2020 年 68 巻 7 号 p. 664-670
2020 年 68 巻 7 号 p. 664-670
Research from the past decade has shown that the buffer capacities of intestinal fluids are much lower than those in the media used for dissolution test of many solid formulations. The purpose of this study was to elucidate the effect of buffer capacity on the dissolution profiles of highly soluble drug products, using metoclopramide (a biopharmaceutics classification system [BCS] class III drug) tablets as a model. The dissolution profiles of three metoclopramide products were obtained in Japanese pharmacopeia dissolution medium (pH 1.2 and 6.8), diluted medium with low buffer capacity comparable to that of gastrointestinal fluid, and other biorelevant media. One product showed slower dissolution in the medium with lower buffer capacity (bio-relevant, diluted compendial solution), but substantially similar dissolution in the compendial test solutions. Disintegration difference was implied to be involved in the different dissolution profiles depending on the medium buffer capacity. This study indicated the importance of media buffer capacity as a factor inducing different dissolution between products of highly soluble active pharmaceutical ingredients. The diluted compendial media would be a useful alternative to biorelevant media for the detection of the different formulation performances depending on the buffer capacities.
The dissolution test is a valuable method for the characterization of the performance of oral solid formulations and other formulations. The dissolution test is utilized for two main purposes: the prediction of dissolution profiles of drug products in a gastrointestinal tract; and for quality control in specification tests. A predictive dissolution test is a potential tool to optimize product formulations and to understand the risk factors associated with changes in formulation characteristics.1) It may also reduce the requirements for a human bioequivalence study; for example, through a biopharmaceutics classification system (BCS)-based waiver. When the dissolution test is used as a quality control test, it is expected to ensure batch-to-batch consistency2) and prevent significant bioinequivalence.3)
To achieve better prediction of drug product dissolution in in vivo conditions, an in vitro dissolution test is conducted under test conditions that mimic several physiological fluids, such as low sheer stress derived from dissolution medium4,5) and a low volume of test medium.6–8) Other important factors shaping the dissolution profiles are characteristics of the dissolution test medium, such as pH, buffer capacity, and presence or absence of bile components, enzymes, and other components.9,10) The buffer capacity is the potential of a buffering medium to mitigate changes in pH following the addition of acidic or basic. There is a significant difference in buffer capacity between human intestinal fluid and dissolution test medium.11–14) The buffer capacity of fluids in the stomach, duodenum, and jejunum ranged from 0.26 to 6.32 µmol/mL/ΔpH in fasted state, and from 0.78 to 5.98 µmol/mL/ΔpH in fed state in the stomach, duodenum, and proximal and mid/distal jejunum.15) Hypo- and a-chlorhydric gastric conditions decreased the buffer capacities of the fluid in the fasted stomach and small intestine.16) In contrast, the buffer capacities of conventionally used dissolution test media are: 34 µmol/mL/ΔpH (pH 6.8, United States Phamacopeia (USP) simulated intestinal fluid),11) 35 µmol/mL/ΔpH (pH 5.0, fed state simulated gastric fluid),11) and 25 µmol/mL/ΔpH (pH 5.8, fed state simulated intestinal fluid).17) Recent studies have proposed the use of dissolution media with even lower buffer capacities.18,19) Both buffer capacity and ionic strength are reported to affect the dissolution profiles of drug products containing less soluble active pharmaceutical ingredients (APIs) because of the change of surface pH and API solubility, although it is considered to have only a slight effect on the dissolution of highly soluble APIs (e.g., BCS class I/III).11,20–22) Thus, the buffer capacity of compendial dissolution test medium is significantly higher than that of intestinal fluid, and the potential risk associated with the use of high buffer capacity medium has been indicated.15)
To evaluate the quality of drug products, dissolution tests of oral solid dosage forms have been conducted with three buffering media (pH 1.2, 4.0, and 6.8) and purified water in Japan.23) The dissolution data showed that some formulations, which included BCS class I or III APIs, showed a much slower dissolution in purified water (<85% in 30 min) compared with those in three buffers (≥ 85% in 30 min). This implied that the ionic strength and buffer capacity affect the dissolution of the formulations containing highly soluble APIs. For a better understanding of the effect of buffer capacity on the dissolution profile of formulations including BCS class I or III APIs, it is necessary to evaluate the dissolution by using a dissolution test medium with low buffer capacity, comparable with that of physiological fluids.
