2021 年 69 巻 10 号 p. 1017-1028
2021 年 69 巻 10 号 p. 1017-1028
Celecoxib, a nonsteroidal anti-inflammatory drug, has been reported to have antitumor and antimetastatic activities, and it has potential for application in cancer treatments. The expression of matrix metalloproteinase (MMP)-2/9 is strongly correlated with cancer malignancy, and inhibition of these MMPs is believed to be effective in improving the antitumor and antimetastatic effects of drugs. We have previously revealed that UTX-121, which converted the sulfonamide of celecoxib to methyl ester, has more potent MMP-2/9 inhibitory activity than celecoxib. Based on these findings, we identified compounds with improved MMP inhibitory activity through a structure–activity relationship (SAR) study, using UTX-121 as a lead compound. Among them, compounds 9c and 10c, in which the methyl group of the p-tolyl group was substituted for Cl or F, showed significantly higher antitumor activity than UTX-121, and suppressed the expression of MMP-2/9 and activation of pro MMP-2. Our findings suggest that compounds 9c and 10c may be potent lead compounds for the development of more effective antitumor drugs targeting MMP.
Celecoxib is a nonsteroidal anti-inflammatory drug that selectively inhibits cyclooxygenase (COX)-2.1) COX is necessary for the production of prostaglandins from arachidonic acid in the arachidonic acid cascade. There are two isozymes for COX, COX-1 and COX-2. COX-1 is constitutively expressed, and involved in the regulation of biological functions. On the other hand, COX-2 is induced by various cytokines at the site of inflammation and produces prostaglandins, which are mainly associated with inflammation and pain.2) Therefore, celecoxib, a selective COX-2 inhibitor, has gained attention as an innovative drug with fewer side effects. Recently, it has been shown that celecoxib possesses antitumor and antimetastatic activities against various cancer types,3–5) suggesting that it is a promising anticancer and antimetastatic agent. Many studies have focused on the antitumor activity of celecoxib, and it has been reported that celecoxib could affect several factors such as Akt, extracellular signal-regulated kinase (ERK), vascular endothelial growth factor (VEGF), and nuclear factor-kappaB (NF-κB).6,7) It has also been suggested that this antitumor activity is mediated by a COX-2-dependent pathway and a COX-2-independent pathway.6,8) However, the detailed molecular mechanism by which celecoxib exhibits its antitumor and antimetastatic activities has not yet been clarified. In addition, the antitumor and antimetastatic activities of celecoxib are relatively modest, and further improvements are desired.
Matrix metalloproteinases (MMPs) are Zn2+-dependent enzymes that play a major role in the degradation of extracellular matrix (ECM).9–11) At present, more than 20 subtypes of MMPs have been identified. Among these MMPs, MMP-2/9 are known as type IV collagenases that degrade type IV collagen in the basement membrane.12) It has been reported that these MMPs are overexpressed in several cancer types.13–16) Hence, the inhibition of these MMPs could contribute to antitumor and antimetastatic effects. Celecoxib has also been reported to have MMP-2/9 inhibitory activities,17,18) but no antitumor or antimetastatic agent utilizing this MMP inhibitory activity of celecoxib has been developed yet.
We previously developed UTX-121, in which the sulfonamide of celecoxib was replaced with a methyl ester.19) Carboxylic acid is a bioisostere of sulfonamide, but it is known to have extremely poor cell permeability owing to its high polarity. Therefore, we designed UTX-121 with a carboxylic acid group protected by a methyl ester. It was found that UTX-121 suppresses the production of MMP-9 and inhibits the activation of pro MMP-2 by disrupting the cell surface expression of MT1-MMP. Moreover, UTX-121 suppressed focal adhesion kinase (FAK)/Akt activation and cell migration, which inhibited cell invasion in Matrigel. However, the MMP inhibitory activity of UTX-121 is modest, and the development of derivatives with more potent activities is desired. Celecoxib has a unique conformation that may contribute to its biological function. We have not yet studied the p-tolyl group of celecoxib, and the modifications of this moiety may lead to enhanced MMP inhibitory effects. In this study, we first investigated various aspects of the methyl ester group in UTX-121. We then focused on the p-tolyl moiety of UTX-121 and explored compounds with higher MMP inhibitory activities using the structure–activity relationship (SAR) method.
We previously developed UTX-121, in which the sulfonamide of celecoxib was converted into a methyl ester (Fig. 1). This revealed that UTX-121 suppressed MMP-9 production and MMP-2 activation more effectively than celecoxib.19) In this study, we investigated the role of methyl esters in UTX-121. Specifically, we designed compounds in which the methyl ester was converted into alcohol (compound 3) or ether (compound 4), to examine the effect of hydrogen bonds formed by the carbonyl group on antitumor and MMP inhibitory activities. Then, to investigate the alkyl chain length of methyl ester, we designed compounds 5–8, which changed the carbon number of the methyl moiety, and evaluated their antitumor and MMP inhibitory activities.

We also focused on the p-tolyl moiety of UTX-121. First, we designed compounds 9c–21c, in which the methyl group of the p-tolyl moiety was substituted with various functional groups with different hydrophobicities, electronic effects, and molecular sizes. Furthermore, we designed and synthesized compounds 22c–30c, in which the phenyl ring of UTX-121 was replaced with other aromatic groups such as cyclohexane or pyridine, and evaluated their antitumor and MMP inhibitory activities.
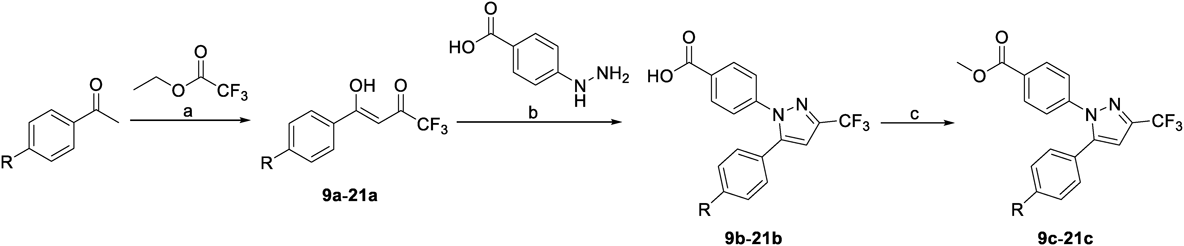
Synthesis of Celecoxib DerivativesCompounds 1 and 2 were synthesized as described previously.19) Compound 2 was reduced using LiAlH4 to give compound 3, and compound 4 was obtained by Williamson ether synthesis. Compounds 5–8 were synthesized by esterification of compound 2 (Chart 1).

