolo[1,5-a]pyrimidin-2-yl)propan-1-amine (7e) (5.0 g, 19.2 mmol) as a brown amorphous, which was used for the next reaction without further purification.
To a solution of 7e (4.8 g, 18.4 mmol) in methanol (100 mL) was added (S)-tert-butyl pyrrolidin-3-ylcarbamate (17.1 g, 91.9 mmol) and trimethylamine (20 mL, 147 mmol). After stirring at 65°C for 4 h, the reaction mixture was poured into water and extracted with chloroform. The organic layer was washed with brine and dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (50–100% ethyl acetate in hexane; 20% methanol in chloroform) to obtain 10a (4.5 g, 12.0 mmol, 65%) as a brown amorphous. 1H-NMR (600 MHz, CDCl3) δ: 0.88–0.99 (3H, m), 1.45 (9H, s), 1.72–1.97 (3H, m), 2.16–2.25 (1H, m), 2.33 (3H, s), 3.53 (1H, dd, J = 10.7, 3.7 Hz), 3.71 (1H, ddd, J = 10.7, 7.8, 5.8 Hz), 3.80 (1H, dt, J = 10.7, 7.4 Hz), 3.88–3.97 (2H, m), 4.30 (1H, br s), 4.71 (1H, br s), 5.91–6.09 (1H, m), 8.02 (1H, s); MS (ESI/APCI dual) m/z: 375 [M + H]+.
Compound 10b was obtained by the same procedure as that described for 10a.
tert-Butyl [(3S)-1-{2-[(1S)-1-Aminoethyl]-6-methylpyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl]carbamate (10b)Pale yellow powder; 1H-NMR (600 MHz, CDCl3) δ: 1.41–1.50 (12H, m), 1.89–1.95 (1H, m), 2.18–2.24 (1H, m), 2.33 (3H, m), 3.53 (1H, dd, J = 11.4, 3.9 Hz), 3.68–3.74 (1H, m), 3.77–3.83 (1H, m), 3.88–3.95 (1H, m), 4.20 (1H, q, J = 6.9 Hz), 4.30 (1H, br s), 4.69 (1H, br s), 6.02 (1H, s), 8.02 (1H, s); MS (ESI/APCI dual) m/z: 361 [M + H]+.
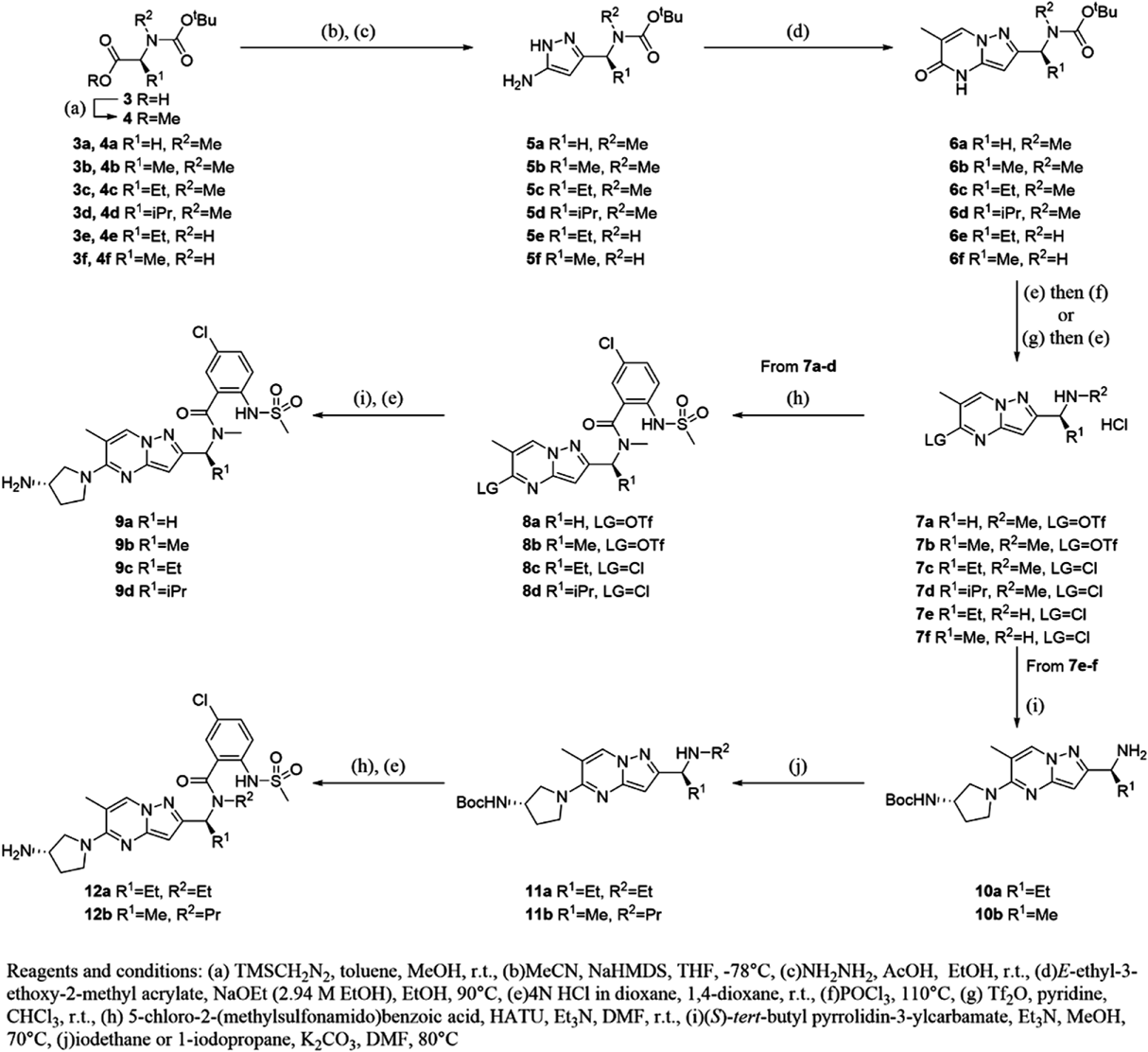
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-N-ethyl-2-[(methanesulfonyl)amino]benzamide Hydrochloride (12a)To a solution of 10a (50 mg, 0.134 mmol) in N,N-dimethylformamide (1.0 mL) was added potassium carbonate (46 mg, 0.334 mmol) and iodoethane (0.011 mL, 0.134 mmol). After stirring at 80°C for 1 h, 5-chloro-2-(methylsulfonamido)benzoic acid (40 mg, 0.160 mmol), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (66 mg, 0.174 mmol) and trimethylamine (0.093 mL, 0.668 mmol) were added to the reaction mixture. After stirring at room temperature for 16 h, the reaction mixture was purified by reversed-phase preparative HPLC to obtain tert-butyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(ethyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (13 mg, 0.021 mmol, 15%) as colorless powder.
To a solution of tert-butyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(ethyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (16 mg, 0.025 mmol) in 1,4-dioxane (1.0 mL) was added 4M hydrogen chloride in 1,4-dioxane (1.0 mL) and the mixture was stirred for 1 h at room temperature. Then, the reaction mixture was concentrated under reduced pressure to obtain 12a (15 mg, 0.026 mmol, 100%) as a colorless powder. 1H-NMR (600 MHz, DMSO-d6) δ: 0.80–0.98 (6H, m), 1.96–2.30 (4H, m), 2.32–2.40 (3H, m), 2.51–2.64 (3H, m), 2.98–3.05 (2H, m), 3.69–3.97 (5H, m), 4.47–4.55 (1H, m), 6.15–6.21 (1H, m), 7.45–7.56 (3H, m), 8.21 (3H, br s), 8.52 (0.5H, s), 9.20 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.85, 13.44, 17.86, 29.22, 36.84, 40.05, 40.80, 46.70, 48.73, 52.57, 58.64, 90.56, 107.98, 126.35, 127.32, 127.70, 129.49, 129.61, 133.63, 134.55, 146.97, 154.69, 155.59, 161.22; HR-MS ESI/APCI dual m/z: 534.2037 [M + H]+ (Calcd for C24H32ClN7O3S: 534.2049).
Compound 12b was obtained by the same procedure as that described for 12a.
