Abstract
Lactoferrin (Lf) nanoparticles have been developed as a carrier of drugs and gene. Two main methods, desolvation technique and emulsification method, for preparation of protein nanoparticles have been reported so far, but most of the previous reports of Lf nanoparticles preparation are limited to emulsification method. In this study, we investigated the optimal conditions by desolvation technique for the preparation of glutaraldehyde-crosslinked bovine Lf (bLf) nanoparticles within the size range of 100–200 nm, and evaluated their properties as a carrier for oral and intravenous drug delivery. The experimental results of dynamic light scattering and Transmission Electron Microscope suggested that glutaraldehyde-crosslinked bLf nanoparticles with 150 nm in size could be produced by addition of 2-propanol as the desolvating solvent into the bLf solution adjusted to pH 6, followed by crosslinking with glutaraldehyde. These cross-linked bLf nanoparticles were found to be compatible to blood components and resistant against rapid degradation by pepsin. Thus, cross-linked bLf nanoparticles prepared by desolvation technique can be applied as a drug carrier for intravenous administration and oral delivery.
Introduction
Lactoferrin (Lf), which is a glycoprotein found in human breast milk as well as most epithelial surface secretions including tears, nasogastric, saliva, and bronchial, has been studied extensively for use as a biomaterial in the field of drug delivery by virtues of its good biocompatibility and comparatively more affordable price.1–3) One example of such attempts was Lf nanoparticles that have been used as a carrier of an antiretroviral drug4) and plasmid DNA (pDNA).5) Most of the reported Lf nanoparticles have been mostly prepared by means of emulsion method,6,7) which enables preparation of 100–200 nm of Lf nanoparticle with good polydispersity index (PDI). However, this method may be cumbersome due to laborious process: olive oil, at first, added into Lf solution containing drug with gentle vortex, (ii) subsequently, the mixtures was sonicated in ice using an ultrasonic homogenizer, (iii) then, the resulting nanoparticles were snap frozen in liquid nitrogen and thawed under ice condition in 4 h, (iv) finally, nanoparticles dispersed in aqueous solution after collection by centrifugation. Besides emulsion method, different physical and chemical approaches for preparing protein-based nanoparticles have been reported, such as desolvation,8) nanospray drying9) and self-assembly.10) Among the aforementioned approaches, desolvation technique is known as a straightforward, rapid and easily applicable method to carry out the whole nanoparticle preparation procedure in one pot. However, to the best of our knowledge, there is no report attempting to prepare and characterize Lf nanoparticle using desolvation technique.
In this study, we attempted optimization of the preparation condition of desolvation technique for producing bovine Lf (bLf) nanoparticles as a drug delivery carrier. We examined the effect of solvents and pH on the physicochemical properties of bLf nanoparticles.11) The biocompatible properties of bLf nanoparticles stabilized by treatment with glutaraldehyde as a crosslinking agent, as a carrier for oral and intravenous drug delivery in vitro were evaluated.
Experimental
Preparation of bLf NanoparticlesNanoparticles were prepared using a previously described desolvation technique with minor modification.11) In short, 8 mL of an organic solvent (ethanol (EtOH), methanol (MeOH), 2-propanol (2-Pro), acetonitrile (AcCN) or acetone) was added into 2 mL of bLf solution (100 mg/mL, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) with a syringe pump at a flow rate of 1 mL/min under magnetic stirring (1250 rpm) at room temperature. The pH of bLf solution was adjusted to 3–9 using HCl or NaOH.
Cross-Linking of bLf Nanoparticles by GlutaraldehydeThe resulting nanoparticles desolvated by 2-Pro or AcCN were stabilized by the addition of 8% glutaraldehyde as a crosslinking agent, followed by stirring for 24 h (500 rpm). The suspension was centrifuged (5000 × g, 20 min), and subsequently supernatant was centrifuged (10000 × g, 20 min). The collected precipitation was re-dispersed in ultrapure water and ultrasonicated for 5 min.
Determination of Physicochemical CharacteristicsThe physicochemical characteristics (particle size, PDI and zeta-potential) of the prepared nanoparticles were determined by dynamic light scattering (DLS, ELS-Z2, Otsuka Electronics Co., Ltd., Osaka, Japan). Transmission Electron Microscope (TEM) images were observed with a FEI Titan Themis at 200 kV after nanoparticles were stained by 2% (w/w) ammonium molybdate.