(a) LiAlH4, THF, room temperature (r.t.), 1.5 h; (b) CH3I, NaH, THF, r.t., 19 h; (c) 5–7: SOCl2, EtOH (or 1-propanol or 2-propanol), r.t., 18–24 h; 8: benzyltriethylammonium chloride, K2CO3, 2-bromo-2-methylpropane, N,N-dimethylacetamide, 55 °C, 19 h.
The synthesis of various derivatives focusing on the p-tolyl moiety of UTX-121 is shown in Charts 2 and 3. Compounds 9b–30b were synthesized through the Claisen condensation reaction, followed by coupling with 4-hydrazinobenzoic acid. Then, compounds 9c–30c were obtained by esterizing compounds 9b–30b using SOCl2.
(a) NaH, THF (or tert-butyl methyl ether), r.t., 0.5–22 h; (b) 2 N HCl, EtOH, 75 °C, 0.5–3 h; (c) SOCl2, MeOH, r.t., 17.5–24 h.

(a) NaH, THF (or tert-butyl methyl ether), r.t., 1–26 h; (b) 2 N HCl, EtOH, 75 °C, 0.5–1.5 h; (c) SOCl2, MeOH, r.t., 18–23.5 h.
After incubating HT-1080 cells with the compounds for 48 h, the WST-8 assay was used to evaluate the antitumor activities of UTX-121 derivatives. IC50 values are shown in Tables 1–3.
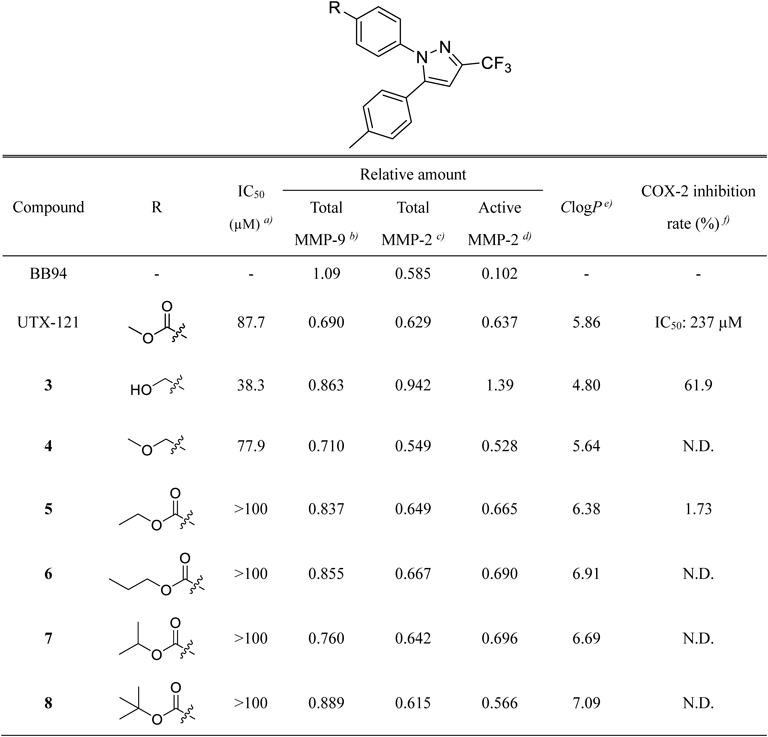 |
a) Antitumor activity in HT-1080 cells. b) Band density of total MMP-9. c) Band density of total MMP-2 (latent + active MMP-2). d) Band density of active MMP-2. e) Calculated using ChemDraw 19.1. f) COX-2 inhibition rate at 200 µM.
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | IC50 (µM) a) | Relative amount | C log P e) | COX-2 inhibition rate (%) f) | ||
| Total MMP-9 b) | Total MMP-2 c) | Active MMP-2 d) | |||||
| BB94 | — | — | 1.09 | 0.585 | 0.102 | — | — |
| UTX-121 | Me | 87.7 | 0.690 | 0.629 | 0.637 | 5.86 | IC50: 237 µM |
| 9c | F | 38.1 | 0.300 | 0.338 | 0.470 | 5.50 | N.D. |
| 10c | Cl | 33.2 | 0.239 | 0.248 | 0.325 | 6.07 | 34.0 |
| 11c | Br | 36.0 | 0.353 | 0.454 | 0.522 | 6.22 | 19.3 |
| 12c | H | 76.6 | 0.533 | 0.718 | 0.847 | 5.36 | 43.9 |
| 13c | Et | >100 | 0.742 | 0.743 | 0.706 | 6.38 | 8.90 |
| 14c | CF3 | >100 | 0.892 | 0.843 | 0.811 | 6.24 | 40.5 |
| 15c | CN | >100 | 0.561 | 0.612 | 0.547 | 4.79 | 78.5 |
| 16c | SMe | >100 | 0.825 | 0.929 | 0.964 | 5.92 | 44.0 |
| 17c | OH | 18.0 | 0.137 | 0.599 | 0.787 | 4.73 | 32.6 |
| 18c | OMe | >100 | 0.756 | 0.796 | 0.872 | 5.30 | 23.1 |
| 19c | NO2 | >100 | 0.911 | 0.941 | 0.957 | 5.10 | 67.6 |
| 20c | NHMe | 34.5 | 0.472 | 0.645 | 0.823 | 4.90 | 47.6 |
| 21c | NMe2 | >100 | 0.807 | 0.913 | 0.888 | 5.55 | 23.7 |
a) Antitumor activity in HT-1080 cells. b) Band density of total MMP-9. c) Band density of total MMP-2 (latent + active MMP-2). d) Band density of active MMP-2. e) Calculated using ChemDraw 19.1. f) COX-2 inhibition rate at 200 µM.
 |
a) Antitumor activity in HT-1080 cells. b) Band density of total MMP-9. c) Band density of total MMP-2 (latent + active MMP-2). d) Band density of active MMP-2. e) Calculated using ChemDraw 19.1. f) COX-2 inhibition rate at 200 µM.
The antitumor activity of compound 4, in which the methyl ester of UTX-121 was converted into methyl ether, was equivalent to that of UTX-121. On the other hand, compound 3, substituted for alcohol, showed higher antitumor activity than UTX-121. Compounds 5–8 showed no significant antitumor activity (Table 1).