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}ethyl]-5-chloro-2-[(methanesulfonyl)amino]-N-propylbenzamide Hydrochloride (12b)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.53 (1H, t, J = 7.2 Hz), 0.77 (2H, t, J = 7.4 Hz), 1.21–1.54 (4H, m), 1.57 (2H, d, J = 7.0 Hz), 1.66 (1H, d, J = 7.0 Hz), 2.00–2.10 (1H, m), 2.20–2.30 (1H, m), 2.34–2.38 (3H, m), 2.85–2.98 (2H, m), 3.04 (3H, s), 3.81–3.96 (5H, m), 4.74–4.82 (0.7H, m), 5.73 (0.3H, br s), 6.13 (1H, s), 7.43–7.58 (3H, m), 8.18 (3H, br s), 8.52 (0.5H, s), 9.34 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 11.49, 17.04, 17.76, 21.44, 29.22, 40.68, 43.95, 46.72, 48.71, 52.59, 52.71, 89.79, 107.84, 125.19, 126.43, 127.48, 129.53, 129.61, 132.18, 134.35, 146.95, 155.45, 156.56, 166.85; HR-MS ESI/APCI dual m/z: 534.2011 [M + H]+ (Calcd for C24H32ClN7O3S: 534.2049).
tert-Butyl [(3S)-1-{6-Methyl-2-[(1S)-1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl]carbamate (13a)To a solution of 10a (4.3 g, 11.4 mmol) in methanol (48 mL) was added ethyl 2,2,2-trifluoroacetate (2.0 g, 14.8 mmol) and trimethylamine (2.5 mL, 18.2 mmol). After stirring overnight at room temperature, the reaction mixture was concentrated under reduced pressure to obtain tert-butyl [(3S)-1-{6-methyl-2-[(1S)-1-(2,2,2-trifluoroacetamido)propyl]pyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl]carbamate (6.5 g, crude) as a pale yellow powder, which was used for the next reaction without further purification.
To a solution of tert-butyl [(3S)-1-{6-methyl-2-[(1S)-1-(2,2,2-trifluoroacetamido)propyl]pyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl]carbamate (5.4 g, 11.5 mmol) in N,N-dimethylformamide (50 mL) was added iodomethane (2.2 mL, 34.4 mmol) and cesium carbonate (15 g, 45.9 mmol). After stirring at 65°C for 2 h, the reaction mixture was poured into water and extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain tert-butyl [(3S)-1-(6-methyl-2-{(1S)-1-[methyl(trifluoroacetyl)amino]propyl}pyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (5.60 g, crude) as a pale yellow oil, which was used for the next reaction without further purification.
To a solution of tert-butyl [(3S)-1-(6-methyl-2-{(1S)-1-[methyl(trifluoroacetyl)amino]propyl}pyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (5.5 g, 11.4 mmol) in tetrahydrofuran (40 mL)–methanol (40 mL) was added 1M sodium hydroxide aq. (40 mL). After stirring for 1 h at room temperature, the reaction mixture was poured into water and extracted with chloroform. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 13a (4.3 g, 11.1 mmol, 98%) as a brown amorphous. 1H-NMR (600 MHz, CDCl3) δ: 0.88 (3H, t, J = 7.4 Hz), 1.45 (9H, s), 1.68–1.98 (3H, m), 2.14–2.25 (1H, m), 2.28–2.42 (6H, m), 3.48–3.63 (2H, m), 3.66–3.85 (2H, m), 3.87–3.98 (1H, m), 4.30 (1H, br s), 4.59–4.78 (1H, m), 5.91–6.07 (1H, m), 7.96–8.10 (1H, m); MS (ESI/APCI dual) m/z: 389 [M + H]+.
Compound 13b was obtained by the same procedure as that described for 13a.
Benzyl [(3S)-1-{6-Methyl-2-[(1S)-1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl]carbamate (13b)Colorless powder; 1H-NMR (400 MHz, CDCl3) δ: 0.82–0.94 (3H, m), 1.70–1.90 (2H, m), 1.90–2.01 (1H, m), 2.17–2.29 (1H, m), 2.32 (3H, s), 2.36 (3H, s), 3.53–3.62 (2H, m), 3.66–3.87 (2H, m), 3.88–4.00 (1H, m), 4.30–4.41 (1H, m), 4.86–4.97 (1H, m), 5.12 (2H, s), 6.03 (1H, s), 7.28–7.41 (5H, m), 8.04 (1H, s); MS (ESI/APCI dual) m/z: 423 [M + H]+.
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-3-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (14a)To a solution of 13a (10 mg, 0.026 mmol) in N,N-dimethylformamide (1.0 mL) was added 3-chloro-2-(methylsulfonamido)benzoic acid (6.4 mg, 0.031 mmol), 1-[bis(dimethylamino)-methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (13 mg, 0.034 mmol), and trimethylamine (0.018 mL, 0.13 mmol). After stirring at room temperature for 3 h, the reaction mixture was purified by reversed-phase preparative HPLC to obtain tert-butyl [(S)-1-(2-{(S)-1-[3-chloro-N-methyl-2-(methylsulfonamido)benzamido]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (12 mg, 0.019 mmol, 72%) as a pale yellow powder.
To a solution of tert-butyl [(S)-1-(2-{(S)-1-[3-chloro-N-methyl-2-(methylsulfonamido)benzamido]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (12 mg, 0.019 mmol) in 1,4-dioxane(1.0 mL) was added 4M hydrogen chloride in 1,4-dioxane (1.0 mL) and the mixture was stirred for 1 h at room temperature. Then, the reaction mixture was concentrated under reduced pressure to obtain 14a (10 mg, 0.019 mmol, 100%) as a colorless powder. 1H-NMR (600 MHz, DMSO-d6) δ: 0.79–1.05 (3H, m), 1.84–2.30 (4H, m), 2.33–2.42 (3H, m), 2.43–2.59 (3H, m), 2.98–3.17 (3H, m), 3.68–4.01 (5H, m), 4.37 (0.5H, br s), 5.75 (0.5H, br s), 6.06–6.25 (1H, m), 7.23–7.67 (3H, m), 8.17–8.27 (3H, m), 8.51 (0.5H, s), 9.43 (0.5H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.43, 17.48, 22.81, 29.28, 31.23, 40.05, 42.69, 46.68, 48.77, 52.55, 90.10, 108.14, 125.89, 128.84, 129.39, 130.20, 130.25, 131.90, 134.53, 146.89, 151.11, 154.89, 166.59; HR-MS ESI/APCI dual m/z: 520.1872 [M + H]+ (Calcd for C23H30ClN7O3S: 520.1892).
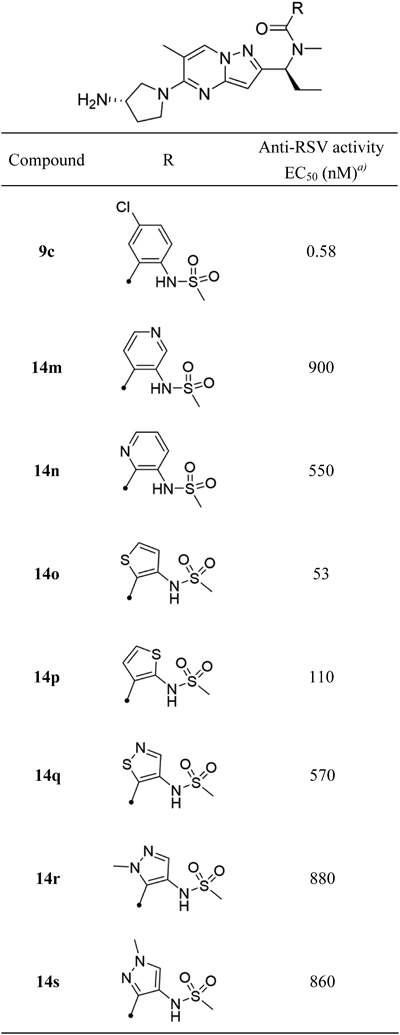
Compounds 14c–e, h–l, o, p, r, s were obtained by the same procedure as that described for 14a.