Structural Analysis of bLfIn case of Native-polyacrylamide gel electrophoresis (PAGE), samples were mixed with a sample buffer solution and were loaded into a 12.5% polyacrylamide gel and detected by staining with Coomassie Blue R-250. Gel image was recorded using a Bio-Rad GS-800 calibrated densitometer. Nanoparticle structure before crosslinking by glutaraldehyde was collapsed by the addition of ultrapure water. Far-UV circular dichroism (CD) spectra (200–250 nm) were recorded with a Jasco-720 spectropolarimeter (Tokyo, Japan) using a 1-mm path length cell.
Digestion Tolerance AssaySample (bLf or bLf nanoparticles) and pepsin from porcine stomach mucosa (FUJIFILM Wako Pure Chemical Corporation) were dissolved in ultrapure water at a final concentration of 10 µM and 2 µg/mL, respectively, and HCl was added to adjust the pH to pH 3.2. At the stipulated times (5, 10, 15, 20, 60 min) after incubation at 37°C, the mixture was collected and the digestion reaction was terminated by ice cooling. The degree of digestion was analysed by Native-PAGE with the same procedure described in “Structural analysis of bLf.”
Blood Compatibility and Hemolysis AssayBlood compatibility and hemolysis assay were performed as reported previously with minor modifications.12,13) In brief, either bLf or bLf nanoparticle was added to fresh blood collected from rats (male, Sprague-Dawley) to produce an 80% v/v concentration. At 0, 4 and 18 h after incubation at 37°C, the number of white blood cells (WBC), red blood cells (RBC) and platelets (PLT) were assessed using an animal blood cell counter (MEK-6458; NIHON KOHDEN Corp., Tokyo, Japan). The results are shown as a percentage of that of saline treatment (negative control).
The rat RBCs harvested by repeatedly washing with saline was mixed with either ultrapure water, PBS, bLf or bLf nanoparticle. At 4 and 18 h after incubation at 37°C, the mixture was centrifuged (3000 rpm, 10 min) and the absorbance (540 nm) of the supernatant was measured. The percent hemolysis was shown as a percentage of that of water treatment (100% hemolysis). These studies were reviewed and approved by the Animal Care and Use committee of Sojo University (Permit #: 2017-P-020).