Table 2 summarizes the antitumor activities of compounds 9c–21c, in which various functional groups were introduced into the methyl group of the p-tolyl moiety. Compound 12c (H) had approximately the same level of antitumor activity as UTX-121, while compounds 9c–11c (F, Cl, Br) and compound 20c (NHMe) showed higher activities than UTX-121. Compound 17c (OH) had the highest antitumor activity among all compounds (IC50: 18.0 µM). Compounds 13c–16c, 18c, 19c, and 21c showed no antitumor activity.
Finally, we investigated the antitumor activities of compounds 22c–30c, in which the phenyl ring of UTX-121 was replaced with other aromatic groups (Table 3). Compounds 22c, 23c, 25c–27c had moderate antitumor activity, with IC50 values of approximately 60–80 µM. The other derivatives did not exhibit any antitumor activity.
MMP Inhibitory ActivityAfter incubating HT-1080 cells with the compounds (25 µM) for 24 h, the effect of UTX-121 derivatives on MMP activity was examined by gelatin zymography. Band densities were measured using ImageJ software, and the relative band densities of various derivatives are shown in Tables 1–3, with dimethyl sulfoxide (DMSO) represented as 1.
Compound 4 reduced the production levels of total MMP-2 and active MMP-2 more strongly than UTX-121, but its inhibitory activity on MMP-9 production was lower than that of UTX-121. Compound 8 showed comparable MMP-2 inhibitory activity to UTX-121, while compounds 5–7 were not sufficient to inhibit MMPs (Table 1).
Next, we investigated the MMP inhibitory activities of compounds 9c–21c, in which the methyl group was replaced with various functional groups (Table 2). Compounds 9c–11c (F, Cl, Br) exhibited potent inhibitory activity against MMPs; in particular, compounds 9c and 10c had the highest activities. Compound 12c suppressed MMP-9 production, and compound 15c suppressed MMP-2/9 production and pro MMP-2 activation to the same extent as UTX-121. The inhibitory effects of compounds 17c and 20c were significant only against MMP-9.
Table 3 shows the MMP inhibitory activities of compounds 22c–30c. In case of compounds 22c and 23c, the inhibitory activity was slightly higher than that of UTX-121 only for total MMP-9. However, compounds 24c–30c did not show any significant MMP inhibitory activity.
COX-2 Inhibitory ActivityWe determined the COX-2 inhibition rate of various derivatives at 200 µM using the COX-2 (human) Inhibitor Screening Assay Kit.
While the IC50 value of celecoxib against COX-2 was 0.020 µM (reported IC50 value: 0.040 µM),1,19) no derivatives showed high COX-2 inhibitory activity. The IC50 value of compound 25c, which showed the highest inhibition rate (95.9%), was 10.1 µM, indicating that COX-2 inhibitory activities of derivatives designed in this study were much lower than that of celecoxib.
Structure–Activity RelationshipWe first investigated the methyl ester of UTX-121. Compounds 3 and 4 were designed to evaluate the effects of methyl ester on antitumor and MMP inhibitory activities. Compound 3 showed significant antitumor activity but weak MMP inhibitory activity, and compound 4 exhibited antitumor and MMP inhibitory activity equivalent to that of UTX-121. Based on these results, the methyl ester (UTX-121) and ether (compound 4), which suppressed MMP-2/9 expression and pro MMP-2 activation, seemed to be the most suitable. We also examined the alkyl chain length of methyl esters. Linker expansions (compounds 5–7) led to diminished antitumor and MMP inhibitory activities. The introduction of the tert-butyl moiety inhibited MMP-2 expression and activation, but did not show any antitumor activity (Table 1). Therefore, we determined that the methyl ester (C1) was the most appropriate for both antitumor and MMP inhibitory activities.
We then focused on the p-tolyl group of UTX-121. Table 2 summarizes the activities of compounds 9c–21c with modifications to the methyl group. Replacements with halogens (compounds 9c–11c) led to a marked improvement in antitumor and MMP inhibitory activities. The C log P of compounds 9c–11c is similar to that of UTX-121 (approximately 6), suggesting that a relatively high lipophilicity is necessary to induce these activities. Compounds 9c and 10c showed potent inhibitory activities, and the introduction of Br (compound 11c) slightly attenuated the potency. This may be due to the atomic size and electronic effects of Br. Since chlorine has the same size as the methyl group and bromine has the same size as the isopropyl group, the size of the binding pocket of the target molecule is limited, and electrostatic interaction is therefore expected. Compounds 17c and 20c also showed higher antitumor activities, but interestingly, only MMP-9 was strongly inhibited. Both these derivatives have lower C log P values compared to the other derivatives, which indicates that either their cellular uptake is reduced, or their binding to the target hydrophobic pocket is insufficient. Additionally, OH and NHMe act as proton donors. Therefore, it is possible that compounds 17c and 20c may act via a different mechanism from that of UTX-121, in which they are taken up by cells and inhibit MMP-2/9 through the suppression of cell surface expression of MT1-MMP.19) The substitution of CN (compound 15c) also resulted in relatively potent MMP inhibitory activity, which was attributed to the ability of CN to act as a bioisostere of halogens.20) However, as previously mentioned, the size of the substituents also seems to be an important factor; it is therefore speculated that the MMP inhibitory activity of compound 15c is not comparable to that of compounds 9c–11c. Moreover, only compound 15c possessed MMP-2/9 inhibitory activities without showing antitumor activity, and the C log P value was low, suggesting that it might have a different mechanism from the other compounds. In addition, no significant correlation was observed between σ values representing the electronic effects of substituents on the benzene ring and various inhibitory activities, suggesting that electronic effects might have no impact either.
Since it was assumed that the electronic effects may not affect the potencies, we also designed derivatives in which the phenyl ring was substituted with cyclic or bicyclic moieties (compounds 22c–30c), and evaluated their activities (Table 3). Replacements with cyclohexene (compound 22c) or cyclohexane (compound 23c) resulted in moderate antitumor activity and the highest MMP inhibitory effect among all compounds 22c–30c. The C log P values of these two derivatives are also close to 6, just like in case of compounds 9c–11c, indicating that a certain degree of lipophilicity is required for antitumor and MMP inhibitory activities. The introduction of various heterocycles (compounds 24c–27c) suppressed the expression of MMP-9 compared to MMP-2. These compounds have lower C log P values than the other derivatives, implying that they might interact via a different mechanism just like in case of compounds 17c and 20c. Compounds 28c–30c showed very low antitumor and MMP inhibitory activities. This may be due to their large molecular size, which prevents them from reaching the target binding site, although their lipophilicity is secured.