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (14c)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.85–1.06 (3H, m), 1.86–2.30 (4H, m), 2.33–2.40 (3H, m), 2.57 (1.5H, s), 2.73 (1.5H, br s), 3.02 (3H, s), 3.76 (2H, br s), 3.82–3.89 (2H, m), 3.90–3.99 (1H, m), 4.55 (0.5H, br s), 5.77 (0.5H, dd, J = 9.7, 5.6 Hz), 6.12 (1H, s), 7.26–7.39 (2H, m), 7.41–7.52 (2H, m), 8.39 (3H, br s), 8.51 (0.5H, s), 9.08 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.71, 17.70, 22.43, 29.24, 31.31, 40.47, 46.81, 48.69, 52.63, 58.26, 89.95, 108.04, 125.11, 125.91, 127.07, 127.70, 129.81, 133.33, 134.73, 146.83, 155.15, 155.47, 169.04; HR-MS ESI/APCI dual m/z: 486.2257 [M + H]+ (Calcd for C23H31N7O3S: 486.2282).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-N-methylbenzamide Hydrochloride (14d)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.75–0.85 (2H, m), 0.95–1.03 (1H, m), 1.85–2.13 (3H, m), 2.20–2.29 (1H, m), 2.36 (3H, s), 2.65 (1H, s), 2.77 (2H, s), 3.72–3.80 (2H, m), 3.82–3.88 (2H, m), 3.91–3.97 (1H, m), 4.67–4.73 (0.5H, m), 5.72–5.81 (0.5H, m), 6.04 (1H, s), 7.37–7.51 (5H, m), 8.43 (3H, br s), 8.51–8.59 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.51, 17.44, 23.49, 29.22, 40.05, 46.72, 48.73, 52.57, 58.40, 89.61, 108.32, 126.61, 128.38, 129.14, 134.65, 136.92, 146.71, 155.11, 155.35, 171.21; HR-MS ESI/APCI dual m/z: 393.2393 [M + H]+ (Calcd for C22H28N6O: 393.2397).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-[(methanesulfonyl)amino]-N,5-dimethylbenzamide Hydrochloride (14e)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.86–0.95 (1.5H, m), 0.99–1.06 (1.5H, m), 1.84–2.29 (4H, m), 2.29–2.38 (6H, m), 2.56 (1.5H, s), 2.72 (1.5H, br s), 2.96 (3H, s), 3.70–4.10 (5H, m), 4.51–4.58 (0.5H, m), 5.74–5.80 (0.5H, m), 6.11 (1H, s), 7.14 (1H, s), 7.22–7.29 (1H, m), 7.31–7.38 (1H, m), 8.35 (3H, br s), 8.51 (0.5H, s), 8.97 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.68, 17.63, 20.31, 22.38, 29.21, 31.20, 40.04, 40.28, 46.74, 48.68, 52.6, 89.92, 107.99, 125.64, 127.10, 130.22, 130.52, 132.59, 134.60, 135.69, 146.82, 155.14, 155.26, 169.05; HR-MS ESI/APCI dual m/z: 500.2414 [M + H]+ (Calcd for C24H33N7O3S: 500.2438).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-[(methanesulfonyl)amino]-N-methyl-5-propylbenzamide Hydrochloride (14h)Colorless powder; 1H-NMR (400 MHz, DMSO-d6) δ: 0.81–1.06 (6H, m), 1.51–1.64 (2H, m), 1.83–2.31 (6H, m), 2.32–2.38 (3H, m), 2.57 (1.5H, s), 2.72 (1.5H, br s), 2.98 (3H, s), 3.45–3.96 (5H, m), 4.48–4.58 (0.5H, m), 5.73–5.81 (0.5H, m), 6.11 (1H, s), 7.14 (1H, s), 7.22–7.31 (1H, m), 7.33–7.41 (1H, m), 8.19–8.35 (3H, m), 8.51 (0.5H, s), 8.95 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.72, 13.53, 17.63, 22.40, 23.92, 29.21, 31.22, 36.32, 40.04, 40.34, 46.71, 48.70, 52.56, 89.94, 107.99, 125.54, 126.54, 127.33, 129.56, 130.80, 133.88, 140.55, 146.96, 154.66, 155.58, 169.15; HR-MS ESI/APCI dual m/z: 528.2731 [M + H]+ (Calcd for C26H37N7O3S: 528.2751).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-[(methanesulfonyl)amino]-N-methyl-5-(propan-2-yl)benzamide Hydrochloride (14i)Pale pink amorphous; 1H-NMR (600 MHz, DMSO-d6) δ: 0.87–0.96 (1.5H, m), 1.00–1.07 (1.5H, m), 1.13–1.24 (6H, m), 1.84–2.30 (4H, m), 2.34–2.38 (3H, m), 2.56 (1.5H, s), 2.72 (1.5H, br s), 2.86–3.02 (4H, m), 3.70–4.07 (5H, m), 4.47–4.56 (0.5H, m), 5.73–5.81 (0.5H, m), 6.12 (1H, s), 7.13–7.24 (1H, m), 7.29–7.40 (2H, m), 8.36 (3H, br s), 8.51 (0.5H, s), 8.98 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.52, 17.65, 22.44, 23.66, 23.72, 29.23, 31.22, 32.71, 40.04, 40.42, 46.72, 48.70, 52.58, 89.98, 108.01, 124.63, 125.26, 125.72, 127.45, 130.82, 132.51, 134.58, 146.94, 155.14, 155.52, 169.19; HR-MS ESI/APCI dual m/z: 528.2740 [M + H]+ (Calcd for C26H37N7O3S: 528.2751).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-(methanesulfonyl)-N-methylbenzamide Hydrochloride (14j)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.98–1.11 (3H, m), 1.85–1.98 (1H, m), 2.01–2.21 (2H, m), 2.21–2.29 (1H, m), 2.31–2.41 (3H, m), 3.23–3.41 (6H, m), 3.69–4.08 (5H, m), 5.68–5.82 (1H, m), 6.03–6.23 (1H, m), 7.38–7.64 (1H, m), 7.64–7.88 (3H, m), 7.97–8.07 (1H, m), 8.27–8.41 (3H, m), 8.50–8.61 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.76, 17.47, 22.24, 24.39, 29.21, 31.72, 45.01, 46.94, 48.72, 52.74, 90.57, 108.19, 127.55, 129.09, 129.80, 133.60, 134.22, 134.84, 136.83, 137.66, 154.84, 155.04, 169.11; HR-MS ESI/APCI dual m/z: 471.2179 [M + H]+ (Calcd for C23H30N6O3S: 471.2173).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-methoxy-N,5-dimethylbenzamide Hydrochloride (14k)Colorless amorphous; 1H-NMR (400 MHz, DMSO-d6) δ: 0.72–0.89 (1.5H, m), 0.92–1.05 (1.5H, m), 1.77–1.95 (1H, m), 2.00–2.17 (2H, m), 2.18–2.31 (4H, m), 2.31–2.40 (3H, m), 3.35–3.67 (3H, m), 3.70–4.05 (8H, m), 4.41–4.49 (0.5H, m), 5.74–5.84 (0.5H, m), 5.88–6.05 (1H, m), 6.84–7.08 (2H, m), 7.13–7.23 (1H, m), 8.43–8.59 (4H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.76, 17.47, 19.86, 23.78, 29.21, 30.05, 46.80, 48.74, 52.64, 55.18, 60.10, 89.78, 108.25, 111.31, 126.56, 129.58, 130.24, 130.30, 134.72, 134.84, 138.28, 152.55, 168.37, 171.74; HR-MS ESI/APCI dual m/z: 437.2639 [M + H]+ (Calcd for C24H32N6O2: 437.2660).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-N,5-dimethyl-2-(trifluoromethyl)benzamide Hydrochloride (14l)Colorless powder; 1H-NMR (400 MHz, DMSO-d6) δ: 0.72–1.06 (3H, m), 1.75–2.30 (4H, m), 2.31–2.46 (6H, m), 3.70–3.99 (5H, m), 4.37–4.43 (0.5H, m), 5.73–5.81 (0.5H, m), 5.82–6.13 (1H, m), 7.18–7.50 (2H, m), 7.65–7.74 (1H, m), 8.33–8.47 (3H, m), 8.50–8.58 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.81, 17.46, 20.70, 22.29, 29.22, 31.25, 46.89, 48.71, 52.71, 58.60, 89.91, 108.14, 121.93, 122.15, 123.02, 124.84, 126.41, 127.58, 128.46, 129.71, 129.91, 134.71, 134.79, 135.52, 142.39, 143.37, 146.45, 154.81, 155.17, 168.11; HR-MS ESI/APCI dual m/z: 475.2402 [M + H]+ (Calcd for C24H29F3N6O: 475.2428).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-3-[(methanesulfonyl)amino]-N-methylthiophene-2-carboxamide Hydrochloride (14o)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.88–0.97 (3H, m), 1.89–2.29 (4H, m), 2.35 (3H, s), 2.81 (3H, br s), 3.06 (3H, br s), 3.69–3.96 (5H, m), 6.09 (1H, s), 7.16 (1H, s), 7.76 (1H, s), 8.23 (3H, s), 8.47 (1H, s), 10.11 (1H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.63, 17.58, 22.95, 29.24, 35.23, 40.29, 46.68, 48.75, 52.55, 58.16, 90.22, 110.98, 119.30, 122.85, 128.06, 128.18, 134.41, 146.89, 154.71, 155.43, 164.07; HR-MS ESI/APCI dual m/z: 492.1808 [M + H]+ (Calcd for C21H29N7O3S2: 492.1846).