StatisticsAll data are expressed as the mean ± standard deviation (S.D.). Statistical analyses for multiple comparisons in the study were determined by ANOVA (two-way ANOVA). A probability value of p < 0.05 was considered statistically significant.
Results and Discussion
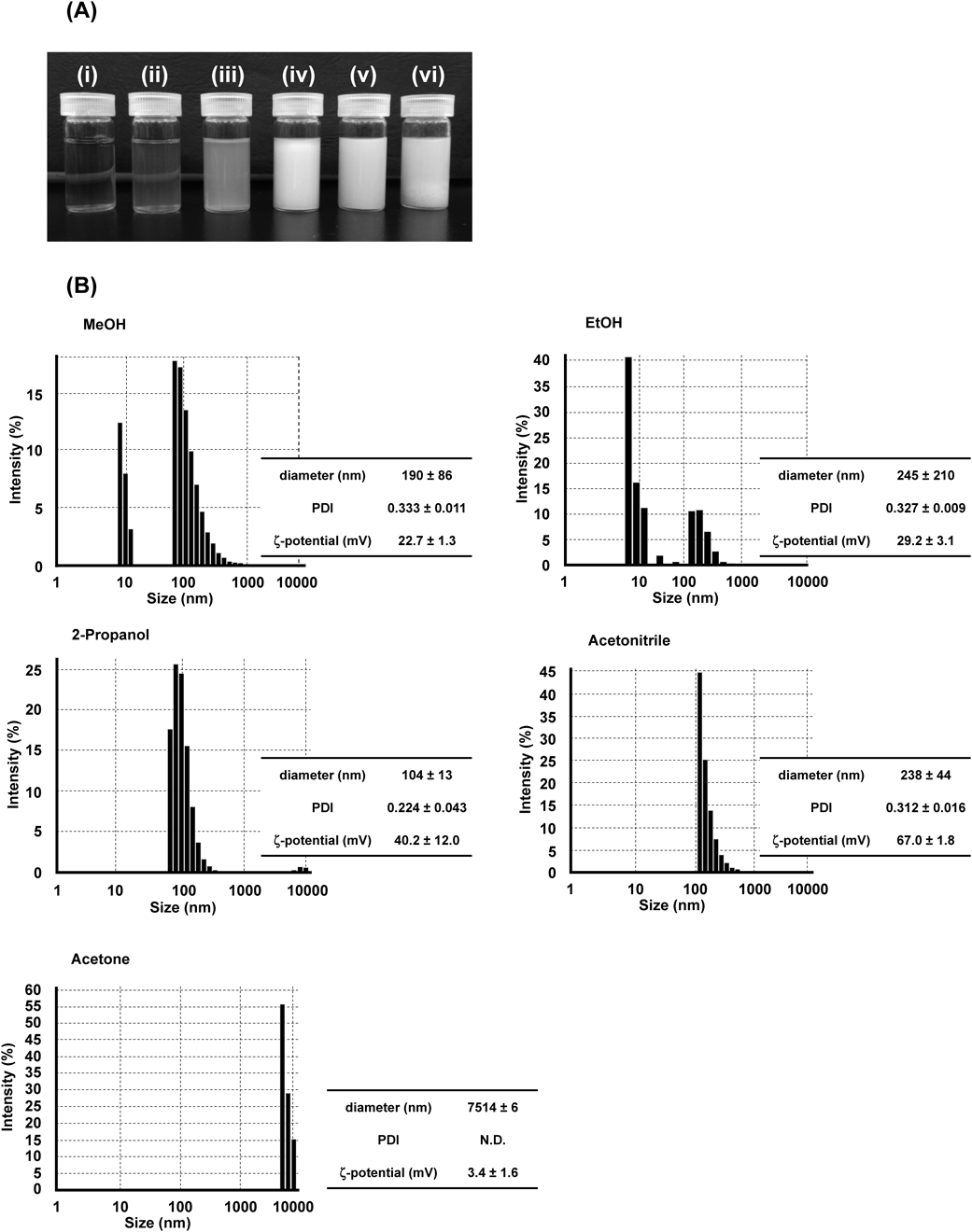
Effects of Solvents Used on Physicochemical Characteristics of bLf Nanoparticles Prepared by Desolvation TechniqueThe solvent effect of EtOH, MeOH, 2-Pro, AcCN and acetone on size and size distribution of protein nanoparticle prepared by desolvation technique was first examined.14,15) As a result, the appearance of bLf nanoparticles was different depending on the desolvating solvent used (Fig. 1A). DLS results clearly showed that desolvation by MeOH and EtOH formed nanoparticle with biphasic size distribution, and desolvation by acetone formed nanoparticles with large size (Fig. 1B). On the other hand, bLf nanoparticles desolvated by 2-Pro and AcCN had relatively uniform size with strong positive charge (Fig. 1B). Storp et al. previously reported that desolvating solvent with high dielectric constant produces smaller size of albumin nanoparticles.16) Similar phenomenon has been reported in a previous study using α-lactalbumin.17) In this study, the particle size of bLf nanoparticles in increasing order was 2-Pro (104 nm) < MeOH (190 nm) < AcCN (238 nm) < EtOH (245 nm) < acetone (7541 nm). However, the dielectric constant of each desolvating solvent was 18.3,18) 32.6,18) 37.5,18) 24.318) and 21 μ,19) for 2-Pro, MeOH, AcCN, EtOH and acetone, respectively. Dielectric constant of the desolvation solvent influences the particle size of bLf nanoparticle in a different manner than albumin and α-lactalbumin nanoparticles. Various factors influenced the formation of protein nanoparticles, particularly the difference of protein properties, such as charge, molecular weight and presence/absence of sugar chain, is likely to contribute to the difference in dielectric constant effect between the present and previous studies.