In addition, the COX-2 inhibitory activity of all derivatives was extremely limited, and there was no correlation between their antitumor activities and MMP inhibitory activities. This indicates that the antitumor and MMP inhibitory activities of these derivatives are probably COX-2 independent, but it is not yet clear whether there is any similarity in the mechanism of celecoxib and UTX-121 derivatives regarding these activities. Since UTX-121 derivatives inhibited the MT1-MMP-mediated MMP-2 activation while the MMP inhibitory activity of celecoxib was poor, it is possible that there is a different target molecule from celecoxib.
Furthermore, it was revealed that there was a weak correlation between the antitumor activity and the MMP inhibitory activity among the derivatives found to have antitumor activity (r = 0.68 (vs. total MMP-9), 0.38 (vs. total MMP-2), 0.32 (vs. active MMP-2)). It is well known that cancer cells degrade the ECM using MMPs to make their proliferation easy (also called tumor jailbreak).21) Therefore, UTX-121 derivatives are thought to inhibit the expansion of the growth space by acting on the targets shared by cell proliferation and MMP expression/activation, leading to their antitumor effects.
In summary, we designed and synthesized UTX-121 derivatives and investigated compounds with higher MMP inhibitory activities using the SAR method. Among them, compounds 9c and 10c showed higher antitumor activity than UTX-121, and markedly inhibited the expression of MMP-2/9 and activation of pro MMP-2. It is necessary to investigate the activity of these compounds in further detail and determine their mechanisms of action. We believe that compounds 9c and 10c could be effective lead compounds in the development of antitumor drugs targeting MMP.
All reactions were carried out under a nitrogen atmosphere. Column chromatography was performed on Kanto Chemical silica gel 60 N (spherical, neutral, 40–50 µm). The purity and retention time (tR) of all final compounds were measured using a JASCO HPLC system equipped with a TSKgel ODS-120H column (4.6 mm I.D. × 15 cm, 5 µm) and a UV detector operated at 220 nm. Gradient elution was performed at a flow rate of 1.0 mL/min with a mixture of acetonitrile solvent and 0.1% trifluoroacetic acid in water. 1H-NMR spectra were measured on a JEOL JNM-ECZ400S (400 MHz) or JNM-ECA500WB (500 MHz). High resolution (HR)MS were recorded on a JEOL JMS-SX 102 A mass spectrometer.
(Z)-1,1,1-Trifluoro-4-hydroxy-4-(p-tolyl)-3-buten-2-one (1)4′-Methylacetophenone (100 µL, 753 µmol) was dissolved in dry tetrahydrofuran (THF) (2.0 mL), and 60% sodium hydride (NaH) (65 mg, 1.63 mmol, 2.2 equivalent (equiv.)) was added under ice-cold. After stirring for 1 h under 0 °C, ethyl trifluoroacetate (135 µL, 1.13 mmol, 1.5 equiv.) was added, and the reaction mixture was stirred for 3.5 h at room temperature. The reaction mixture was evaporated, added ice–water (5 mL), acidified to pH 6 with 1 N hydrochloric acid (HCl), and extracted 3 times with ethyl acetate (EtOAc). The organic layer was washed with water, dried over magnesium sulfate (MgSO4), filtered, and concentrated. The residue was washed with n-hexane, and evaporated. The solid was dissolved in dichloromethane (CH2Cl2), and evaporated to afford 1 (140 mg, 608 µmol, 81%). 1H-NMR (400 MHz, CDCl3) δ: 7.86 (2H, d, J = 8.4 Hz), 7.31 (2H, d, J = 8.4 Hz), 6.55 (1H, s), 2.45 (3H, s).
4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic Acid (2)4-Hydrazinobenzoic acid (361 mg, 2.37 mmol, 1.1 equiv.) was dissolved in dry ethanol (EtOH) (30 mL), added 2 N HCl (1.3 mL) and 1 (503 mg, 2.19 mol). After stirring for 0.5 h at 75 °C, the reaction mixture was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated to afford 2 (741 mg, 2.14 mmol, 98%). 1H-NMR (400 MHz, (CD3)2CO) δ: 8.10 (2H, d, J = 8.8 Hz), 7.51 (2H, d, J = 8.8 Hz), 7.24 (4H, s), 7.00 (1H, s), 2.35 (3H, s).
(4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)phenyl)methanol (3)Lithium aluminum hydride in THF (200 µL, 200 µmol, 1.4 equiv.) was slowly added to dry THF (500 µL), and 2 (in 1.5 mL THF) (51 mg, 147 µmol) was added under ice-cold. After stirring for 0.5 h at 0 °C, the reaction mixture was stirred for 1.5 h at room temperature. The reaction mixture was quenched with reverse osmosis (RO) water and 15% sodium hydroxide (NaOH) aq., filtered with celite, and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2 only) to afford 3 (37 mg, 111 µmol, 76%). Purity: 96.4%; tR: 29.1 min; 1H-NMR (400 MHz, CDCl3) δ: 7.36 (2H, d, J = 8.0 Hz), 7.31 (2H, d, J = 8.8 Hz), 7.12 (4H, q, J = 8.8, 12.4 Hz), 6.72 (1H, s), 4.73 (2H, d, J = 6.0 Hz), 2.35 (3H, s); HRMS (electrospray ionization-time-of-flight (ESI-TOF)) m/z [M + H]+: 333.1196 (Calcd for C18H16F3N2O: 333.1215).
1-(4-(Methoxymethyl)phenyl)-5-(p-tolyl)-3-(trifluoromethyl)-1H-pyrazole (4)Sixty percent NaH (23 mg, 575 µmol, 3.3 equiv.) was dissolved in dry THF (2.0 mL), and 3 (in 2.0 mL THF) (57 mg, 172 µmol) was added on ice bath. After stirring for 1.5 h on ice bath, methyl iodide (32.2 µL, 517 µmol, 3.0 equiv.) was added. After stirring for 19 h at room temperature, the reaction mixture was quenched with DI water (1.0 mL), extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (n-hexane/EtOAc = 10 : 1) to afford 4 (38 mg, 110 µmol, 64%). Purity: 96.3%; tR: 32.5 min; 1H-NMR (400 MHz, CDCl3) δ: 7.31 (4H, q, J = 8.4, 15.6 Hz), 7.11 (4H, q, J = 8.8, 10.4 Hz), 6.71 (1H, s), 4.47 (2H, s), 3.40 (3H, s), 2.35 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 347.1387 (Calcd for C19H18F3N2O: 347.1371).