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-[(methanesulfonyl)amino]-N-methylthiophene-3-carboxamide Hydrochloride (14p)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.78–1.02 (3H, m), 1.84–2.33 (4H, m), 2.35 (3H, s), 2.63–2.75 (3H, m), 3.01 (3H, s), 3.68–4.17 (5H, m), 4.61–4.73 (0.5H, m), 5.65–5.78 (0.5H, m), 6.05–6.20 (1H, m), 7.00 (1H, s), 7.39 (1H, s), 8.25 (3H, br s), 8.36–8.55 (1H, m), 9.87 (0.5H, br s), 10.62 (0.5H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.54, 17.57, 22.86, 29.21, 31.00, 40.04, 40.63, 46.72, 48.70, 52.58, 90.37, 108.01, 120.51, 125.14, 132.95, 134.10, 136.23, 146.72, 152.13, 155.28, 165.59; HR-MS ESI/APCI dual m/z: 492.1812 [M + H]+ (Calcd for C21H29N7O3S2: 492.1846).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-4-[(methanesulfonyl)amino]-N,1-dimethyl-1H-pyrazole-5-carboxamide Hydrochloride (14r)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.80–0.94 (1.5H, m), 0.95–1.05 (1.5H, m), 1.84–2.29 (4H, m), 2.33–2.37 (3H, m), 2.63–2.80 (3H, m), 2.81–2.96 (3H, m), 3.70–3.80 (5H, m), 3.81–3.97 (3H, m), 4.61–4.75 (0.5H, m), 5.67–5.75 (0.5H, m), 6.05–6.10 (0.5H, m), 7.47 (1H, s), 8.21–8.30 (3.5H, m), 8.47–8.54 (0.5H, m), 9.07 (0.5 h, brs); 13C-NMR (151 MHz, DMSO-d6) δ: 10.70, 17.43, 24.04, 29.21, 30.09, 37.95, 40.02, 40.36, 46.69, 48.70, 52.54, 90.05, 108.17, 116.35, 119.53, 131.63, 134.50, 146.86, 154.46, 155.36, 161.29; HR-MS ESI/APCI dual m/z: 490.2321 [M + H]+ (Calcd for C21H31N9O3S: 490.2343).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-4-[(methanesulfonyl)amino]-N,1-dimethyl-1H-pyrazole-3-carboxamide Hydrochloride (14s)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.86–0.95 (3H, m), 1.82–2.28 (4H, m), 2.32 (3H, s), 2.71 (1.5H, s), 2.92 (1.5H, s), 2.96 (3H, s), 3.68–3.76 (2H, m), 3.79–3.94 (6H, m), 5.73–5.78 (0.5H, m), 5.87–5.93 (0.5H, m), 6.03 (0.5H, s), 6.07 (0.5H, s), 7.78–7.82 (1H, m), 8.31 (3H, br s), 8.44 (0.5H, s), 8.49 (0.5H, s), 9.00 (0.5H, s), 9.15 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.45, 17.48, 22.43, 29.20, 31.19, 38.93, 40.05, 46.83, 48.73, 52.67, 56.81, 90.12, 108.28, 120.50, 125.79, 134.71, 138.31, 146.35, 155.13, 155.19, 163.51; HR-MS ESI/APCI dual m/z: 490.2316 [M + H]+ (Calcd for C21H31N9O3S: 490.2343).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-4-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (14b)To a solution of 13a (50 mg, 0.13 mmol) in N,N-dimethylformamide (1.0 mL) was added 4-chloroanthranilic acid (27 mg, 0.15 mmol), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (64 mg, 0.17 mmol) and trimethylamine (0.090 mL, 0.64 mmol). After stirring at room temperature for 3 h, the reaction mixture was purified by reversed-phase preparative HPLC to obtain tert-butyl ((S)-1-{2-[(S)-1-(2-amino-4-chloro-N-methylbenzamido)propyl]-6-methylpyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl)carbamate (52 mg, 0.10 mmol, 75%) as a colorless powder.
To a solution of tert-butyl ((S)-1-{2-[(S)-1-(2-amino-4-chloro-N-methylbenzamido)propyl]-6-methylpyrazolo[1,5-a]pyrimidin-5-yl}pyrrolidin-3-yl)carbamate (52 mg, 0.10 mmol) in chloroform (2.0 mL) was added trimethylamine (0.14 mL, 0.97 mmol) and methanesulfonyl chloride (0.022 mL, 0.29 mmol) at 0°C. After stirring for 10 min at 0°C, the reaction mixture was concentrated under reduced pressure. Ethanol (2.0 mL) and 2.94 mol/L sodium ethoxide in ethanol (0.16 mL, 0.48 mmol) were added to the residue. After stirring at room temperature for 15 min, the reaction mixture was poured into water and extracted with chloroform. The organic layer was dried over ISOLUTE® Phase Separator and concentrated under reduced pressure. The residue was purified by reversed-phase preparative HPLC to obtain tert-butyl [(S)-1-(2-{(S)-1-[4-chloro-N-methyl-2-(methylsulfonamido)benzamido]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (40 mg, 0.065 mmol, 67%) as a colorless powder.
To a solution of tert-butyl [(S)-1-(2-{(S)-1-[4-chloro-N-methyl-2-(methylsulfonamido)benzamido]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (40 mg, 0.065 mmol) in 1,4-dioxane (1.0 mL) was added 4M hydrogen chloride in 1,4-dioxane (1.0 mL) and the mixture was stirred for 1 h at room temperature. Then the reaction mixture was concentrated under reduced pressure to obtain 14b (36 mg, 0.063 mmol, 98%) as a colorless powder. 1H-NMR (600 MHz, DMSO-d6) δ: 0.86–0.92 (1.5H, m), 1.02 (1.5H, t, J = 7.2 Hz), 1.86–2.30 (4H, m), 2.33–2.38 (3H, m), 2.58 (1.5H, s), 2.73 (1.5H, br s), 3.01–3.12 (3H, m), 3.68–4.15 (5H, m), 4.56 (0.5H, dd, J = 9.9, 4.5 Hz), 5.73 (0.5H, dd, J = 10.1, 5.6 Hz), 6.11 (1H, s), 7.39 (2H, s), 7.49 (1H, d, J = 11.6 Hz), 8.23 (3H, br s), 8.51 (0.5H, s), 9.28 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.70, 17.65, 22.40, 29.23, 31.34, 40.05, 40.77, 46.72, 48.70, 52.60, 89.98, 108.03, 124.17, 125.66, 128.83, 129.32, 130.36, 133.88, 134.58, 146.98, 154.96, 155.54, 168.14; HR-MS ESI/APCI dual m/z: 520.1872 [M + H]+ (Calcd for C23H30ClN7O3S: 520.1892).
Compounds 14f, g, m, n, q were obtained by the same procedure as that described for 14b.
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-ethyl-2-[(methanesulfonyl)amino]-N-methylbenzamide Hydrochloride (14f)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.86–0.95 (1.5H, m), 1.00–1.06 (1.5H, m), 1.14–1.22 (3H, m), 1.84–2.29 (4H, m), 2.33–2.38 (3H, m), 2.57 (1.5H, s), 2.58–2.66 (2H, m), 2.72 (1.5H, br s), 2.97 (3H, s), 3.71–3.99 (5H, m), 4.50–4.57 (0.5H, m), 5.74–5.80 (0.5H, m), 6.12 (1H, s), 7.16 (1H, br s), 7.26–7.32 (1H, m), 7.34–7.40 (1H, m), 8.35 (3H, br s), 8.51 (0.5H, s), 8.98 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.51, 15.41, 17.64, 22.41, 27.37, 29.22, 31.23, 40.05, 40.35, 46.73, 48.69, 52.59, 89.95, 108.00, 125.69, 126.77, 129.02, 130.73, 132.58, 134.57, 141.87, 146.93, 155.15, 155.53, 169.12; HR-MS ESI/APCI dual m/z: 514.2559 [M + H]+ (Calcd for C25H35N7O3S: 514.2595); chiral HPLC, 99% ee (CHIRALCEL OZ-3 5 µm 4.6 mm × 150 mm; flow, 1 mL/min, 60% ethanol in hexane; detection wavelength, 254 nm), (S)-isomer tR = 9.37 min, (R)-isomer tR = 7.31 min.