In this study, we prepared dissolution test media with a low buffer capacity by simply diluting the media described in Japanese Pharmacopoeia and then adding sodium chloride to maintain ionic strength. By using metoclopramide (a BCS class III drug)24) immediate release products, which show slow dissolution only in purified water but not in other buffers, the usefulness of the diluted compendial dissolution test media as an alternative to biorelevant media with low buffer capacity and the effect of buffer capacity on the dissolution of highly soluble drug products were investigated.
Three metoclopramide immediate release tablets formulations, Primperan Tablets 5 (Lot#S014G01; Astellas Pharma Inc., Tokyo, Japan), Terperan Tablets (Lot#L166A; ASKA Pharmaceutical. Co., Ltd., Tokyo, Japan), and Metoclopramide Tablets 5 mg [Takata] (Lot#U001 and #V004; TAKATA Pharmaceutical Co., Ltd., Saitama, Japan), were obtained from local distributors in Japan. The excipient components of the products are shown in Table 1. Metoclopramide hydrochloride was purchased from Sigma-Aldrich Japan Co. LLC (Tokyo, Japan), pepsin was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and FaSSIF/FeSSIF/FaSSGF powders were purchased from Biorelevant.com Ltd. (London, U.K.).
| No. | Product name | Lot No. | Manufacturer | Excipients |
|---|---|---|---|---|
| No.1 | Primperan® Tablets 5 | S014G01 | Astellas Pharma Inc. | Lactose hydrate, Corn starch, Hydroxypropylcellulose (HPC), Microcrystalline cellulose, Light anhydrous silicic acid, Magnesium stearate (StMg), Hypromellose, Macrogol, Talc, Precipitated calcium carbonate |
| No.2 | Terperan® Tablets 5 mg | L166A | ASKA Pharmaceutical. Co., Ltd. | Carnauba wax, Microcrystalline cellulose, Titanium oxide, Magnesium stearate (StMg), Talc, Corn starch, Lactose hydrate, Hypromellose, Macrogol6000, Methylcellulose |
| No.3 | Metoclopramide Tablets 5 mg 「Takata」 | U001, V004 | TAKATA Pharmaceutical Co., Ltd. | Lactose hydrate, Microcrystalline cellulose, povidone, Magnesium stearate (StMg), Hypromellose, propylene glycol, Titanium oxide, Hydroxypropylcellulose (HPC), Carnauba wax |
Compendial pH 1.2, 4.0, and 6.8 buffer solutions were prepared in accordance with the Japanese Pharmacopoeia (JP). Dissolution media with a low buffer capacity was prepared by dilution of the compendial dissolution test media. To maintain the ionic strength of a diluted buffer equal to that in the undiluted dissolution medium, NaCl was added to diluted dissolution medium. A pH 6.8 buffer solution listed in United States Pharmacopoeia, phosphate and maleate buffer, other low buffer capacity media,18) were also prepared. The pH, ionic strength, and composition of buffer solutions used in this study are summarized in Table 2. Fasted State Simulated Gastric Fluid (FaSSGF), Fasted State Simulated Intestinal Fluid (FaSSIF), and FaSSGF, simulating hypochlorhydric and achlorhydric gastric conditions (FaSSGF(hypo) and FaSSGF(achlo)) were prepared according to the previous literature.9,25)
| pH | Ionic strength (mmol/L) | Conc. (mmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCl | NaOH | NaCl | Na2HPO4 | KH2PO4 | NaH2PO4 | C6H8O7 (citric acid) | C4H4O4 (maleic acid) | |||
| pH 1.2 (JP1) | 1.2 | 115.7 | 81.49 | 34.22 | ||||||
| pH 1.2 (1/15 dilution) + NaCl | 2.7 | 115.7 | 5.43 | 110.28 | ||||||
| pH 1.2 (1/30 dilution) + NaCl | 2.6 | 115.7 | 2.72 | 112.99 | ||||||
| pH 1.2 (1/30 dilution) | 2.6 | 3.9 | 2.72 | 1.14 | ||||||
| pH 4.0 | 4.0 | 57.8 | 19.28 | 15.36 | ||||||
| pH 6.8 (JP2) | 6.9 | 50.0 | 12.50 | 12.49 | ||||||
| pH 6.8 (1/2 dilution) + NaCl | 6.9 | 50.0 | 25.0 | 6.25 | 6.25 | |||||