Ethyl 4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (5)2 (50 mg, 144 µmol) was dissolved in dry EtOH, and added SOCl2 (52.0 µL, 721 µmol, 5.0 equiv.) under ice-cold. After stirring for 24 h at room temperature, the reaction mixture was quenched with saturated sodium hydrogen carbonate (NaHCO3) aq. (1.5 mL), and evaporated. The residue was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (n-hexane/EtOAc = 10 : 1) to afford 5 (20 mg, 53.4 µmol, 37%). Purity: 96.8%; tR: 34.7 min; 1H-NMR (400 MHz, CDCl3) δ: 8.04 (2H, d, J = 7.2 Hz), 7.39 (2H, d, J = 7.2 Hz), 7.13 (4H, q, J = 8.0, 19.2 Hz), 6.73 (1H, s), 4.38 (2H, q, J = 7.2, 14.4 Hz), 2.37 (3H, s), 1.40 (3H, t, J = 7.2 Hz); HRMS (ESI-TOF) m/z [M + H]+: 375.1328 (Calcd for C20H18F3N2O2: 375.1320).
Propyl 4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (6)The title compound was synthesized from 2 (51 mg, 147 µmol) in 68% yield using the method described for the preparation of 5. 1-Propanol was used as a solvent instead of EtOH. Purity: 99.0%; tR: 35.8 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.8 Hz), 7.39 (2H, d, J = 8.8 Hz), 7.13 (4H, q, J = 8.4, 20.4 Hz), 6.73 (1H, s), 4.28 (2H, t, J = 7.2 Hz), 2.37 (3H, s), 1.85–1.75 (2H, m), 1.03 (3H, t, J = 7.2 Hz); HRMS (ESI-TOF) m/z [M + H]+: 389.1481 (Calcd for C21H20F3N2O2: 389.1477).
Isopropyl 4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (7)The title compound was synthesized from 2 (52 mg, 150 µmol) in 22% yield using the method described for the preparation of 5. 2-Propanol was used as a solvent instead of EtOH. Purity: 95.1%; tR: 35.8 min; 1H-NMR (400 MHz, CDCl3) δ: 8.02 (2H, d, J = 8.8 Hz), 7.38 (2H, d, J = 8.8 Hz), 7.12 (4H, q, J = 8.0, 20.8 Hz), 6.73 (1H, s), 5.30–5.20 (1H, m), 2.37 (3H, s), 1.37 (6H, d, J = 6.4 Hz); HRMS (ESI-TOF) m/z [M + H]+: 389.1484 (Calcd for C21H20F3N2O2: 389.1477).
tert-Butyl 4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (8)2 (50 mg, 144 µmol), benzyltriethylammonium chloride (36 mg, 158 µmol, 1.1 equiv.) and potassium carbonate (K2CO3) (496 mg, 3.59 mmol, 25 equiv.) were dissolved in N,N-dimethylacetamide (1.0 mL), and added 2-bromo-2-methylpropane (723 µL, 6.48 mmol, 45 equiv.). After stirring for 19 h at 55 °C, the reaction mixture was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (n-hexane/EtOAc = 4 : 1) to afford 8 (52 mg, 129 µmol, 90%). Purity: 96.2%; tR: 37.0 min; 1H-NMR (400 MHz, CD3OD) δ: 7.99 (2H, d, J = 8.0 Hz), 7.40 (2H, d, J = 8.8 Hz), 7.18 (4H, q, J = 8.0, 18.4 Hz), 6.91 (1H, s), 2.35 (3H, s), 1.60 (9H, s); HRMS (ESI-TOF) m/z [M + H]+: 403.1615 (Calcd for C22H22F3N2O2: 403.1633).
Methyl 4-(5-(4-Fluorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (9c)4′-Fluoroacetophenone (300 µL, 2.48 mmol) was dissolved in dry THF (6.0 mL) under ice-cold, and 60% NaH (223 mg, 5.58 mmol, 2.3 equiv.) was added. After stirring for 1 h under 0 °C, ethyl trifluoroacetate (444 µL, 3.72 mmol, 1.5 equiv.) was added, and the reaction mixture was stirred for 1 h at room temperature. The reaction mixture was acidified to pH 6 with 1 N HCl, extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was reprecipitated with EtOAc and n-hexane to afford (Z)-1,1,1-trifluoro-4-(4-fluorophenyl)-4-hydroxy-3-buten-2-one (9a) (373 mg, 1.59 mmol, 64%). 1H-NMR (400 MHz, (CD3)2CO) δ: 8.00 (2H, t, J = 7.2 Hz), 7.14 (2H, t, J = 8.8 Hz), 6.23 (1H, s).
4-(5-(4-Fluorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (9b) was synthesized from 9a (196 mg, 837 µmol) in 94% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.11 (2H, d, J = 9.2 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.43 (2H, q, J = 4.8, 8.4 Hz), 7.21 (2H, t, J = 8.8 Hz), 7.05 (1H, s).
9b (316 mg, 902 µmol) was dissolved in dry methanol (MeOH), and added SOCl2 (309 µL, 4.29 mmol, 4.7 equiv.) under ice-cold. After stirring for 22 h at room temperature, the reaction mixture was quenched with sat. NaHCO3 aq., and evaporated. The residue was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (n-hexane/EtOAc = 10 : 1) to afford 9c (196 mg, 538 µmol, 60%). Purity: 97.3%; tR: 32.1 min; 1H-NMR (400 MHz, CDCl3) δ: 8.04 (2H, d, J = 6.8 Hz), 7.38 (2H, d, J = 6.8 Hz), 7.20 (2H, t, J = 7.2 Hz), 7.05 (2H, t, J = 7.8 Hz), 6.75 (1H, s), 3.93 (3H, s); 13C-NMR (126 MHz, CDCl3) δ: 166.1, 163.3 (d, 1JCF = 251 Hz), 144.1, 144.0 (q, 2JCF = 38.4 Hz), 142.6, 130.9 (d, 3JCF = 8.4 Hz), 130.7, 130.1, 125.2, 125.1, 121.2 (q, 1JCF = 269 Hz), 116.3 (d, 2JCF = 21.7 Hz), 106.4, 52.5; 19F-NMR (373 MHz, CDCl3) δ: −62.3, −110.6; HRMS (ESI-TOF) m/z [M + H]+: 365.0915 (Calcd for C18H13F4N2O2: 365.0913).