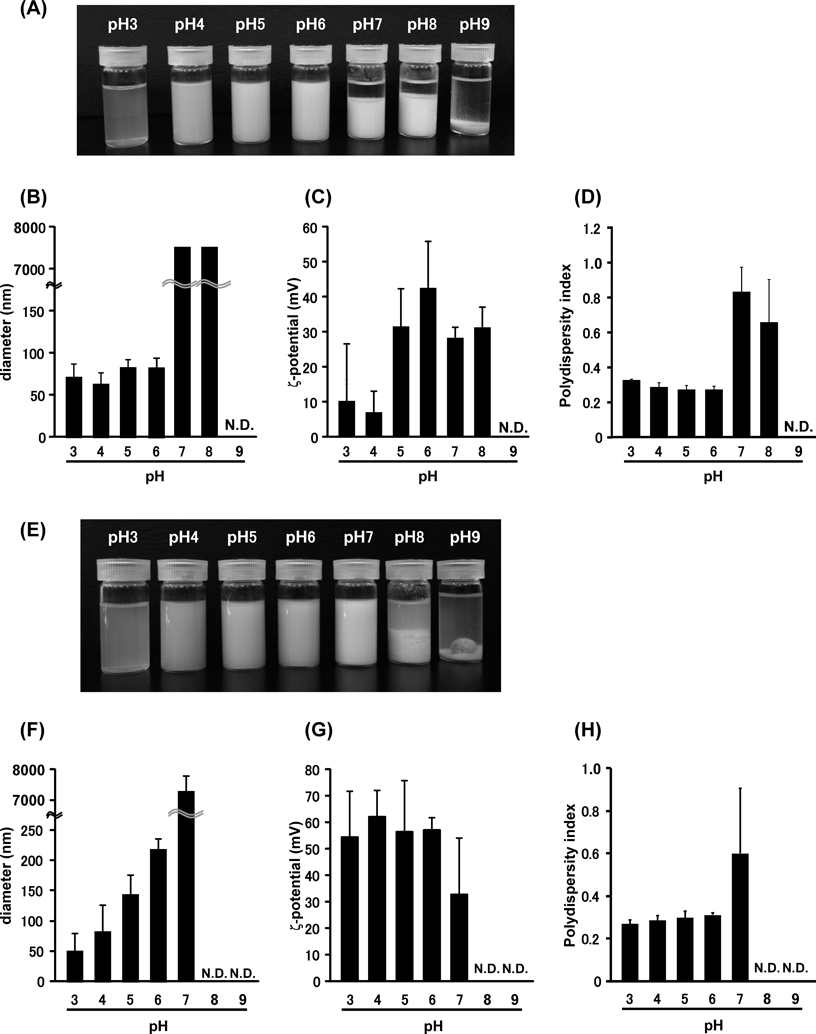
Effects of pH of bLf Solution on the Physicochemical Characteristics of bLf Nanoparticles Prepared by Desolvation TechniqueIt was reported that the size of albumin nanoparticles prepared by desolvation technique became smaller as pH increased.11,14) Thus, we next investigated pH effect on the size of bLf nanoparticle prepared by desolvation technique. Based on the experiment results shown in Fig. 1, 2-Pro and AcCN, which formed uniform size of bLf nanoparticle, were chosen as the desolvating solvents for further studies. Different from albumin nanoparticle,14) bLf precipitated when the pH of bLf solutions, with addition of either 2-Pro or AcCN, was above 7 (Figs. 2A, E). In accordance with the appearance of the particles, particle diameters measured by DLS were large (or undetected) with high PDI values (Figs. 2B–D and F–H). On the other hand, the particles with size ranging below 200 nm were produced by desolvation technique at pH 3–6, and these particles had positive charge and constant PDI (Figs. 2B–D and F–H). The reason for the different tendency between albumin nanoparticle and bLf nanoparticle is unclear, but difference in the protein isoelectric points (albumin = 4.7, Lf = 8) might have contributed significantly in the response of protein to form nanoparticles. In addition, albumin nanoparticle prepared in the previous studies used EtOH as a desolvating solvent,11,14) whereas we prepared bLf nanoparticle using 2-Pro and AcCN in this study. Thus, the difference of desolvating solvent might also have an effect on the formation of nanoparticles under different pH conditions. In this study, pH 6 was used in desolvation of bLf solution for further investigations. Furthermore, the turbidity of bLf nanoparticles suspension desolvated by AcCN which was evaluated from appearance correlated with the size of nanoparticles (Figs. 2E, 2F). Such correlation was not observed in the nanoparticles prepared by the other experimental conditions (Figs. 1A and 1B, Figs. 2A and 2B).
