Regular Articles
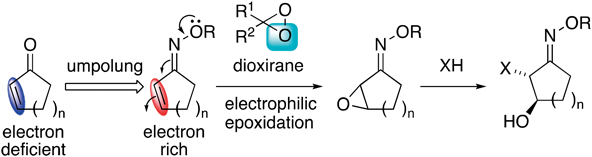
Electrophilic Epoxidation of α,β-Unsaturated Oximes with Dioxiranes and Ring Opening of the Epoxides
Keywords:
α,β-unsaturated oxime,
umpolung,
dimethyldioxirane,
epoxidation,
Shi asymmetric epoxidation
2021 Volume 69 Issue 10 Pages 1010-1016
Details