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-2-[(methanesulfonyl)amino]-5-methoxy-N-methylbenzamide Hydrochloride (14g)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.85–0.97 (1.5H, m), 0.99–1.06 (1.5H, m), 1.82–2.30 (4H, m), 2.33–2.38 (3H, m), 2.56 (1.5H, s), 2.71 (1.5H, br s), 2.87–2.99 (3H, m), 3.72–3.81 (5H, m), 3.82–3.89 (2H, m), 3.90–3.98 (1H, m), 4.47–4.58 (0.5H, m), 5.73–5.80 (0.5H, m), 6.09–6.16 (1H, m), 6.78–6.92 (1H, m), 6.97–7.06 (1H, m), 7.31–7.39 (1H, m), 8.36 (3H, br s), 8.51 (0.5H, s), 8.96 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.73, 17.62, 22.47, 29.22, 31.13, 40.05, 40.21, 46.72, 48.69, 52.59, 55.55, 89.99, 108.04, 111.66, 112.38, 115.00, 125.39, 128.76, 134.55, 135.23, 146.93, 155.33, 157.56, 168.62; HR-MS ESI/APCI dual m/z: 516.2378 [M + H]+ (Calcd for C24H33N7O4S: 516.2387).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-3-[(methanesulfonyl)amino]-N-methylpyridine-4-carboxamide Hydrochloride (14m)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.84–0.93 (1.5H, m), 0.97–1.05 (1.5H, m), 1.86–2.30 (4H, m), 2.34–2.39 (3H, m), 2.58 (1.5H, s), 2.75 (1.5H, br s), 3.05–3.12 (3H, m), 3.69–3.99 (5H, m), 4.44–4.49 (0.5H, m), 5.70–5.77 (0.5H, m), 6.12–6.17 (1H, m), 7.44–7.50 (1H, m), 8.27–8.33 (3H, m), 8.34–8.38 (0.5H, m), 8.51–8.53 (0.5H, m), 8.53–8.57 (1H, m), 8.66–8.72 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.72, 17.61, 22.40, 29.21, 31.12, 34.11, 41.21, 46.84, 48.70, 52.62, 90.09, 108.15, 122.60, 123.47, 134.64, 142.44, 144.43, 146.60, 146.84, 154.54, 155.18, 165.99; HR-MS ESI/APCI dual m/z: 487.2217 [M + H]+ (Calcd for C22H30N8O3S: 487.2234).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-3-[(methanesulfonyl)amino]-N-methylpyridine-2-carboxamide Hydrochloride (14n)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.88–0.94 (1.5H, m), 0.98–1.06 (1.5H, m), 1.87–2.30 (4H, m), 2.32–2.38 (3H, m), 2.59 (1.5H, s), 2.75 (1.5H, s), 3.06–3.11 (3H, m), 3.65–3.97 (5H, m), 4.55–4.61 (0.5H, m), 5.72–5.80 (0.5H, m), 6.10–6.16 (1H, m), 7.46–7.55 (1H, m), 7.87–7.93 (1H, m), 8.24 (3H, br s), 8.36 (0.5H, s), 8.44–8.49 (1H, m), 8.52 (0.5H, s), 9.39 (0.5H, s), 10.18 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.62, 17.77, 22.40, 26.96, 29.19, 30.86, 40.89, 46.86, 48.74, 52.64, 90.09, 108.07, 124.69, 133.52, 134.70, 145.55, 145.88, 146.62, 147.95, 155.14, 155.36, 167.38; HR-MS ESI/APCI dual m/z: 487.2200 [M + H]+ (Calcd for C22H30N8O3S: 487.2234).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-4-[(methanesulfonyl)amino]-N-methyl-1,2-thiazole-5-carboxamide Hydrochloride (14q)Colorless powder; 1H-NMR (600 MHz, DMSO-d6) δ: 0.84–0.91 (1.5H, m), 0.95–1.01 (1.5H, m), 1.88–2.31 (4H, m), 2.35 (3H, s), 2.73 (1.5H, s), 2.78 (1.5H, s), 3.07 (3H, s), 3.68–3.95 (5H, m), 4.98–5.04 (0.5H, m), 5.70–5.76 (0.5H, m), 6.09–6.14 (1H, m), 8.21 (3H, br s), 8.44 (0.5H, s), 8.52 (0.5H, s), 8.81 (0.5H, s), 8.86 (0.5H, s), 9.62 (0.5H, s), 9.93 (0.5H, s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.63, 17.50, 22.45, 29.22, 31.05, 40.05, 46.72, 48.75, 52.57, 57.66, 90.44, 108.10, 132.16, 134.59, 138.59, 141.04, 146.71, 154.57, 155.31, 163.77; HR-MS ESI/APCI dual m/z: 493.1780 [M + H]+ (Calcd for C20H28N8O3S2: 493.1799).
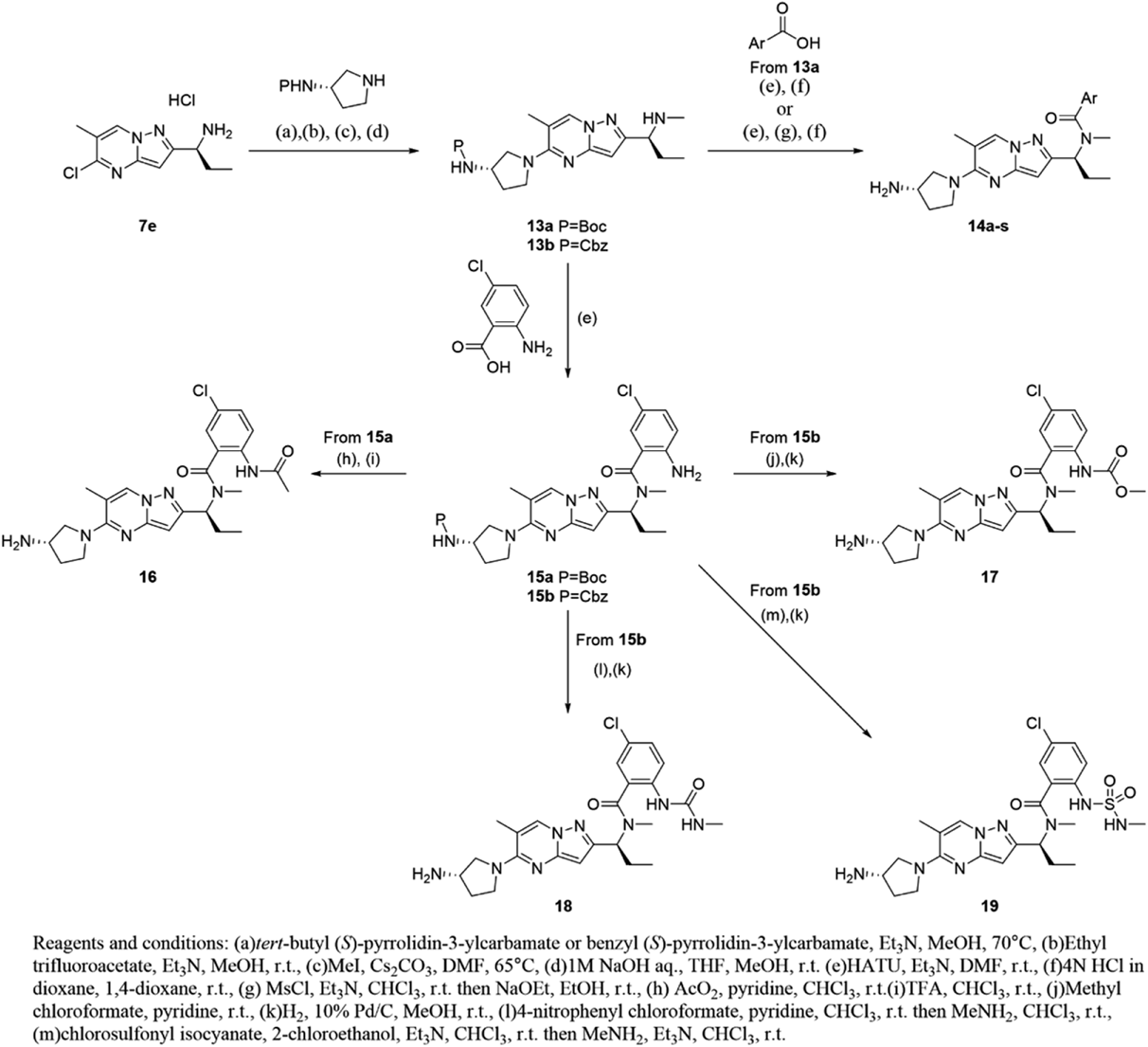
tert-Butyl [(3S)-1-(2-{(1S)-1-[(2-Amino-5-chlorobenzoyl)(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (15a)To a solution of 13a (50 mg, 0.129 mmol) and 2-amino-5-chloro-benzoic acid (24 mg, 0.142 mmol) in N,N-dimethylformamide (1.0 mL) was added trimethylamine (0.090 mL, 0.64 mmol) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (59 mg, 0.154 mmol). After stirring at room temperature for 1 h, the reaction mixture was purified by reversed-phase preparative HPLC to obtain 15a (60 mg, 0.117 mmol, 91%) as a colorless amorphous. 1H-NMR (400 MHz, CDCl3) δ: 0.87–1.19 (3H, m), 1.46 (9H, s), 1.85–2.30 (4H, m), 2.35 (3H, s), 2.83 (3H, br s), 3.49–3.60 (1H, m), 3.66–3.86 (2H, m), 3.88–3.99 (1H, m), 4.23–4.48 (3H, m), 4.67 (1H, br s), 6.07 (1H, s), 6.58–6.68 (1H, m), 7.04–7.14 (1H, m), 7.22–7.30 (1H, m), 8.04 (1H, s); MS (ESI/APCI dual) m/z: 542 [M + H]+.
Compound 15b was obtained by the same procedure as that described for 15a.
Benzyl [(3S)-1-(2-{(1S)-1-[(2-Amino-5-chlorobenzoyl)(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (15b)Colorless amorphous; 1H-NMR (400 MHz, CDCl3) δ: 0.85–1.15 (3H, m), 1.88–2.29 (4H, m), 2.32 (3H, s), 2.70–2.96 (3H, m), 3.52–3.63 (1H, m), 3.67–3.87 (2H, m), 3.89–4.01 (1H, m), 4.28–4.43 (1H, m), 4.89–5.01 (1H, m), 5.12 (2H, s), 6.06 (1H, s), 6.58–6.67 (1H, m), 7.04–7.22 (1.5H, m), 7.29–7.41 (5.5H, m), 8.04 (1H, s); MS (ESI/APCI dual) m/z: 576 [M + H]+.