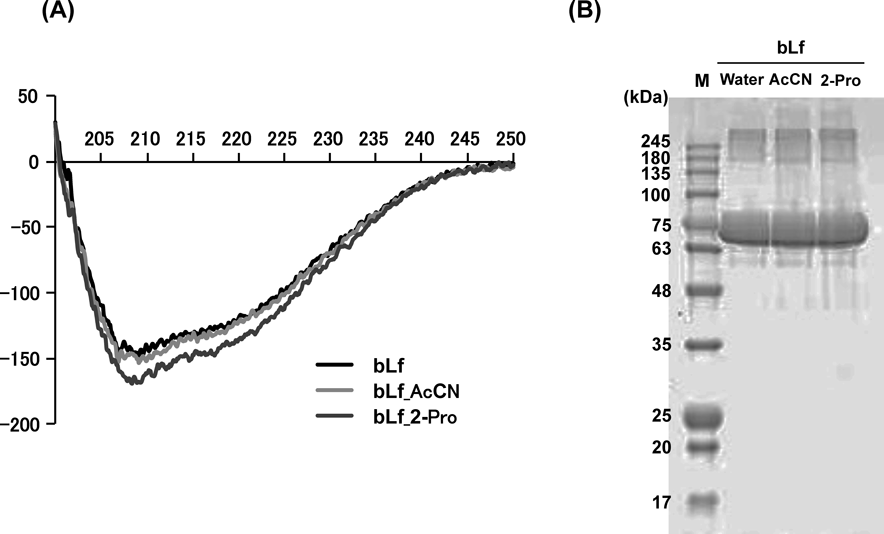
Evaluation of bLf Structure after DesolvationSince the desolvating solvent may cause denaturation and fragmentation of bLf, the effects of 2-Pro and AcCN on bLf structure were evaluated by Native-PAGE and far-UV CD spectra and compared with those of untreated bLf. As a result, the characteristics of the far-UV CD spectra for bLf after 2-Pro and AcCN treatments were almost similar to that of untreated bLf (Fig. 3A). Furthermore, in Native-PAGE, a strong single band was observed around 75 kDa for untreated bLf and bLf after 2-Pro and AcCN treatments (Fig. 3B). These results suggest that denaturation and fragmentation in bLf appear to be negligible in the desolvating process by 2-Pro and AcCN.
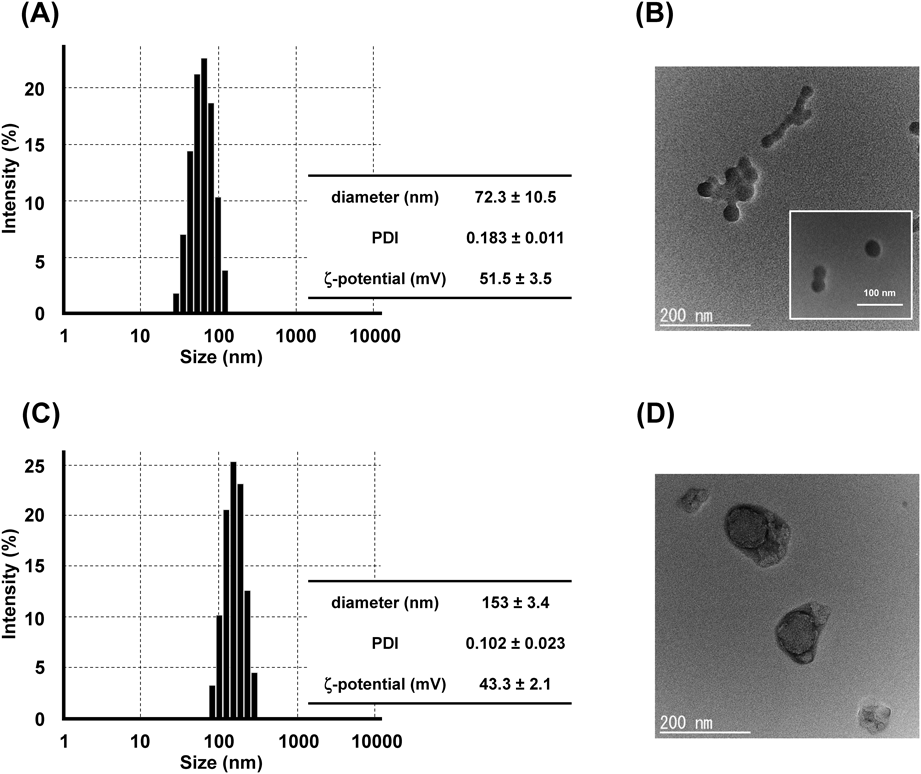
Physicochemical Characterization of bLf Nanoparticles Cross-Linked by GlutaraldehydeTo use bLf nanoparticles for oral and intravenous drug delivery, organic solvents should be removed. However, the presence of organic solvent is essential for the maintenance of bLf nanoparticles structure. Thus, protein nanoparticles prepared by desolvation technique were generally cross-linked with a crosslinker such as glutaraldehyde or genipin,8,20) the resulting cross-linked nanoparticles can remain stably dispersed in aqueous phase. In this study, we used glutaraldehyde as a crosslinker. DLS measurement showed that the diameter of nanoparticles desolvated by AcCN decreased from 238 to 72 nm after cross-linking (Fig. 4A). However, TEM image showed that nanoparticles with size ranging 10–20 nm looked like forming aggregation (Fig. 4B). On the other hand, DLS measurement showed that the diameter of nanoparticles desolvated by 2-Pro slightly increased from 100 to 150 nm after cross-linking (Fig. 4C). Corresponded with the results of DLS, the nanoparticle with around 150 nm in size were observed by TEM (Fig. 4D). Glutaraldehyde is capable of forming interparticle and intraparticle cross-link via lysine residues on the surface