Methyl 4-(5-(4-Chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (10c)(Z)-4-(4-Chlorophenyl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (10a) was synthesized from 4′-chloroacetophenone (500 µL, 3.85 mmol) in 53% yield using the method described for the preparation of 9a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.89 (2H, d, J = 8.4 Hz), 7.49 (2H, d, J = 8.8 Hz), 6.54 (1H, s).
4-(5-(4-Chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (10b) was synthesized from 10a (404 mg, 1.61 mmol) in 64% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.12 (2H, d, J = 8.8 Hz), 7.53 (2H, d, J = 8.8 Hz), 7.47 (2H, d, J = 8.4 Hz), 7.40 (2H, d, J = 8.8 Hz), 7.10 (1H, s).
10c was synthesized from 10b (152 mg, 414 µmol) in 72% yield using the method described for the preparation of 9c. Purity: 95.0%; tR: 33.5 min; 1H-NMR (400 MHz, CDCl3) δ: 8.05 (2H, d, J = 9.2 Hz), 7.38 (2H, d, J = 8.8 Hz), 7.33 (2H, d, J = 8.4 Hz), 7.15 (2H, d, J = 8.4 Hz), 6.77 (1H, s), 3.93 (3H, s); 13C-NMR (126 MHz, CDCl3) δ: 166.1, 144.1 (q, 2JCF = 38.4 Hz), 143.9, 142.5, 135.8, 130.8, 130.2, 129.4, 127.4, 125.2, 125.1, 121.1 (q, 1JCF = 269 Hz), 106.5, 52.6; 19F-NMR (471 MHz, CDCl3) δ: −62.3; HRMS (ESI-TOF) m/z [M + H]+: 381.0642 (Calcd for C18H13ClF3N2O2: 381.0618).
Methyl 4-(5-(4-Bromophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (11c)4′-Bromoacetophenone (302 mg, 1.52 mmol) was dissolved in dry THF (4.0 mL), and 60% NaH (85 mg, 2.13 mmol, 1.4 equiv.) was added under ice-cold. After stirring for 1 h under 0 °C, ethyl trifluoroacetate (267 µL, 2.24 mmol, 1.5 equiv.) was added, and the reaction mixture was stirred for 0.5 h at room temperature. The reaction mixture was acidified to pH 6 with 1 N HCl, extracted two times with EtOAc, and washed with brine to afford (Z)-4-(4-bromophenyl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (11a) (343 mg, 1.16 mmol, 76%). 1H-NMR (400 MHz, (CD3)2CO) δ: 7.84 (2H, d, J = 8.4 Hz), 7.55 (2H, d, J = 8.8 Hz), 6.26 (1H, s).
4-(5-(4-Bromophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (11b) was synthesized from 11a (153 mg, 519 µmol) in 90% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.12 (2H, d, J = 9.2 Hz), 7.62 (2H, d, J = 8.8 Hz), 7.54 (2H, d, J = 8.8 Hz), 7.33 (2H, d, J = 8.8 Hz), 7.10 (1H, s).
11c was synthesized from 11b (104 mg, 252 µmol) in 54% yield using the method described for the preparation of 9c. Purity: 96.3%; tR: 33.8 min; 1H-NMR (400 MHz, (CD3)2CO) δ: 8.09 (2H, d, J = 8.8 Hz), 7.61 (2H, d, J = 8.4 Hz), 7.54 (2H, d, J = 9.2 Hz), 7.32 (2H, d, J = 8.8 Hz), 7.10 (1H, s), 3.91 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 425.2155 (Calcd for C18H13BrF3N2O2: 425.0113).
Methyl 4-(5-Phenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (12c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-phenyl-3-buten-2-one (12a) was synthesized from acetophenone (1.00 mL, 8.57 mmol) quantitatively using the method described for the preparation of 11a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.95 (2H, d, J = 7.2 Hz), 7.50 (1H, t, J = 7.6 Hz), 7.42 (2H, t, J = 8.0 Hz), 6.35 (1H, s).
4-(5-Phenyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (12b) was synthesized from 12a (395 mg, 1.83 mmol) in 95% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.09 (2H, d, J = 8.4 Hz), 7.51 (2H, d, J = 8.8 Hz), 7.45–7.35 (5H, m), 7.04 (1H, s).
12c was synthesized from 12b (97 mg, 292 µmol) in 52% yield using the method described for the preparation of 9c. Purity: 94.4%; tR: 32.2 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.8 Hz), 7.41–7.32 (5H, m), 7.22 (J = 6.4 Hz, 2H, d), 6.77 (1H, s), 3.92 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 347.1004 (Calcd for C18H14F3N2O2: 347.1007).
Methyl 4-(5-(4-Ethylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (13c)Sixty percent NaH (166 mg, 4.15 mmol, 2.1 equiv.) was dissolved in dry tert-butyl methyl ether (6.0 mL), and added p-ethylacetophenone (300 µL, 2.01 mmol) under ice-cold. After stirring for 1 h under 0 °C, ethyl trifluoroacetate (361 µL, 3.02 mmol, 1.5 equiv.) was added, and the reaction mixture was stirred for 2.5 h at room temperature. The reaction mixture was acidified to pH 6 with 1 N HCl, extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was reprecipitated with EtOAc and n-hexane to afford (Z)-4-(4-ethylphenyl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (13a) (193 mg, 790 µmol, 39%). 1H-NMR (400 MHz, (CD3)2CO) δ: 7.91 (2H, d, J = 8.4 Hz), 7.30 (2H, d, J = 7.6 Hz), 6.36 (1H, s), 2.68 (2H, q, J = 7.6, 15.2 Hz), 1.22 (3H, t, J = 7.6 Hz).
4-Hydrazinobenzoic acid (69 mg, 453 µmol, 1.1 equiv.) was dissolved in dry EtOH (5.0 mL), added 2 N HCl (260 µL) and 13a (99 mg, 405 µmol). After stirring for 1.5 h at 75 °C, the reaction mixture was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was dissolved in EtOAc, and n-hexane was added to remove the precipitated solid by filtration. The filtrate was evaporated to afford 4-(5-(4-ethylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (13b) (118 mg, 328 µmol, 81%). 1H-NMR (400 MHz, CD3OD) δ: 8.05 (2H, d, J = 8.8 Hz), 7.42 (2H, d, J = 8.8 Hz), 7.21 (4H, q, J = 8.8, 14.8 Hz), 6.91 (1H, s), 2.66 (2H, q, J = 7.6, 15.2 Hz), 1.22 (3H, t, J = 7.6 Hz).