| pH 6.8 (1/10 dilution) + NaCl | 6.9 | 50.0 | 45.0 | 1.25 | 1.25 | |||||
| pH 6.8 (1/10 dilution) | 7.0 | 5.0 | 1.25 | 1.25 | ||||||
| Purified water | 5.7 | 0.0 | ||||||||
| 0.2% NaCl | 5.7 | 34.2 | 34.22 | |||||||
| pH 6.8 (USP) | 6.8 | 72.4 | 22.4 | 50 | ||||||
| Phospate buffer | 6.5 | 106.9 | 1.2 | 101.3 | 5 | |||||
| Maleate buffer | 6.4 | 103.9 | 12.3 | 97.7 | 7 | |||||
The buffer capacities of the test media were measured in just one pH direction by dropwise addition of either NaOH (for all test solutions) or HCl (for test solutions except for acidic buffer solutions).16) To the dissolution medium (600 mL, 37°C), 0.5 M NaOH or 0.5 M HCl aqueous solutions were added while monitoring the pH values by using a pH meter (LAQUAact D-74, HORIBA, Ltd., Kyoto, Japan). The buffer capacity was calculated from the volume of NaOH or HCl solution added.
Dissolution TestsDissolution tests were conducted in accordance with the Japanese Pharmaceutical Codex III. Metoclopramide tablets (n = 6) were dissolved in an USP apparatus II (paddle) (Toyama Sangyo Co., Ltd., Osaka, Japan) at 50 rpm in 900 mL volume of test medium at 37 ± 0.5°C. Sampling for all dissolution tests was performed at 5, 10, 15, 30, 45, and 60 min. At each sampling time, 20 mL of medium was removed and filtered through a 0.45 µm polytetrafluoroethylene (PTFE) filter (Chromatodisk 25P, GL Sciences Inc., Tokyo, Japan), substituting 20 mL of fresh, prewarmed medium to replace the sample volume removed. pH control was not performed during the dissolution test with maleate buffer solutions.26) The pH values of dissolution test medium before and after the dissolution test were measured.
The concentration of metoclopramide was determined by using HPLC system (Prominence, Shimadzu Corporation, Kyoto, Japan). The mobile phase comprised 550 mL of sodium dodecyl sulfate (5 mM) aqueous solution, 450 mL acetonitrile, and 3 mL acetic acid. A Mightysil RP-18 GP column (150 × 4.6 mm, 5-µm, Kanto Chemical Co., Tokyo, Japan) was used at 25°C. The flow rate was set to adjust the retention time of metoclopramide to approximately 5 min and the injection volume was 50 µL. Measurements were performed using a detection wavelength of 275 nm.
f2 CalculationsDissolution similarities were statistically evaluated by using the similarity factor (f2) in accordance with the U.S. Food and Drug Administration (FDA) guidance.27)
The buffer capacities of various test media resistant to acidic and basic pH change were measured. The buffer capacity in the basic direction for pH 1.2 dissolution medium was approximately 70 mmol/L/ΔpH, and dilution of the dissolution medium decreased buffer capacity and ionic strength, depending on the dilution rate. Buffer capacities of 15 and 30-fold diluted pH 1.2 solution were below 5 mmol/L/ΔpH, which was comparable with the buffering capacity of human stomach fluid reported previously15) (Table 3). The buffer capacities in the basic direction for pH 4.0 and 6.8 media were approximately 12.8 and 9.8 mmol/L/ΔpH, respectively. A 10-fold dilution of pH 6.8 buffer media yielded a buffer capacity of 1.0 mmol/L/ΔpH. In the pH 1.2 and 6.8 solutions, the addition of NaCl increased the ionic strength but did not alter the buffer capacity. The buffer capacities of purified water and 0.2% NaCl aqueous solution were close to zero. The buffer capacities of the phosphate and maleate buffers were below 3 mmol/L/ΔpH, which were similar to that of human intestinal fluid from the duodenum or jejunum.15) The buffer capacities in the acidic direction were comparable with in the basic direction for each solution.
