2020 年 68 巻 7 号 p. 560-566
2020 年 68 巻 7 号 p. 560-566
Bone metastases can cause high morbidity and mortality, often developing as they advance, especially in patients with prostate and breast cancers. Most drugs are rarely distributed to the bone and are hence pharmacologically ineffective in treating bone metastases. The development of drug targeting technologies is required for the efficient treatment of bone metastases. To date, numerous bone-targeting ligands, including tetracyclines, bisphosphonates, aspartic acid, and aptamers have been developed and used for bone-targeted delivery of anti-tumor drugs, peptide/protein drugs, nucleic acid drugs, and diagnostic imaging agents. The conjugates of drugs with bone-targeting ligands were first developed in the field of bone drug targeting systems; macromolecular carriers and nanoparticles modified with these bone-targeting ligands have also been developed. Additionally, antibodies to prostate-specific membrane antigen (PSMA) and human epidermal growth factor receptor 2 (HER2) are used in active targeting bone metastatic prostate cancer and breast cancer, respectively. Some conjugates using antibodies to PSMA and HER2 were developed and used in clinical trials. In this review, recent challenges in the development of bone-targeted delivery systems and strategies for the treatment of bone metastasis have been summarized. Future development of novel drug formulations in order to optimize targeted drug delivery in the treatment of bone metastasis have also been discussed.
Bone metastases can cause high morbidity and mortality, especially in patients with prostate and breast cancers.1) Therapeutic measures involve surgery, hormone therapy, and bone-modifying agent therapy, such as treatment with bisphosphonate (BP) and denosumab, a fully human monoclonal antibody to receptor activator of nuclear factor-kappaB (NF-κB) ligand (RANKL). In addition, analgesics are used to alleviate pain and paralysis caused by spinal cord compression of bone metastatic cancer cells. Furthermore, chemotherapy is initiated in life-threatening circumstances of multiple bone metastases.2)
However, these therapies are rarely able to cure bone metastasis, because bone-modifying agents mainly retard skeletal events and are not the primary treatment method. Further, delivery of most anti-tumor drugs to the bone is limited due to lower blood flow to the bone than that in organs. This may be because of the unique histological features of the bone, which acts as a blood-bone marrow barrier formed by the lining cells that prevent the entry of large exogenous substances from the bone surface.3,4) Therefore, selective targeting of therapeutic agents to the bone and metastatic cancer is needed to treat bone diseases (Fig. 1). Since the first research articles were published on drug targeting to the bone in the early 1990s,5) research into drug targeting to the bone and metastatic cancer has grown exponentially. This has led to published clinical trials and an active community of researchers in the field today.
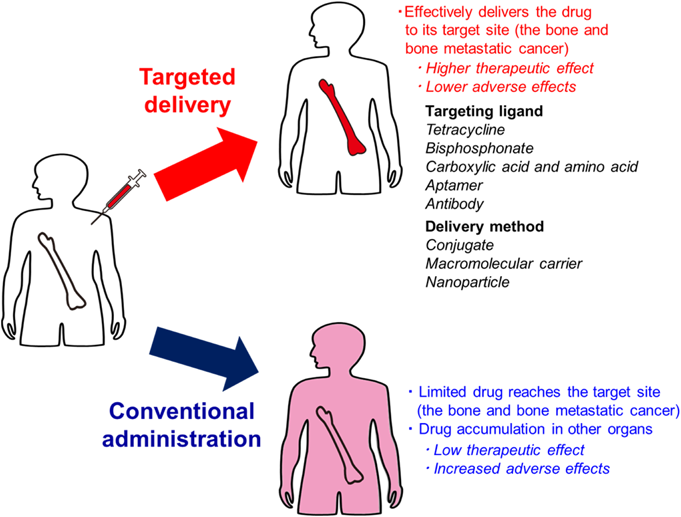
(Color figure can be accessed in the online version.)
Of the various strategies available, targeting ligands with high affinity for hydroxyapatite (HAP), a bone matrix, appears to be good approach for drug targeting. So far, tetracycline,5–7) BP,8,9) and carboxylic acid (e.g., aspartic acid)10–12) have been reported as bone-targeting ligands with high affinity for HAP. In contrast, the peptide sequence Ser-Asp-Ser-Ser-Asp (SDSSD)13) and its aptamers14) have recently been reported as a new targeting-ligands for osteoblasts. Furthermore, prostate-specific membrane antigen (PSMA) antibody15) and human epidermal growth factor receptor 2 (HER2) antibody16) are useful as targeting ligands for the treatment of bone metastatic prostate cancer and breast cancer, respectively. The use of these targeting ligands has the potential for efficient bone-targeted drug delivery and treatment of metastatic cancers.