13b (90 mg, 250 µmol) was dissolved in dry MeOH, and added SOCl2 (90.0 µL, 1.25 mmol, 5.0 equiv.) under ice-cold. After stirring for 23 h at room temperature, the reaction mixture was quenched with sat. NaHCO3 aq., extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (n-hexane/EtOAc = 10 : 1) to afford 13c (57 mg, 152 µmol, 61%). Purity: 99.8%; tR: 34.0 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.8 Hz), 7.40 (2H, d, J = 8.4 Hz), 7.15 (4H, q, J = 8.0, 19.6 Hz), 6.74 (1H, s), 3.93 (3H, s), 2.66 (2H, q, J = 8.0, 15.2 Hz), 1.25 (3H, t, J = 7.6 Hz); HRMS (ESI-TOF) m/z [M + H]+: 375.1323 (Calcd for C20H18F3N2O2: 375.1320).
Methyl 4-(3-(Trifluoromethyl)-5-(4-(trifluoromethyl)phenyl)-1H-pyrazol-1-yl)benzoate (14c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(4-(trifluoromethyl)phenyl)-3-buten-2-one (14a) was synthesized from 4′-(trifluoromethyl)acetophenone (200 µL, 982 µmol) in 72% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.18 (2H, d, J = 8.0 Hz), 7.80 (2H, d, J = 8.8 Hz), 6.42 (1H, s).
4-Hydrazinobenzoic acid (112 mg, 736 µmol, 1.2 equiv.) was dissolved in dry EtOH (5.0 mL), added 2 N HCl (450 µL) and 14a (180 mg, 633 µmol). After stirring for 2 h at 75 °C, the reaction mixture was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was reprecipitated with EtOAc and n-hexane to afford 4-(3-(trifluoromethyl)-5-(4-(trifluoromethyl)phenyl)-1H-pyrazol-1-yl)benzoic acid (14b) (168 mg, 420 µmol, 66%). 1H-NMR (400 MHz, (CD3)2CO) δ: 8.12 (2H, d, J = 8.4 Hz), 7.78 (2H, d, J = 8.4 Hz), 7.62 (2H, d, J = 8.0 Hz), 7.53 (2H, d, J = 8.4 Hz), 7.20 (1H, s).
14c was synthesized from 14b (115 mg, 317 µmol) in 68% yield using the method described for the preparation of 9c. Purity: 94.4%; tR: 33.1 min; 1H-NMR (400 MHz, CDCl3) δ: 8.07 (2H, d, J = 8.4 Hz), 7.62 (2H, d, J = 8.8 Hz), 7.38 (2H, d, J = 8.8 Hz), 7.35 (2H, d, J = 8.4 Hz), 6.84 (1H, s), 3.94 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 415.0997 (Calcd for C19H13F6N2O2: 415.0881).
Methyl 4-(5-(4-Cyanophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (15c)(Z)-4-(4,4,4-Trifluoro-1-hydroxy-3-oxo-1-buten-1-yl)benzonitrile (15a) was synthesized from 4-acetylbenzonitrile (200 mg, 1.38 mmol) in 92% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.15 (2H, d, J = 8.0 Hz), 7.87 (2H, d, J = 8.4 Hz), 6.42 (1H, s).
4-(5-(4-Cyanophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (15b) was synthesized from 15a (145 mg, 601 µmol) in 61% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, CD3OD) δ: 8.09 (2H, d, J = 8.4 Hz), 7.75 (2H, d, J = 8.0 Hz), 7.48 (2H, d, J = 8.4 Hz), 7.44 (2H, d, J = 8.4 Hz), 7.12 (1H, s).
15c was synthesized from 15b (110 mg, 308 µmol) in 56% yield using the method described for the preparation of 13c. Purity: 96.9%; tR: 30.5 min; 1H-NMR (400 MHz, CDCl3) δ: 8.08 (2H, d, J = 8.4 Hz), 7.65 (2H, d, J = 8.8 Hz), 7.36 (4H, q, J = 8.8, 12.0 Hz), 6.86 (1H, s), 3.94 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 372.0980 (Calcd for C19H13F3N3O2: 372.0960).
Methyl 4-(5-(4-(Methylthio)phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (16c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(4-(methylthio)phenyl)-3-buten-2-one (16a) was synthesized from 4′-(methylthio)acetophenone (100 mg, 602 µmol) in 30% yield using the method described for the preparation of 11a. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.07 (2H, d, J = 8.8 Hz), 7.43 (2H, d, J = 8.4 Hz), 6.88 (1H, s), 2.60 (3H, s).
4-(5-(4-(Methylthio)phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (16b) was synthesized from 16a (36 mg, 137 µmol) in 73% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, CD3OD) δ: 8.07 (2H, d, J = 7.2 Hz), 7.43 (2H, d, J = 8.0 Hz), 7.22 (4H, q, J = 8.8, 17.6 Hz), 6.94 (1H, s), 2.48 (3H, s).
16c was synthesized from 16b (36 mg, 95.1 µmol) in 53% yield using the method described for the preparation of 9c. Purity: 95.0%; tR: 33.3 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 9.2 Hz), 7.39 (2H, d, J = 8.8 Hz), 7.17 (2H, d, J = 8.4 Hz), 7.11 (2H, d, J = 8.4 Hz), 6.73 (1H, s), 3.92 (3H, s), 2.48 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 393.0850 (Calcd for C19H16F3N2O2S: 393.0885).
Methyl 4-(5-(4-Hydroxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (17c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(4-hydroxyphenyl)-3-buten-2-one (17a) was synthesized from p-hydroxyacetophenone (204 mg, 1.50 mmol) in 15% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 9.34 (1H, br s), 7.95 (2H, d, J = 8.8 Hz), 6.92 (2H, d, J = 8.8 Hz).
4-Hydrazinobenzoic acid (44 mg, 289 µmol, 1.6 equiv.) was dissolved in dry EtOH (3.0 mL), added 2 N HCl (130 µL) and 17a (43 mg, 185 µmol). After stirring for 3 h at 75 °C, EtOAc was added and the precipitate was removed by filtration. The filtrate was evaporated, and the residue was reprecipitated with EtOAc and n-hexane to afford 4-(5-(4-hydroxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (17b) (21 mg, 60.3 µmol, 33%). 1H-NMR (400 MHz, CD3OD) δ: 8.06 (2H, d, J = 8.4 Hz), 7.42 (2H, d, J = 8.4 Hz), 7.10 (2H, d, J = 8.8 Hz), 6.84 (1H, s), 6.77 (2H, d, J = 8.4 Hz).