| pH | Buffer capacity | ||
|---|---|---|---|
| (mmol/L/ΔpH) acidic direction | (mean ± S.D.) bacis direction | ||
| pH 1.2 (JP) | 1.2 | N.D. | 67.8 ± 1.1 |
| pH 1.2 (1/15 dilution) + NaCl | 2.7 | N.D. | 4.5 ± 0.0 |
| pH 1.2 (1/30 dilution) + NaCl | 2.6 | N.D. | 2.0 ± 0.0 |
| pH 1.2 (1/30 dilution) | 2.6 | N.D. | 2.3 ± 0.0 |
| pH 4.0 | 4.0 | 12.8 ± 0.4 | 11.4 ± 0.0 |
| pH 6.8 (JP) | 6.9 | 9.8 ± 0.0 | 9.2 ± 0.0 |
| pH 6.8 (1/2 dilution) + NaCl | 6.9 | 4.9 ± 0.1 | 4.8 ± 0.0 |
| pH 6.8 (1/10 dilution) + NaCl | 6.9 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| pH 6.8 (1/10 dilution) | 7.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| pH 6.8 (USP) | 6.8 | 17.9 ± 0.1 | 21.4 ± 0.2 |
| Phospate buffer | 6.5 | 1.1 ± 0.0 | 2.3 ± 0.0 |
| Maleate buffer | 6.4 | 3.6 ± 0.0 | 1.5 ± 0.1 |
| Purified water | 5.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 0.2% NaCl | 5.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
N.D., not determined.
The dissolution profiles of three metoclopramide products were evaluated (Fig. 1). Products No. 1 and No. 2 showed very rapid dissolution (more than 85% in 15 min) and products No. 3 showed rapid dissolution (more than 85% in 30 min) in the pH 1.2, 4.0, and 6.8 media. Differences in dissolution profiles among three buffer solutions were not observed in any products. In contrast, the delayed dissolution of all the three products was observed when purified water was used as the dissolution test medium. The dissolution rates in 0.2% NaCl solution were similarly slow; therefore, low ionic strength was not thought to be the cause for slow dissolution observed when purified water was used. The pH changes in dissolution medium during the dissolution tests were observed in purified water and 0.2% NaCl aqueous solution, but not in other dissolution media (Supplementary Table 1). The bulk pH of purified water and 0.2% NaCl aqueous solution increased by approximately 0.7–1.3 after the dissolution test.

Each result represents the mean ± standard deviation (S.D.) of six tablets.
The disintegration aspects of tablets at the initiation of the dissolution test in pH 6.8 dissolution medium were shown in Fig. 2. Tablets of product No. 1 and 2 disintegrated easily, but a small amount of solid residue was observed from product No. 1. Tablets of product No. 3 tablet did not disintegrate sufficiently during the time of dissolution test.

White arrows indicate the position of tablet. (Color figure can be accessed in the online version.)
The dissolution profiles of the three products were evaluated in acidic and neutral buffer solutions and their diluted solutions. The dissolution rates of product No. 1 and No. 2 were very rapid in 15- and 30-fold diluted pH 1.2 media, as well as in the original pH 1.2 medium (Fig. 3). In contrast, dissolution of product No. 3 was delayed depending on dilution rate and the f2 values were below 50 in 15- and 30-fold diluted pH 1.2 medium (Table 4). The buffer capacity of the diluted solution was low, but bulk pH was not changed in all of the dissolution media (data not shown).