In this review, recent challenges in the development of bone-targeting systems and strategies for bone metastases have been summarized. Future developments in drug formulation have also been discussed in order to optimize drug targeting.
Several studies have been published using tetracycline, BP, carboxylic acids, amino acids, and aptamers for the development of bone-targeted drug delivery systems. Table 1 shows a list of bone-targeting ligands in the preclinical and clinical stages.
 |
(Color figure can be accessed in the online version.)
Tetracycline has been used as an antibiotic since 1947.6) Tetracycline affects Gram-positive bacteria such as staphylococci and pneumococci, Gram-negative bacteria such as Shigella and Escherichia coli, and infections such as rickettsia and chlamydia. In 1965, tetracycline was reported to have high affinity towards HAP and caused children’s teeth to stain yellow.7) Although its high affinity to HAP is a side effect of tetracycline, this property makes bone-targeting possible. Tetracycline was the first compound reported as a bone-targeting ligand, and the mechanism is related to a chelate bond between oxygen atoms in the tetracycline structure and calcium contained in HAP7) (Table 1-a). Orme et al. first studied the bone-targeting of estradiol using chemical modification and demonstrated that estradiol conjugated with tetracycline showed high affinity for HAP.5) However, the pharmacokinetics and pharmacological effects were not examined.
2.2. Bisphosphonate (BP)BPs, a class of drugs characterized by a phosphate-carbon-phosphate backbone, are physiological regulators of calcification and osteoclastic bone resorption.17–19) A number of BPs are used for the treatment and prevention of bone diseases and disorders of calcium metabolism, including osteoporosis, Paget’s disease, and hypercalcemia.20,21) It was reported that BPs showed high affinity to HAP and were specifically distributed to the bone after intravenous injection. BPs have been the most popular targeting ligands for bone-targeting delivery systems (Table 1-b), since Fujisaki et al. reported bisphosphonic prodrug as a novel method for site-specific and controlled delivery of drugs to the bone.8,9) Fujisaki et al. and Bauss reported BP prodrugs of 17-estradiol through various different types of linkers and demonstrated that the linkers played a key role in drug stability during circulation and affect bone-targeting efficacy.9,22) Among BPs, alendronate (ALN) and zoledronate have been used as bone-targeting ligands. ALN in particular, is a popular bone-targeting ligand because it has a primary amine and can be conjugated with drugs and drug carriers through various chemical linkers. Thus far, various BP conjugates and BP-modified protein drugs and nanoparticles have been reported.23–25)
2.3. Carboxylic Acids and Amino AcidsOsteocalcin (OC), a carboxylic acid-rich protein, reportedly absorbed onto the HAP surface, forms the rigid bone tissue.26) OC forms a tight globular structure that contains various carboxylic acids, such as γ-carboxylated glutamic acids (Gla), aspartic acid (Asp), and glutamic acid (Glu). It was also reported that several types of oligopeptides, including (Asp)n, (Glu)n, and (Asp-Ser-Ser)n, accumulated in the bone after intravenous injection.27–29) It was also reported that estradiol conjugated with hexa-Asp peptide predominantly accumulated in the bone after intravenous injection12) (Table 1-c). Due to carboxylic acids, such as Asp and Glu, being biocompatible compounds, they could be promising as moieties for drug targeting to the bone tissues. We reported that the efficient bone-targeting of the macromolecular carrier polyamidoamine (PAMAM)-dendrimer was successfully achieved via the combination of Asp and a polyethylene glycol (PEG) modification (Table 1-d). The bone-targeting efficacy of PEG-Asp modified PAMAM was noted to be much higher than that of PEG-Glu modified PAMAM. Furthermore, we found that a polymeric Asp sequence structure was necessary for bone affinity.10) This is probably because the Asp polymer sequence can form a chelate complex with Ca2+.10)
Sun et al. suggested that SDSSD selectively binds to osteoblasts via periostin (osteoblast-specific factor (OSF)-2). Phage display was used to select peptides that could target both mouse and human osteoblasts, and the peptide sequence SDSSD, was identified.13) The binding affinity of SDSSD for periostin was confirmed by affinity chromatography and cell-binding experiments, suggesting that the SDSSD peptide targeted osteoblasts in a ligand-receptor specific manner (Table 1-e).