2-Acetamido-N-[(1S)-1-{5-[(3S)-3-aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-N-methylbenzamide Hydrochloride (16)To a solution of 15a (42 mg, 0.081 mmol) in chloroform (3.0 mL) was added acetic anhydride (0.022 mL, 0.24 mmol) and pyridine (0.033 mL, 0.40 mmol). After stirring at room temperature for 4 h, the reaction mixture was concentrated under reduced pressure and purified by reversed-phase preparative HPLC to obtain tert-butyl [(3S)-1-(2-{(1S)-1-[(2-acetamido-5-chlorobenzoyl)(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (26 mg, 0.045 mmol, 60%) as a colorless amorphous.
To a solution of tert-butyl [(3S)-1-(2-{(1S)-1-[(2-acetamido-5-chlorobenzoyl)(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (24 mg, 0.045 mmol) in chloroform (0.50 mL) was added trifluoroacetic acid (0.50 mL). After stirring at room temperature for 1 h, the reaction mixture was poured into sat. sodium bicarbonate aq. and extracted with chloroform. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 16 (25 mg, 0.052 mmol, quant.) as a colorless amorphous. 1H-NMR (600 MHz, CDCl3) δ: 0.79–0.99 (1.5H, m), 1.03–1.13 (1.5H, m), 1.74–1.83 (1H, m), 1.89–2.08 (2H, m), 2.10–2.26 (4H, m), 2.34–2.42 (3H, m), 2.73 (1.5H, s), 2.91 (1.5H, s), 3.40–3.47 (1H, m), 3.66–3.79 (2H, m), 3.85–3.94 (2H, m), 4.79–4.88 (0.5H, m), 5.90–5.97 (0.5H, m), 5.98–6.08 (1H, m), 7.23 (0.5H, br s), 7.31–7.37 (1H, m), 8.01 (1H, br s), 8.06–8.21 (1H, m), 8.67 (0.5H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.73, 17.64, 22.23, 27.51, 31.03, 33.22, 47.49, 50.34, 52.61, 56.97, 89.77, 107.96, 126.35, 127.94, 129.02, 129.33, 133.10, 133.99, 134.15, 147.35, 154.63, 155.61, 167.37, 168.74; HR-MS ESI/APCI dual m/z: 484.2200 [M + H]+ (Calcd for C24H30ClN7O2: 484.2222).
Methyl (2-{[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl](methyl)carbamoyl}-4-chlorophenyl)carbamate (17)To a solution of 15b (60 mg, 0.10 mmol) in pyridine (1.0 mL) was added methyl chloroformate (0.16 mL, 2.1 mmol). After stirring at room temperature for 4 h, the reaction mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (OH, 10–100% ethyl acetate in hexane) to obtain benzyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methoxycarbonyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (26 mg, 0.041 mmol, 40%) as a colorless amorphous.
To a solution of benzyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methoxycarbonyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (26 mg, 0.041 mmol) in methanol (2.1 mL) was added 10% palladium on activated carbon (13 mg). The reaction was flushed with hydrogen and stirred under hydrogen atmosphere at room temperature for 1 h. The reaction mixture was filtered through membrane filter and concentrated under reduced pressure to obtain 17 (19 mg, 0.037 mmol, 91%) as a colorless amorphous. 1H-NMR (400 MHz, CDCl3) δ: 0.79–1.12 (3H, m), 1.83–2.28 (4H, m), 2.28–2.38 (3H, m), 2.67–2.74 (1.5H, m), 2.77–2.86 (1.5H, m), 3.56–3.67 (1H, m), 3.70–3.80 (3H, m), 3.82–4.06 (4H, m), 4.67–4.82 (0.5H, m), 5.72–5.88 (0.5H, m), 5.91–6.07 (1H, m), 7.19–7.24 (0.5H, m), 7.27–7.38 (1H, m), 7.77–8.23 (2H, m), 8.54–8.68 (0.5H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.63, 17.52, 22.23, 29.60, 31.17, 40.05, 46.70, 48.88, 52.07, 52.89, 89.85, 107.94, 126.49, 126.89, 127.52, 128.20, 129.33, 133.51, 134.49, 147.19, 154.33, 154.91, 155.55, 167.57; HR-MS ESI/APCI dual m/z: 500.2145 [M + H]+ (Calcd for C24H30ClN7O3: 500.2171).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-N-methyl-2-[(methylcarbamoyl)amino]benzamide (18)To a solution of 15b (50 mg, 0.087 mmol) in chloroform (0.87 mL) was added pyridine (0.028 mL, 0.347 mmol) and 4-nitrophenyl chloroformate (19 mg, 0.095 mmol). After stirring at room temperature for 1 h, the reaction mixture was concentrated under reduced pressure.
To a solution of the residue in chloroform (0.87 mL) was added methylamine (0.071 mL, 0.694 mmol). After stirring at room temperature for 2 h, the reaction mixture was added to water and extracted with chloroform. The organic layer was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (OH, 10–100% ethyl acetate in hexane) to obtain benzyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methylcarbamoyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (44 mg, 0.070 mmol, 81%) as a colorless amorphous.
To a solution of benzyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methylcarbamoyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (44 mg, 0.070 mmol) in methanol (3.5 mL) was added 10% palladium on activated carbon (22 mg). The reaction was flushed with hydrogen and stirred under hydrogen atmosphere at room temperature for 1 h. After stirring at room temperature for 1 h, the reaction mixture was filtered through a membrane filter and concentrated under reduced pressure to obtain 18 (30 mg, 0.060 mmol, 85%) as a colorless amorphous. 1H-NMR (400 MHz, CDCl3) δ: 0.82–1.15 (3H, m), 1.75–1.88 (1H, m), 1.88–2.23 (3H, m), 2.32–2.41 (3H, m), 2.62–2.92 (6H, m), 3.41–3.53 (1H, m), 3.67–3.79 (2H, m), 3.82–3.94 (2H, m), 4.69–4.85 (0.5H, m), 4.93–5.07 (0.5H, m), 5.51–5.64 (0.5H, m), 5.83–5.94 (0.5H, m), 5.95–6.10 (1H, m), 7.10–7.34 (2H, m), 7.97–8.21 (2H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.79, 17.66, 23.45, 26.02, 31.39, 33.24, 47.49, 50.36, 57.01, 58.84, 89.37, 107.94, 123.00, 124.34, 124.83, 125.91, 126.13, 129.00, 134.15, 147.25, 154.47, 155.43, 155.65, 167.99; HR-MS ESI/APCI dual m/z: 499.2306 [M + H]+ (Calcd for C24H31ClN8O2: 499.2331).
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-N-methyl-2-[(methylsulfamoyl)amino]benzamide (19)To a solution of chlorosulfonyl isocyanate (0.020 mL, 0.231 mmol) in chloroform (1.2 mL) was added 2-chloroethanol (0.031 mL, 0.462 mmol). After stirring at room temperature for 1 h, the reaction mixture was added trimethylamine (0.032 mL, 0.231 mmol) and 15b (67 mg, 0.115 mmol). After stirring at room temperature for 6h, the reaction mixture was concentrated under reduced pressure. To a solution of the residue in chloroform (1.2 mL) was added trimethylamine (0.048 mL, 0.346 mmol) and methylamine (0.46 mL, 0.923 mmol). After stirring at 120°C under microwave irradiation for 30 min, the reaction mixture was added to water and extracted with chloroform. The organic layer was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (OH, 10–100% ethyl acetate in hexane) to obtain benzyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methylsulfamoyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (23 mg, 0.035 mmol, 30%) as a colorless amorphous.
To a solution of benzyl [(3S)-1-(2-{(1S)-1-[{5-chloro-2-[(methylsulfamoyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-5-yl)pyrrolidin-3-yl]carbamate (22 mg, 0.032 mmol) in methanol (1.6 mL) was added 10% palladium on activated carbon (11 mg). The reaction mixture was flushed with hydrogen and stirred under hydrogen atmosphere at room temperature for 1 h. The reaction mixture was filtered through a membrane filter and concentrated under reduced pressure to obtain 19 (16 mg, 0.030 mmol, 92%) as a colorless amorphous. 1H-NMR (400 MHz, CDCl3) δ: 1.00–1.16 (3H, m), 1.77–2.23 (4H, m), 2.34–2.40 (3H, m), 2.60–2.65 (1.5H, m), 2.71 (2H, s), 2.75–2.83 (2.5H, m), 3.39–3.54 (1H, m), 3.67–3.78 (2H, m), 3.82–3.95 (2H, m), 4.60–4.70 (1H, m), 5.92–6.04 (1H, m), 7.22–7.25 (0.5H, m), 7.30–7.39 (1H, m), 7.63–7.74 (1H, m), 8.36 (0.5H, br s); 13C-NMR (151 MHz, DMSO-d6) δ: 10.63, 17.87, 22.35, 28.17, 31.21, 31.97, 47.21, 49.86, 52.90, 55.67, 89.61, 107.84, 122.29, 126.45, 127.28, 129.51, 130.19, 133.57, 134.35, 147.29, 154.47, 155.69, 167.59; HR-MS ESI/APCI dual m/z: 535.1982 [M + H]+ (Calcd for C23H31ClN8O3S: 535.2001).