Dissolution tests were conducted with pH 1.2 medium, 15-fold diluted pH 1.2 medium, and 15-fold diluted pH 1.2 medium containing sodium chloride. Each result represents the mean ± S.D. of six tablets.
| pH 1.2 | pH 1.2 (1/15 dilution) + NaCl | pH 1.2 (1/30 dilution) + NaCl | |
|---|---|---|---|
| No.1 | 15 min, >85% | Similar (15 min, >85%) | Similar (15 min, >85%) |
| No.2 | 15 min, >85% | Similar (15 min, >85%) | Similar (15 min, >85%) |
| No.3 | 30 min, >85% | Not-similar (f2 = 48.8) | Not-similar (f2 = 35.3) |
The effect of buffer capacity on the dissolution rate was evaluated in compendial pH 6.8 buffering medium and its diluted solutions (Fig. 4). The dissolution of product No. 2 was slightly retarded in 10-fold diluted pH 6.8 buffer solutions, but exceeded 85% within 15 min in all three buffering media. The dissolution rates of products No. 1 and No. 3 were strongly affected by the buffer capacity of the medium. Product No. 1 showed very rapid dissolution (>85% in 15 min) in pH 6.8 medium and rapid dissolution (>85% in 30 min) in 2-fold diluted pH 6.8 medium. In 10-fold diluted pH 6.8 medium, the dissolution of product No. 1 did not exceed 85%, even within 45 min, and the f2 value was 36.3 (Table 5). The dissolution rate of product No. 3 was also delayed in diluted pH 6.8 medium and did not reach 85% within 60 min in 10-fold diluted pH 6.8 medium. The dissolution profiles of product No. 3 in both low buffering capacity media were not judged to be similar to that in pH 6.8 medium because the f2 values were below 50. Apparent changes in the bulk pH during dissolution test were not observed in the diluted dissolution media (data not shown).
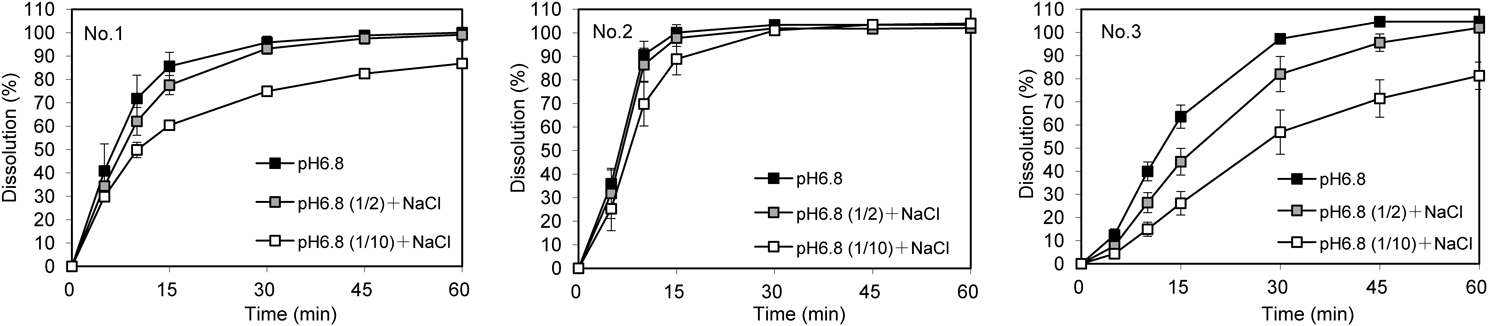
Dissolution tests were conducted with pH 6.8 medium, 2-fold diluted pH 6.8 medium, and 10-fold diluted pH 6.8 medium containing sodium chloride. Each result represents the mean ± S.D. of six tablets.