of nanoparticles.21) Thus, in the case of nanoparticle desolvated by AcCN, glutaraldehyde might cross-link intraparticularly and interparticularly, whereas for nanoparticles prepared by 2-Pro, glutaraldehyde might crosslink intraparticularly only. From the view point of drug carrier, the size of cross-linked bLf nanoparticles desolvated by 2-Pro (around 150 nm) is the ideal size for the nanoparticles to remain in the circulatory system for long period after intravenous administration14) and to accumulate at the inflamed site in the colon.22,23) Based on these facts, we concluded that glutaraldehyde cross-linked bLf nanoparticles desolvated by 2-Pro were optimal bLf nanoparticles prepared by desolvation technique.
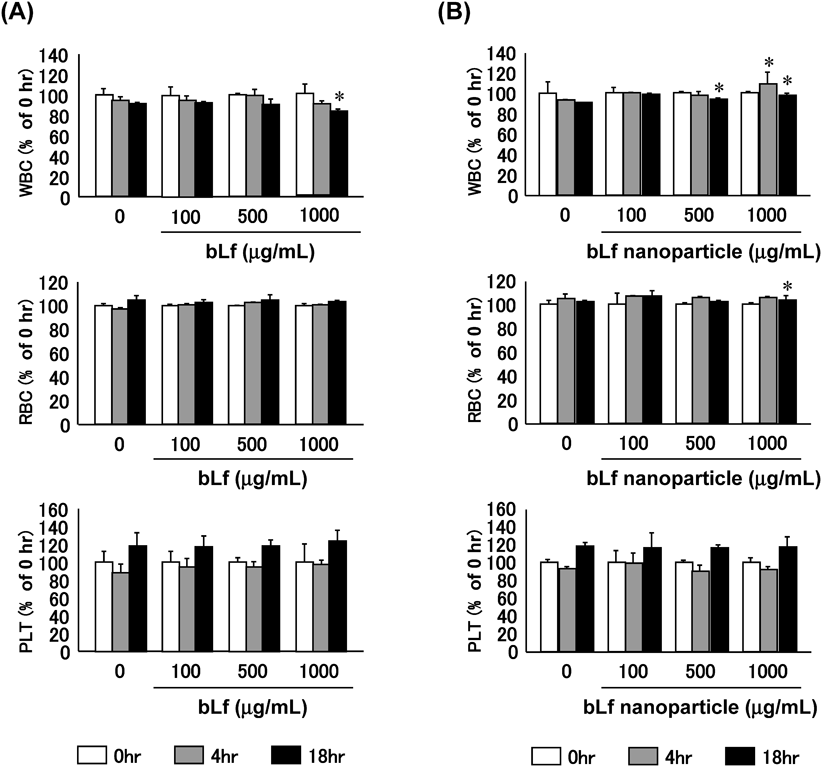
Blood Compatibility in VitroBlood compatibility is essential for bLf nanoparticle to be considered for intravenous drug delivery. Especially, cationic nanoparticles can electrostatically interact with blood components, resulting in hemolysis.24) Thus, the compatibility of cross-linked bLf nanoparticles with blood components (WBC, RBC and PLT) in vitro was evaluated. After incubating different concentrations of cross-linked bLf nanoparticles with whole blood at 37°C in vitro, obvious changes in WBC and RBC were not observed up to 18 h of incubation, comparable to the results of control experiments with saline (Fig. 5B). On the other hand, the numbers of PLT were slightly increased at 18 h after incubation at all concentration. Similar changes in WBC, RBC and PLT were observed after incubation of bLf with whole blood (Fig. 5A). Furthermore, even though cross-linked bLf nanoparticles were positively charged, they have negligible effect on hemolysis (data not shown). This result is consistent with the changes of RBC number. These results indicate that no abnormal interaction occurred between cross-linked bLf nanoparticle and blood components, supporting the potential use of cross-linked bLf nanoparticle for intravenous drug delivery.