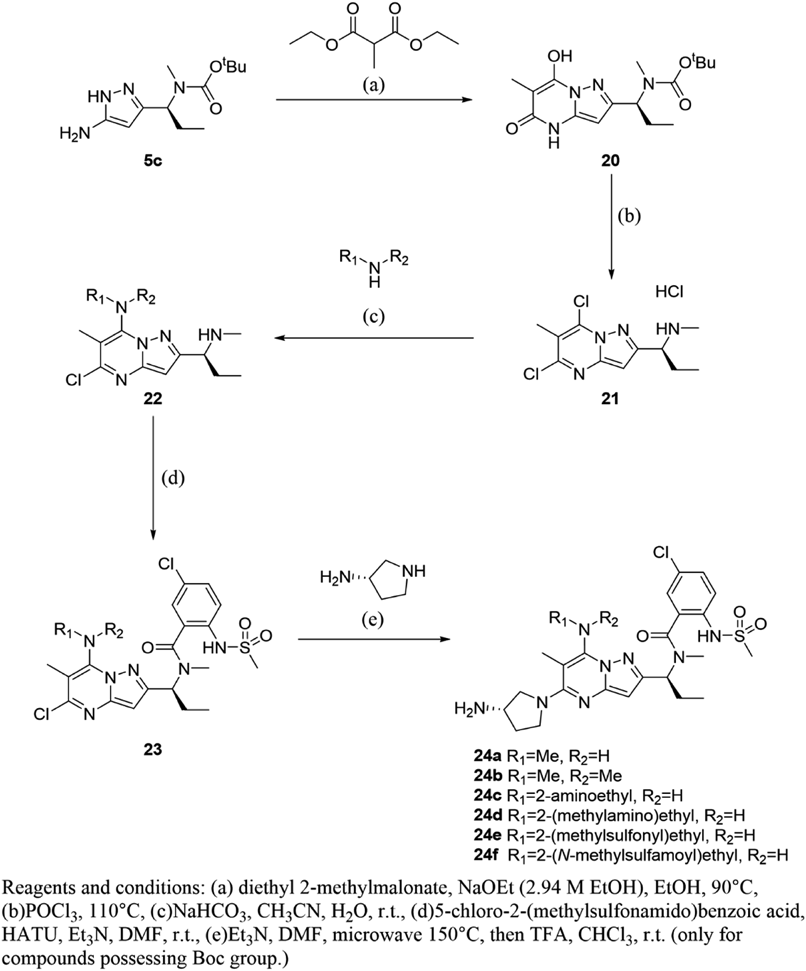
tert-Butyl [(1S)-1-(7-Hydroxy-6-methyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidin-2-yl)propyl]methylcarbamate (20)To a solution of 5c (0.55 g 2.16 mmol) in ethanol (10 mL) was added diethyl 2-methylmalonate (0.55 mL, 3.24 mmol) and 2.94 M sodium ethoxide in ethanol (3.7 mL, 10.81 mmol), and the mixture was stirred at 90°C for 5 h. The reaction mixture was concentrated under reduced pressure. The residue was acidified with 1 N hydrogen chloride aq. and extracted with chloroform. The organic layer was dried over ISOLUTE® Phase Separator and concentrated under reduced pressure. The residue was added to diethyl ether, precipitated, and collected to obtain 20 (0.62 g, 1.92 mmol, 89%) as a pale yellow powder. 1H-NMR (400 MHz, CDCl3) δ: 0.90–1.01 (3H, m), 1.49 (9H, s), 1.69–1.75 (3H, m), 1.83–1.97 (1H, m), 2.10–2.22 (1H, m), 2.67 (3H, s), 3.58–3.69 (1H, m), 4.98–5.28 (1H, m), 5.73 (1H, br s), 9.16 (1H, br s); MS (ESI/APCI dual) m/z: 337 [M + H]+.
(1S)-1-(5,7-Dichloro-6-methylpyrazolo[1,5-a]pyrimidin-2-yl)-N-methylpropan-1-amine Hydrochloride (21)The mixture of 20 (4.6 g, 13.6 mmol) and phosphorus oxychloride (13 mL, 136.1 mmol) was stirred at 110°C for 3 h, and the reaction mixture was concentrated under reduced pressure. After purifying by silica gel column chromatography (NH, 1–5% methanol in chloroform), 4M hydrogen chloride in AcOEt was added to the residue and the mixture was concentrated under reduced pressure to obtain 21 (1.8 g, 5.77 mmol, 42%) as a colorless powder. 1H-NMR (400 MHz, DMSO-d6) δ: 0.73–0.87 (3H, m), 1.94–2.20 (2H, m), 2.45 (3H, s), 2.48 (3H, s), 4.34–4.46 (1H, m), 7.05 (1H, s), 9.38 (2H, br s); MS (ESI/APCI dual) m/z: 273 [M + H]+.
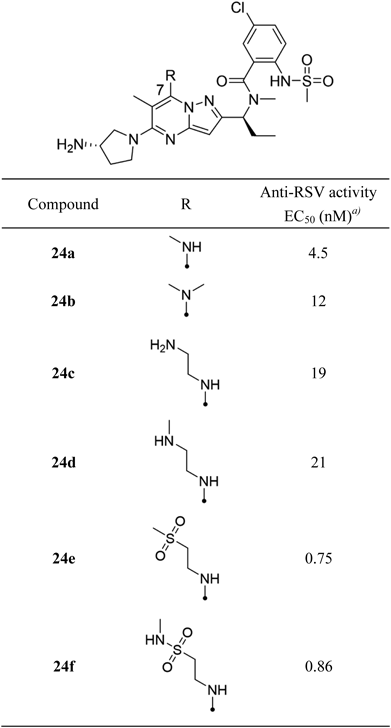
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methyl-7-(methylamino)pyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (24a)To a solution of 21 (0.12 g, 0.388 mmol) in acetonitrile (1.0 mL) and water (1.0 mL) was added 9.8 M methylamine/methanol (0.20 mL, 1.94 mmol) and sodium bicarbonate (0.33 g, 3.88 mmol). After stirring at room temperature for 22 h, the reaction mixture was added to 20% potassium carbonate aq. and extracted with chloroform. The organic layer was dried over ISOLUTE® Phase Separator and concentrated under reduced pressure to obtain 5-chloro-N,6-dimethyl-2-[(1S)-1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-7-amine (0.10 g, 0.372 mmol, 96%) as a colorless powder.
To a solution of 5-chloro-N,6-dimethyl-2-[(1S)-1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-7-amine (97 mg, 0.361 mmol) and 5-chloro-2-(methylsulfonamido)benzoic acid (99 mg, 0.397 mmol) in N,N-dimethylformamide (1.0 mL) was added trimethylamine (0.25 mL, 1.80 mmol) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (0.17 g, 0.433 mmol). After stirring at room temperature for 1 h, the reaction mixture was purified by reversed-phase preparative HPLC to obtain 5-chloro-N-{(1S)-1-[5-chloro-6-methyl-7-(methylamino)pyrazolo[1,5-a]pyrimidin-2-yl]propyl}-2-[(methanesulfonyl)amino]-N-methylbenzamide (0.13 g, 0.904 mmol, 90%) as a colorless powder.
To a solution of 5-chloro-N-{(1S)-1-[5-chloro-6-methyl-7-(methylamino)pyrazolo[1,5-a]pyrimidin-2-yl]propyl}-2-[(methanesulfonyl)amino]-N-methylbenzamide (0.12 mg, 0.252 mmol) in 1-methyl-2-pyrrolidone (1.0 mL) was added trimethylamine (0.35 mL, 2.52 mmol) and (S)-pyrrolidin-3-amine (0.11 mL, 1.26 mmol). After stirring at 150°C under microwave irradiation for 30 min, the reaction mixture was purified by reversed-phase preparative HPLC to obtain 24a (41 mg, 0.30 mmol, 30%) as a pale yellow powder. 1H-NMR (400 MHz, CDCl3) δ: 0.94–1.02 (3H, m), 1.74–2.19 (4H, m), 2.23 (3H, s), 2.80 (3H, s), 2.97 (3H, s), 3.21–3.28 (3H, m), 3.29–3.38 (1H, m), 3.52–3.81 (4H, m), 4.49–4.60 (1H, m), 6.03 (1H, s), 6.07–6.16 (1H, m), 7.32–7.42 (2H, m), 7.52–7.58 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.81, 14.95, 22.85, 27.45, 31.63, 32.72, 40.49, 47.77, 49.96, 52.49, 55.29, 84.19, 90.42, 123.82, 126.23, 127.44, 128.60, 129.12, 133.28, 146.87, 148.18, 154.13, 159.73, 169.12; HR-MS ESI/APCI dual m/z: 549.2143 [M + H]+ (Calcd for C24H33ClN8O3S: 549.2158).
Compounds 24b, e, f were obtained by the same procedure as that described for 24a.