| pH 6.8 | pH 6.8 (1/2 dilution) + NaCl | pH 6.8 (1/10 dilution) + NaCl | |
|---|---|---|---|
| No.1 | 15 min, >85% | Similar (f2 = 56.8) | Not-similar (f2 = 36.3) |
| No.2 | 15 min, >85% | Similar (15 min, >85%) | Similar (15 min, >85%) |
| No.3 | 30 min, >85% | Not-similar (f2 = 43.6) | Not-similar (f2 = 26.2) |
The effect of buffer capacity on the dissolution rate was evaluated in other low buffer capacity media (Fig. 5). All three products showed similar dissolution profiles in pH 6.8 (JP) and pH 6.8 (USP) buffers. Slower dissolution of products No. 1 and No. 3 was observed in both the phosphate and maleate buffer solutions compared to that in pH 6.8 (JP) and pH 6.8 (USP) buffers. The dissolution profiles of product No. 1 and 3 in maleate buffer were judged to be dissimilar to those in pH 6.8 (JP) medium (Table 6). The effect of buffer capacity on the dissolution rate of product No. 2 was limited and the product showed very rapid dissolution (>85% in 15 min) in all buffering media. Although the pH was not controlled during the dissolution tests, the apparent bulk pH change in the maleate and phosphate buffers was not observed as well as other dissolution test media (data not shown).

Dissolution tests were conducted with pH 6.8 medium (JP), pH 6.8 medium (USP), and phosphate and maleate buffer media. Each result represents the mean ± S.D. of six tablets.
| pH 6.8 (JP) | pH 6.8 (USP) | Phospahate buffer | Maleate buffer | |
|---|---|---|---|---|
| No.1 | 15 min, >85% | Similar (15 min, >85%) | Similar (f2 = 56.8) | Not-similar (f2 = 44.1) |
| No.2 | 15 min, >85% | Similar (15 min, >85%) | Similar (15 min, >85%) | Similar (15 min, >85%) |
| No.3 | 30 min, >85% | Similar (f2 = 96.0) | Similar (f2 = 51.8) | Not-similar (f2 = 37.0) |
The dissolution of product No. 3 was evaluated in multiple biorelevant media, which included bile components and/or enzymes to simulate the environment of the stomach and the small intestine in the fasted state (Table 7). Rapid dissolution was observed in FaSSGF and FaSSIF, probably because the buffer capacity was greater than 10 mmol/L/ΔpH. In contrast, slower dissolution of the formulation was observed in the biorelevant media for simulating the contents of the fasted stomach under hypochlorhydric and achlorhydric conditions with low buffer capacity (Fig. 6).
| pH | Buffer capacity# (mmol/L/ΔpH) | Conc. (mM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pepsin (mg/mL) | Sodium taurocholate | Phosphatidyl-choline | HCl | NaOH | NaCl | KH2PO4 | C4H4O4 (maleic acid) | |||
| FaSSGF | 1.6 | — | — | 0.08 | 0.02 | q.s. to pH 1.6 | — | 34.2 | — | — |
| FaSSIF | 6.8 | 12 | — | 3 | 0.75 | — | 13.8 | — | 28.7 | — |
| FaSSGF(hypo) | 5.0 | 0.8 | 0.1 | 0.08 | 0.02 | — | q.s. to pH 5.0 | 15.7 | — | 1.0 |
| FaSSGF(achlo) | 7.0 | 0.7 | 0.1 | 0.08 | 0.02 | — | q.s. to pH 7.0 | 7.0 | — | 3.0 |
#, Buffer capacity values were cited from reference #9 and #25. —, not applicable.

Dissolution tests were conducted with Fasted State Simulated Gastric Fluid (FaSSGF), Fasted State Simulated Intestinal Fluid (FaSSIF), FaSSGF to simulate the hypochlorhydric and achlorhydric gastric conditions (FaSSGF(hypo) and FaSSGF(achlo)). Each result represents the mean ± S.D. of six tablets.