2.4. AptamerAptamer is a nucleic acid molecule or peptide that specifically binds to a molecule.30) Nucleic acid aptamers are being studied for biotechnological applications and for their use as biological drugs capable of molecular recognition instead of antibodies. Ligang et al. found the aptamer CH6 by cell-systematic evolution of ligands by exponential enrichment specifically targets both rat and human osteoblasts14) (Table 1-f). They prepared CH6 aptamer-functionalized lipid nanoparticles (LNPs) encapsulating an osteogenic pleckstrin homology domain-containing family O member 1 (Plekho1) siRNA (CH6-LNPs-siRNA) and demonstrated its potential for osteoblast-specific Plekho1 gene silencing. This promoted bone formation, in rodents with osteopenia.
To date, various bone-drug targeting systems using conjugates, macromolecular carriers, and nanoparticles have been investigated to treat bone metastasis. Table 2 shows representative reports of bone-drug targeting for treating bone metastasis.
 |
Structural parts shown in red indicate bone-targeting ligands. BP, bisphosphonate; 5-FdU, 5F-deoxyuridine; SOD, superoxide dismutase; HPMA copolymer, N-(2-hydroxypropyl) methacrylamide copolymer; PEG, polyethylene glycol; DSPE-PEG, N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine. (Color figure can be accessed in the online version.)
For the treatment of bone metastases, methotrexate targeting efficacy could be achieved by conjugating BP with methotrexate (Table 2, Structure 1).31) BP conjugated proteasome inhibitors and a 5F-deoxyuridine-ALN conjugate showed increased affinity towards HA and cytotoxicity to cancer cells (Table 2, Structure 2).32,33)
Nakatake et al. created a dialkyl BP-platinum complex to efficiently target, and utilize the antitumor effects of platinum, to treat bone metastases (Table 2, Structure 3).34) A doxorubicin-BP conjugate with cathepsin-B or acid-sensitive linkers quickly released the drugs, but achieved sufficient stability in human plasma. Although the pharmacological effects were not evaluated, the maximal tolerated dose of doxorubicin-BP conjugate with acid-sensitive hydrazone bond (Table 2, Structure 4) was 3-fold higher than that of conventional doxorubicin.35)
3.2. Targeting Ligand-Peptide/Protein Drug ConjugatesTo date, numerous BP-modified peptide and protein drugs have been studied for the treatment of bone diseases. Although bone-targeting of osteoprotegerin,36) salmon calcitonin,37,38) and superoxide dismutase (SOD)25) has been reported using BP modification, linkers for the conjugation and degree of modification of BP are important factors for efficient bone-targeting of these drugs.
Zhang et al. and Wright et al. reported a new method for conjugating BP to protein drugs through cleavable linkages.39,40) They synthesized disulfide-linked fetuin-thiol BP conjugates which was readily cleaved by thiols in physiological conditions. Cleavable linkages are advantageous for the maintenance of protein drug activities.39,40)
To establish a rational molecular design for BP-modified proteins for efficient bone targeting, we performed a pharmacokinetic study using a series of ALN modified proteins with various molecular weights and varying degrees of modification (Table 2, Structure 5).25) We demonstrated that efficient bone targeting of glutathione reductase and serum albumin, two high-molecular weight proteins with prolonged blood circulation, was successfully achieved by ALN modification. In addition, a ratio of approximately 3–4 ALN molecules per protein is an optimal degree of modification for bone targeting. In contrast, low-molecular-weight proteins, such as SOD and lysozyme, require modification with both 3–4 ALN/protein and PEG, for efficient delivery to the bone. We also demonstrated that PEG-SOD-ALN effectively suppressed the tumor growth in the leg bone after intravenous injection in a bone metastasis model in mice.25)
3.3. Targeting Ligand Modified Macromolecular CarriersSome researchers reported bone-targeting of anti-tumor drugs using BP-modified macromolecular carriers.41–43) This appears to be a good approach to control drug distribution because macromolecular carriers have several functional groups whereby drugs, targeting ligands, and functional moieties can be chemically conjugated.44–48)
Holmberg et al. synthesized an ALN-modified oxidized dextran conjugated with aminoguanidine and demonstrated its osteoclast inhibiting properties. Although the pharmacological effect of the dextran conjugate has yet to be analyzed in vivo, it appears to be a promising drug candidate with high efficacy for the treatment of bone metastasis.41)
PEG-PAMAM-ALN, a bone-targeted dendritic polymer, in which the PAMAM dendrimer is covalently bounded to ALN and PEG, has been reported as a bone-targeting carrier (Table 2, Structure 6). Approximately 20% of the dose of methotrexate loaded on PEG-PAMAM-ALN accumulated in the bone 3 h after intravenous administration in mice, which was 18-fold higher than the administration of methotrexate alone.42)
Segal et al. synthesized ALN, and the potent anti-angiogenic agent TNP-470 with N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer (Table 2, Structure 7). Anti-angiogenic and anti-tumor activity of the HPMA copolymer-ALN-TNP-470 was found to be much higher than the combination of free ALN and TNP-470 in a xenogeneic model of human osteosarcoma.43)
In addition to these BP-modified macromolecular carriers, an Asp-modified macromolecular carrier may also be effective as a bone-targeting carrier for treating bone metastasis. Recently, we demonstrated that PEG-Asp modified PAMAM, specifically accumulated on the eroded surface of bone, a pattern associated with the pathogenesis of bone metastasis (Table 2, Structure 8).10) Although bone-targeting of anti-tumor drugs using PEG-Asp modified PAMAM has not been examined, PEG-Asp modified PAMAM is a promising bone-targeting drug carrier for the treatment of bone metastasis.