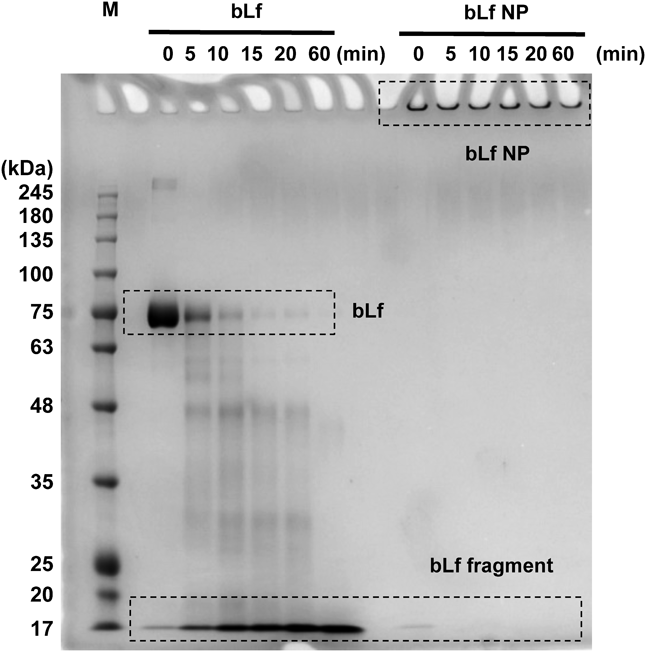
Pepsin Tolerance AssayIn case of bLf nanoparticle for oral drug delivery, digestion tolerance against gastric enzymes is required to be evaluated because proteins-based nanoparticles will be degraded by enzymes of the stomach before reaching the target site in gastrointestinal tract.25) Thus, pepsin tolerance of the cross-linked bLf nanoparticles was examined in vitro. When untreated bLf was incubated with pepsin, the Native PAGE band derived from bLf disappeared in a time dependent manner and the band of around 17 kDa gradually became stronger, indicating that bLf has been digested by pepsin (Fig. 6). In contrast, the strong band derived from cross-linked bLf nanoparticles was observed at the top of gel due to the fact that nanoparticles got stuck by virtues of size (Fig. 6). Furthermore, no degradation was observed even after 60 min incubation. These results indicated that cross-linked bLf nanoparticles had sufficient resistance against pepsin. In addition to pepsin in stomach, there are other proteolytic enzymes responsible for protein degradation in the gastrointestinal tract.26) Therefore, these enzymes could trigger the release of the embedded drug in bLf nanoparticle in gastrointestinal tract.
Conclusion
Desolvation technique is a straightforward, rapid and easily applicable method in the preparation of protein nanoparticles. In this study, we optimized the preparation condition of bLf nanoparticles with uniform size by desolvation technique. 2-Pro was found to be a suitable desolvating solvent for addition into bLf solution at pH 6, and then glutaraldehyde added as a crosslinker. The steps of this method are simple compared to emulsion method reported previously. In addition, the resulting cross-linked bLf nanoparticles possess good blood compatibility and tolerance against digestion by pepsin. Since the particles with nano-order size (100–200 nm) accumulate at the tumor and inflamed site after intravenous administration, and at the inflamed site in the colon after oral administration,14,22,23) Lf nanoparticle prepared in this study has great potential to be applied for delivery of doxorubicin, methotrexate and 5-aminosalicylic acid for the treatment of cancer and colitis as protein based-nanoparticles of these drugs have been formulated for the treatment of those diseases.8,23) At the very least, the results obtained in this study suggested that cross-linked bLf nanoparticles prepared by desolvation technique can be a new nanocarrier for intravenous and oral drug delivery.