For the appropriate prediction of in vivo dissolution profiles, dissolution tests in media that chemically and physically mimic physiological conditions offer a promising approach. This study investigated the effect of the buffer capacity of the media on the dissolution rate of formulations of highly soluble drugs (BCS class III) by using diluted compendial test medium. The buffer capacity of compendial acidic solution was proven to be 10-fold higher compared with that of human gastric fluid, as recently reported.15,16) Excessive quantities of hydrogen ion in the test media would contribute to such high buffer capacity. USP acidic buffer (simulated gastric fluid, SGF) (0.1 N HCl) and FaSSGF should also have high buffer capacities because their solutions includes a comparable hydrogen ion concentration to that of JP acidic buffer solution (0.081 N HCl). The Japanese guideline for bioequivalence studies of generic products specifies a dissolution test medium with comparably low buffer capacity, e.g., four-fold diluted McIlvaine buffer in the weak acid to neutral pH range.28) Although this medium was designed to evaluate the dissolution under low buffer capacity condition, the capacities is still much higher than those of physiological fluid from the duodenum and jejunum.15) Therefore, it is reasonable to apply dissolution test media other than the commonly used compendial buffer to investigate the dissolution of drug products under low buffer capacity. This study also indicated that appropriate dilution and NaCl addition into compendial dissolution test medium was an alternative easy preparation method for dissolution test medium with intended low buffer capacity and ionic strength.
By using the example of metoclopramide tablets, this study showed that there may be a large effect of buffer capacity on some highly soluble APIs. The buffer capacity of the medium has been recognized as a determining factor for the dissolution of less soluble drugs, such as carvedilol,11) quetiapine fumarate,20) and diclofenac sodium.9) The differences were explained partly by possible changes in dissolution medium pH induced by dissolved drug and excipients.29) However, in this study, when using a highly soluble drug, significant changes in the bulk pH were not observed. Cristofoletti et al. suggested a local change in surface pH (pH0) around drug substances or excipients, due to the lack of conjugative base in a medium with low buffer capacity, was an alternative mechanism for the dissolution changes of poorly soluble drugs.18) As the effect of buffer capacity on the dissolution rate varied between products No. 1–3, the different formulation characteristics among the products, such as excipients, were inferred as a cause for the delay in dissolution of products No. 1 and 3. Distinctive excipients included in products No. 1 or No. 3 were light anhydrous silicic acid and precipitated calcium carbonate, or povidone and propylene glycol, respectively. Some interaction between the excipients and metoclopramide may affect the dissolution behavior. Indeed, disintegration patterns varied slightly among the three products in the widely used excipient combinations. Tablets of product No. 1 and No. 3 did not disintegrate sufficiently; therefore, the excipient difference may affect the extent of media inflow and outflow around the formulation and result in a dissolution difference. Further investigation is required to understand the effect of buffer capacity on the dissolution by using non-disintegrating tablets.
The slower dissolution of some metoclopramide tablets compared to those in USP buffer or conventional biorelevant media were observed in the diluted compendial media. The product No. 3 also showed slow dissolution in the recently proposed biorelevant media. Dissolution of other formulations in the biorelevant media with low buffer capacity would be an interesting topic for further study. These results has provided two important suggestions. First, use of dissolution media with a sufficiently low buffer capacity, comparable to that of physiological fluid, is necessary to detect dissolution-delayed products including highly soluble APIs. Second, diluted compendial media is a good alternative to biorelevant media to evaluate the effect of low buffer capacity on the dissolution rate. Diluted media are easy to prepare because the simple dilution and addition of sodium chloride to a compendial buffer is the only preparation required. Therefore, diluted compendial medium is useful for routine dissolution tests as it is an easily available dissolution medium with low buffer capacity.
This study showed that media with a buffer capacity comparable with that of physiological fluid could affect the dissolution rate of drug products with highly soluble APIs. The dissolution profile obtained from a simple dilution of compendial medium provided information on slower dissolution similar to those obtained in the biorelevant media. This indicated that the tests using lower buffer capacity media, including diluted compendial buffer, rationally reduce the risk of different dissolution properties due to the difference in buffer capacities between compendial medium and physiological fluid.
This work was partly supported by the Research on Regulatory Harmonization and Evaluation of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics of the Japan Agency for Medical Research and Development, AMED, under Grant Number JP19mk0101103.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.