3.4. Targeting Ligand Modified NanoparticlesTo construct versatile bone-targeting systems based on BP (ALN and zoledronate) and Asp modification, some groups reported BP and Asp modified nanoparticles encapsulating various drugs as bone-targeting carriers for the treatment of metastases.49–51)
Xi et al. reported curcumin loaded micelles using ALN-hyaluronic acid-octadecanoic acid (ALN-HA-C18) as an amphiphilic material (Table 2, Structure 9). The curcumin loaded ALN modified the micelles which subsequently exhibited much higher cytotoxic activity against MG-63 cells than free curcumin. Further, curcumin loaded ALN-HA-C18 micelles effectively suppressed the increase in the number of tumor cells in osteosarcoma bearing mice as compared with curcumin alone.49)
Swami et al. reported bone-targeting of bortezomib using ALN modified poly(d,l-lactic-co-glycolic acid) (PLGA) nanoparticles. They demonstrated that bortezomib-loaded ALN modified PLGA nanoparticles significantly enhanced survival and decreased the tumor in mouse models of multiple myeloma. They also observed that bortezomib-loaded ALN modified PLGA nanoparticles improved the bone microenvironment and enhanced bone strength and volume.50)
Another BP, zoledronate, was also used for bone-targeting of nanoparticles. Zoledronate-modified PLGA nanoparticles were developed as bone-targeting nanocarriers of methotrexate and showed higher accumulation in bone than unmodified nanoparticles. Additionally, biodistribution studies demonstrated that zoledronate-modified PLGA nanoparticles were selectively targeted to the bone with methotrexate.51)
Bone-targeting of liposome using Asp modification was also reported.11) We developed PEG-conjugated Asp-modified liposomes (PEG-Asp-Lipo) as a bone-targeting carrier of paclitaxel (PTX) by using Asp-modified 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE-Asp) for the prevention of bone metastasis (Table 2, Structure 10). We found that fluorescein isothiocyanate (FITC)-labeled PEG-Asp-Lipo predominantly accumulated on eroded and quiescent bone surfaces. In a metastatic bone tumor mouse model, tumor growth was significantly inhibited by an intravenous injection of PEG-Asp-liposomal PTX.11)
PSMA is a well-characterized target that is selectively overexpressed in prostate cancer cells.52) The PSMA antibody-drug conjugate (ADC), in which monomethyl auristatin E (microtubule disrupting agent) is conjugated to PSMA antibody, showed potent and selective antitumor activity in mouse models of advanced prostate cancer.53) PSMA ADC was evaluated to determine its safety, pharmacokinetics, and preliminary antitumor effects in subjects with treatment-refractory prostate cancer in a Phase 1 study.15)
The targeting to prostate cancer based on PSMA is also applied for the diagnosis of metastatic prostate cancer. PSMA targeted imaging agents for magnetic resonance imaging (MRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT) of prostate cancer have been developed.54) 18F-FSU-880, a imaging probe targeting PSMA, was clinically investigated for safety, distribution, dose estimation, and lesion accumulation.55)
Bone metastases with breast cancer are very common, found in approximately 70% of people with the metastatic disease. Overexpression of HER2 is associated with a more aggressive disease course and worse clinical outcomes in patients with breast cancer. Trastuzumab was first developed as a monoclonal antibody targeting HER2 and significantly improved the morbidity and mortality of patients with metastatic HER2+ breast cancer.56) Recently, the HER2-targeted antibody-drug conjugate trastuzumab emtansine (T-DM1) has been approved for the treatment of metastatic HER2+ breast cancer, because it has exhibited significant antitumor activity against HER2+ advanced breast cancer in the preclinical and phase 1–3 clinical study.16)
Trastuzumab has also been used for imaging diagnosis of metastatic breast cancer. 89Zr-trastuzumab was clinically evaluated as a PET imaging probe in patients with metastatic breast cancer. In this clinical trial, 89Zr-trastuzumab showed significant tumor uptake and visualization of HER2+ metastatic lesions.57,58)