N-[(1S)-1-{5-[(3S)-3-Aminopyrrolidin-1-yl]-7-(dimethylamino)-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (24b)Pink powder; 1H-NMR (400 MHz, CDCl3) δ: 0.96–1.04 (3H, m), 1.67–1.77 (1H, m), 1.93–2.06 (2H, m), 2.09–2.20 (4H, m), 2.86–2.95 (6H, m), 3.11–3.19 (6H, m), 3.26–3.34 (1H, m), 3.57–3.70 (4H, m), 3.74–3.85 (0.3H, m), 4.58–4.69 (0.7H, m), 6.05 (1H, s), 7.24–7.33 (1H, m), 7.36–7.41 (1H, m), 7.58–7.63 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.83, 15.57, 22.73, 30.73, 31.87, 40.54, 41.38, 47.59, 50.02, 52.59, 55.49, 58.32, 90.22, 94.88, 124.36, 126.29, 127.12, 128.66, 129.02, 133.45, 148.28, 149.48, 153.91, 159.41, 168.98; HR-MS ESI/APCI dual m/z: 563.2274 [M + H]+ (Calcd for C25H35ClN8O3S: 563.2314).
N-[(1S)-1-(5-[(3S)-3-Aminopyrrolidin-1-yl]-7-{[2-(methanesulfonyl)ethyl]amino}-6-methylpyrazolo[1,5-a]pyrimidin-2-yl)propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (24e)Colorless powder; 1H-NMR (400 MHz, CDCl3) δ: 0.99 (3H, br s), 1.40–2.18 (4H, m), 2.24 (3H, br s), 2.81 (3H, br s), 2.97 (3H, br s), 3.01 (3H, br s), 3.21–4.09 (9H, m), 4.53 (1H, br s), 6.06 (2H, br s), 7.21–7.45 (2H, m), 7.49–7.61(1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.83, 14.57, 22.75, 30.67, 31.57, 38.61, 40.54, 40.96, 47.83, 49.92, 52.47, 54.08, 55.21, 86.26, 90.46, 124.04, 126.29, 127.22, 128.64, 129.00, 133.39, 146.29, 147.31, 154.39, 159.25, 169.08; HR-MS ESI/APCI dual m/z: 641.2060 [M + H]+ (Calcd for C26H37ClN8O5S2: 641.2090).
N-[(1S)-1-(5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methyl-7-{[2-(methylsulfamoyl)ethyl]amino}pyrazolo[1,5-a]pyrimidin-2-yl)propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (24f)Colorless powder; 1H-NMR (400 MHz, CDCl3) δ: 0.98 (3H, t, J = 7.0 Hz), 1.70–1.82 (1H, m), 1.94–2.06 (2H, m), 2.08–2.19 (4H, m), 2.21 (3H, br s), 2.82 (6H, br s), 2.97 (3H, s), 3.26–3.80 (7H, m), 3.98 (2H, q, J = 6.6 Hz), 4.53 (1H, t, J = 7.2 Hz), 4.82 (1H, br s), 6.05 (1H, s), 6.21 (1H, br s), 7.32–7.44 (2H, m), 7.57 (1H, d, J = 8.7 Hz); 13C-NMR (151 MHz, DMSO-d6) δ: 10.83, 14.61, 22.75, 27.57, 28.52, 30.67, 31.53, 40.54, 47.83, 49.84, 49.92, 52.51, 55.19, 85.94, 90.48, 124.06, 126.29, 127.22, 128.64, 129.02, 133.39, 146.45, 147.29, 154.39, 159.31, 169.08; HR-MS ESI/APCI dual m/z: 656.2170 [M + H]+ (Calcd for C26H38ClN9O5S2: 656.2199).
N-[(1S)-1-{7-[(2-Aminoethyl)amino]-5-[(3S)-3-aminopyrrolidin-1-yl]-6-methylpyrazolo[1,5-a]pyrimidin-2-yl}propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (24c)To a solution of 21 (120 mg, 0.38 mmol) in acetonitrile (1.0 mL) and water (1.0 mL) was added 1-Boc-ethylenediamine (0.30 mL, 1.91 mmol) and sodium bicarbonate (0.32 g, 3.81 mmol). After stirring at room temperature for 20 h, the reaction mixture was poured into sat. sodium bicarbonate aq. and extracted with chloroform. The organic layer was dried over ISOLUTE® Phase Separator and concentrated under reduced pressure. The residue was purified by reversed-phase preparative HPLC to obtain tert-butyl [2-({5-chloro-6-methyl-2-[(1S)-1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-7-yl}amino)ethyl]carbamate (40 mg, 0.10 mmol, 27%) as a colorless amorphous.
To a solution of tert-butyl [2-({5-chloro-6-methyl-2-[(1S)-1-(methylamino)propyl]pyrazolo[1,5-a]pyrimidin-7-yl}amino)ethyl]carbamate (40 mg, 0.10 mmol) and 5-chloro-2-(methylsulfonamido)benzoic acid (30 mg, 0.12 mmol) in N,N-dimethylformamide (1.0 mL) was added trimethylamine (0.070 mL, 0.50 mmol) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (50 mg, 0.13 mmol). After stirring at room temperature for 2 h, the reaction mixture was purified by reversed-phase preparative HPLC to obtain tert-butyl {2-[(5-chloro-2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-7-yl)amino]ethyl}carbamate (33 mg, 0.052 mmol, 52%) as a colorless amorphous.
To a solution of tert-butyl {2-[(5-chloro-2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-7-yl)amino]ethyl}carbamate (33 mg, 0.052 mmol) in 1-methyl-2-pyrrolidone (1.0 mL) was added trimethylamine (0.12 mL, 0.83 mmol) and (S)-pyrrolidin-3-amine (0.037 mL, 0.42 mmol). After stirring at 150°C under microwave irradiation for 30 min, the reaction mixture was purified by reversed-phase preparative HPLC to obtain tert-butyl {2-[(5-[(3S)-3-aminopyrrolidin-1-yl]-2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-7-yl)amino]ethyl}carbamate (14 mg, 0.021 mmol, 40%) as a colorless powder.
To a solution of tert-butyl {2-[(5-[(3S)-3-aminopyrrolidin-1-yl]-2-{(1S)-1-[{5-chloro-2-[(methanesulfonyl)amino]benzoyl}(methyl)amino]propyl}-6-methylpyrazolo[1,5-a]pyrimidin-7-yl)amino]ethyl}carbamate (14 mg, 0.021 mmol) in chloroform (1.0 mL) was added trifluoroacetic acid (1.0 mL). After stirring at room temperature for 1 h, the reaction mixture was poured into sat. sodium bicarbonate aq. and extracted with chloroform. The organic layer was dried over ISOLUTE® Phase Separator and concentrated under reduced pressure to obtain 24c (11 mg, 0.019 mmol, 91%) as a colorless powder. 1H-NMR (400 MHz, CDCl3) δ: 0.94–1.03 (3H, m), 1.65–2.27 (7H, m), 2.82 (3H, s), 2.91–3.03 (5H, m), 3.22–3.31 (1H, m), 3.51–3.83 (7H, m), 4.52–4.63 (1H, m), 6.06 (1H, s), 6.08–6.17 (1H, m), 7.32–7.43 (2H, m), 7.52–7.60 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.73, 13.99, 22.37, 30.22, 32.90, 40.05, 41.14, 43.59, 47.95, 50.38, 51.41, 56.97, 88.43, 90.58, 118.61, 120.78, 125.71, 126.07, 128.38, 131.26, 132.44, 146.51, 154.07, 158.99, 170.57; HR-MS ESI/APCI dual m/z: 578.2381 [M + H]+ (Calcd for C25H36ClN9O3S: 578.2423).
Compound 24d was obtained by the same procedure as that described for 24c.
N-[(1S)-1-(5-[(3S)-3-Aminopyrrolidin-1-yl]-6-methyl-7-{[2-(methylamino)ethyl]amino}pyrazolo[1,5-a]pyrimidin-2-yl)propyl]-5-chloro-2-[(methanesulfonyl)amino]-N-methylbenzamide (24d)Colorless powder; 1H-NMR (400 MHz, CDCl3) δ: 0.94–1.02 (3H, m), 1.70–1.79 (1H, m), 1.94–2.24 (6H, m), 2.48 (3H, s), 2.83 (3H, s), 2.86–2.99 (5H, m), 3.22–3.31 (1H, m), 3.53–3.81 (7H, m), 4.52–4.65 (1H, m), 6.02–6.12 (2H, m), 7.32–7.43 (2H, m), 7.53–7.59 (1H, m); 13C-NMR (151 MHz, DMSO-d6) δ: 10.75, 14.19, 22.61, 30.47, 32.01, 34.41, 40.05, 42.56, 47.85, 50.06, 50.93, 51.85, 55.87, 79.14, 90.54, 122.11, 125.99, 127.20, 128.50, 128.80, 132.00, 146.75, 147.45, 154.23, 159.09, 170.04; HR-MS ESI/APCI dual m/z: 592.2546 [M + H]+ (Calcd for C26H38ClN9O3S: 592.2580).