Acknowledgment
This work was supported by a Research Grant from the THE NAGAI FOUNDATION TOKYO, from Sojo University, from Keio University.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1) Liu S., Zhang S. M., Ju R. J., Xiao Y., Wang X., Song X. L., Gu L. Y., Cheng L., Li X. T., Chen G. R., Eur. J. Pharm. Sci., 106, 185–197 (2017).
- 2) Fang J. H., Chiu T. L., Huang W. C., Lai Y. H., Hu S. H., Chen Y. Y., Chen S. Y., Adv. Healthc. Mater., 5, 688–695 (2016).
- 3) Meng Q., Wang A., Hua H., Jiang Y., Wang Y., Mu H., Wu Z., Sun K., Int. J. Nanomedicine, 13, 705–718 (2018).
- 4) Madugulla L., Ravula A. R., Kondapi A. K., Yenugu S., Syst. Biol. Reprod. Med., 65, 205–213 (2019).
- 5) Kumari S., Kondapi A. K., Int. J. Biol. Macromol., 108, 401–407 (2018).
- 6) Ahmed F., Kumari S., Kondapi A. K., Pharm. Res., 35, 178 (2018).
- 7) Kumari S., Bhattacharya D., Rangaraj N., Chakarvarty S., Kondapi A. K., Rao N. M., Nanomedicine (Lond), 13, 2579–2596 (2018).
- 8) Kimura K., Yamasaki K., Nishi K., Taguchi K., Otagiri M., Cancer Chemother. Pharmacol., 83, 1113–1120 (2019).
- 9) Yamasaki K., Kwok P. C. L., Fukushige K., Prud’homme R. K., Chan H.-K., Int. J. Pharm., 420, 34–42 (2011).
- 10) Jiang Y., Liang M., Svejkar D., Hart-Smith G., Lu H., Scarano W., Stenzel M. H., Chem. Commun., 50, 6394–6397 (2014).
- 11) Kimura K., Yamasaki K., Nakamura H., Haratake M., Taguchi K., Otagiri M., Chem. Pharm. Bull., 66, 382–390 (2018).
- 12) Okamoto Y., Taguchi K., Yamasaki K., Sakuragi M., Kuroda S., Otagiri M., J. Pharm. Sci., 107, 436–445 (2018).
- 13) Taguchi K., Lu H., Jiang Y., Hung T. T., Stenzel M. H., J. Mater. Chem. B, 6, 6278–6287 (2018).
- 14) Langer K., Balthasar S., Vogel V., Dinauer N., von Briesen H., Schubert D., Int. J. Pharm., 257, 169–180 (2003).
- 15) Jun J. Y., Nguyen H. H., Paik S. Y. R., Chun H. S., Kang B. C., Ko S., Food Chem., 127, 1892–1898 (2011).
- 16) Von Storp B., Engel A., Boeker A., Ploeger M., Langer K., J. Microencapsul., 29, 138–146 (2012).
- 17) Etorki A. M., Gao M., Sadeghi R., Maldonado-Mejia L. F., Kokini J. L., J. Food Sci., 81, E2511–E2520 (2016).
- 18) Streng W. H., “Characterization of Compounds in Solution,” Springer U.S., Boston, MA, 2001, pp. 181–218.
- 19) Mohammad-Beigi H., Shojaosadati S. A., Morshedi D., Mirzazadeh N., Arpanaei A., Iranian J. Biotechnol., 14, 45–50 (2016).
- 20) Feng X., Dai H., Ma L., Yu Y., Tang M., Li Y., Hu W., Liu T., Zhang Y., Foods, 8, E479 (2019).
- 21) Marquié C., J. Agric. Food Chem., 49, 4676–4681 (2001).
- 22) Collnot E.-M., Ali H., Lehr C.-M., J. Control. Release, 161, 235–246 (2012).
- 23) Iwao Y., Tomiguchi I., Domura A., Mantaira Y., Minami A., Suzuki T., Ikawa T., Kimura S., Itai S., Eur. J. Pharm. Biopharm., 125, 141–147 (2018).
- 24) Ong Z. Y., Yang C., Cheng W., Voo Z. X., Chin W., Hedrick J. L., Yang Y. Y., Acta Biomater., 54, 201–211 (2017).
- 25) Ensign L. M., Cone R., Hanes J., Adv. Drug Deliv. Rev., 64, 557–570 (2012).
- 26) Gupta S., Jain A., Chakraborty M., Sahni J. K., Ali J., Dang S., Drug Deliv., 20, 237–246 (2013).