Adjei et al. found that small neutral nanoparticles (approx. 150 nm) were also approx. 7-fold more effective in being distributed in the bone marrow compared with larger nanoparticles (approx. 320 nm). They demonstrated that nanoparticles that predominantly accumulated in the bone marrow improved nanoparticle-mediated anticancer drug delivery to sites with bone metastasis, thereby inhibiting cancer progression and preventing bone loss.59)
Trifolium-like platinum nanoparticles (TPNs) were developed for photothermal therapy in the treatment of bone metastasis. TNPs effectively suppressed increases in the number of cancer cells upon exposure to near-infrared light. Moreover, these nanoparticles effectively inhibited tumor growth and prevented osteolysis in a bone metastasis model.60)
It was reported that PEGylated LNP formulations facilitate tumor uptake and intracellular processing through an enhanced permeation and retention effect (EPR).61) Yamamoto et al. tried clusterin siRNA delivery using LNP to treat prostate cancer.62) Clusterin is a cytoprotective chaperone induced by the androgen receptor pathway inhibition which facilitates adaptive survival pathway signaling and treatment resistance.63) Therefore, clusterin is a therapeutic target for the treatment of advanced prostate cancer. LNP containing clusterin siRNA had the potential to suppress tumor growth in prostate cancer through the reduction of clusterin expression. Although therapeutic efficacy is yet to be examined in a bone metastasis model, this formulation could be promising for the treatment of prostate cancer with bone metastasis.
This review summarizes the recent challenges faced in the development of a targeted drug delivery system in metastatic cancer for the treatment of bone metastasis. From the various bone-targeting ligands available, tetracycline was the first compound to be reported as a bone-targeting ligand, but its safety must be considered as it may cause side effects such as pigmentation and pediatric growth inhibition. Therefore, tetracycline use has been limited. Conversely, BP is the most popular bone-targeting ligand and has been used by several researchers for bone-targeting using BP modification. However, BP may also cause side effects such as renal disorders and osteonecrosis of the jaw. Therefore, we think that an increase in the use of Asp and aptamers as bone-targeting ligands can be expected due to high bone selectivity and safety, because these bone-targeting ligands are made of biological materials and are biocompatible compounds. Although SDSSD and aptamers, ligands for osteoblasts, have not yet been used for the treatment of bone metastases, targeted drug delivery using these ligands has the potential for effective treatment.64) Antibodies to PSMA and HER2 are also promising ligands that directly address bone metastatic prostate cancer and breast cancer, respectively.
Of the available bone-targeted drug delivery methods, the use of a macromolecular carrier or nanoparticle carrier system is the most promising approach to achieve targeted drug delivery to the bone and metastatic cancer, because biodistribution of these drug carriers can be controlled through chemical modification.44–48,65,66) Additionally, this approach has already been successful in a variety of therapeutic agents, including chemotherapeutic compounds, protein drugs, antisense oligonucleotides, and genes, in the field of drug delivery.48,65,66)
However, selectivity of existing targeted drug carriers to bone and metastatic cancers remains a challenge, including high uptake clearance at non-target sites such as the liver and kidney. The key to a successful targeted drug delivery system involve: 1) detailed understanding of the physiological and anatomical characteristics of bone and metastatic site; 2) optimization of characteristics of the drug carrier (size, surface charge, and degree of modification) based on the pharmacokinetic properties of carriers including cellular localization; and 3) controlled rate of drug release from carriers. We hope that efficient targeted drug carriers that meet these requirements will be developed for the treatment of bone metastasis in the near future.
The authors are very grateful to Professor Makiya Nishikawa for his help and guidance. This work was supported in part by JSPS KAKENHI Grant Number 19H04473.
The authors declare no conflict of interest.