17c was synthesized from 17b (20 mg, 57.4 µmol) in 66% yield using the method described for the preparation of 13c. Purity: 95.9%; tR: 28.5 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.4 Hz), 7.40 (2H, d, J = 8.4 Hz), 7.09 (2H, d, J = 8.4 Hz), 6.80 (2H, d, J = 8.0 Hz), 6.71 (1H, s), 3.93 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 363.0976 (Calcd for C18H14F3N2O3: 363.0956).
Methyl 4-(5-(4-Methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (18c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(4-methoxyphenyl)-3-buten-2-one (18a) was synthesized from 4′-methoxyacetophenone (105 mg, 699 µmol) in 89% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.97 (2H, d, J = 8.8 Hz), 6.98 (2H, d, J = 9.2 Hz), 6.33 (1H, s), 3.86 (3H, s).
4-(5-(4-Methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (18b) was synthesized from 18a (127 mg, 516 µmol) in 71% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.06 (2H, d, J = 8.8 Hz), 7.43 (2H, d, J = 8.4 Hz), 7.20 (2H, d, J = 9.2 Hz), 6.92 (2H, d, J = 8.4 Hz), 6.88 (1H, s), 3.80 (3H, s).
18c was synthesized from 18b (115 mg, 317 µmol) in 68% yield using the method described for the preparation of 9c. Purity: 98.9%; tR: 31.7 min; 1H-NMR (400 MHz, CDDl3) δ: 8.03 (2H, d, J = 8.0 Hz), 7.40 (2H, d, J = 8.8 Hz), 7.14 (2H, d, J = 8.8 Hz), 6.86 (2H, d, J = 9.2 Hz), 6.71 (1H, s), 3.93 (3H, s), 3.82 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 377.1113 (Calcd for C19H16F3N2O3: 377.1113).
Methyl 4-(5-(4-Nitrophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (19c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(4-nitrophenyl)-3-buten-2-one (19a) was synthesized from 4′-nitroacetophenone (211 mg, 1.28 mmol) in 94% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.25 (2H, d, J = 8.8 Hz), 8.15 (2H, d, J = 8.4 Hz), 6.33 (1H, s).
4-(5-(4-Nitrophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (19b) was synthesized from 19a (182 mg, 697 µmol) in 90% yield using the method described for the preparation of 14b. 1H-NMR (400 MHz, CD3OD) δ: 8.24 (2H, d, J = 9.2 Hz), 8.08 (2H, d, J = 8.8 Hz), 7.55 (2H, d, J = 9.2 Hz), 7.45 (2H, d, J = 8.4 Hz), 7.16 (1H, s).
19c was synthesized from 19b (185 mg, 490 µmol) in 46% yield using the method described for the preparation of 9c. Purity: 97.8%; tR: 31.0 min; 1H-NMR (400 MHz, CDCl3) δ: 8.22 (2H, d, J = 8.8 Hz), 8.08 (2H, d, J = 8.8 Hz), 7.40 (4H, q, J = 8.8, 11.6 Hz), 6.90 (1H, s), 3.94 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 392.0880 (Calcd for C18H13F3N3O4: 392.0858).
Methyl 4-(5-(4-(Methylamino)phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (20c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(4-(methylamino)phenyl)-3-buten-2-one (20a) was synthesized from 4′-(methylamino)acetophenone (104 mg, 697 µmol) in 91% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, CD3OD) δ: 7.84 (2H, d, J = 8.0 Hz), 6.57 (2H, d, J = 8.4 Hz), 6.27 (1H, s), 2.82 (3H, s).
4-Hydrazinobenzoic acid (73 mg, 480 µmol, 1.1 equiv.) was dissolved in dry EtOH (5.0 mL), added 2 N HCl (250 µL) and 20a (104 mg, 424 µmol). After stirring for 2.5 h at 75 °C, the reaction mixture was extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (CH2Cl2/MeOH = 10 : 1) to afford 20b (28 mg, 77.4 µmol, 18%). 1H-NMR (500 MHz, CD3OD) δ: 8.04 (2H, d, J = 8.5 Hz), 7.43 (2H, d, J = 8.5 Hz), 7.00 (2H, d, J = 8.5 Hz), 6.77 (1H, s), 6.54 (2H, d, J = 7.0 Hz), 2.76 (3H, s).
20c was synthesized from 20b (28 mg, 77.5 µmol) in 58% yield using the method described for the preparation of 9c. Purity: 98.1%; tR: 29.4 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.8 Hz), 7.43 (2H, d, J = 9.2 Hz), 7.01 (2H, d, J = 9.2 Hz), 6.65 (1H, s), 6.52 (2H, d, J = 8.8 Hz), 3.92 (3H, s), 2.85 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 376.1276 (Calcd for C19H17F3N3O2: 376.1273).
Methyl 4-(5-(4-(Dimethylamino)phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (21c)(Z)-4-(4-(Dimethylamino)phenyl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (21a) was synthesized from p-(dimethylamino)acetophenone (198 mg, 1.21 mmol) in 72% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.84 (2H, d, J = 8.8 Hz), 6.66 (2H, d, J = 8.4 Hz, 2H), 6.24 (1H, s), 3.00 (6H, s).
4-(5-(4-(Dimethylamino)phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (21b) was synthesized from 21a (202 mg, 779 µmol) in 85% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.06 (2H, d, J = 8.4 Hz), 7.44 (2H, d, J = 8.8 Hz), 7.08 (2H, d, J = 9.2 Hz), 6.80 (1H, s), 6.69 (2H, d, J = 9.2 Hz), 2.96 (6H, s).
21c was synthesized from 21b (198 mg, 528 µmol) in 74% yield using the method described for the preparation of 13c. Purity: 96.9%; tR: 31.4 min; 1H-NMR (400 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.8 Hz), 7.44 (2H, d, J = 8.8 Hz), 7.05 (2H, d, J = 9.2 Hz), 6.66 (1H, s), 6.61 (2H, d, J = 8.8 Hz), 3.93 (3H, s), 2.98 (6H, s); HRMS (ESI-TOF) m/z [M + H]+: 390.1430 (Calcd for C20H19F3N3O2: 390.1429).
Methyl 4-(5-(Cyclohex-1-en-1-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (22c)(Z)-4-(Cyclohex-1-en-1-yl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (22a) was synthesized from 1-acetyl-1-cyclohexene (100 µL, 778 µmol) in 18% yield using the method described for the preparation of 9a. 1H-NMR (400 MHz, CD3OD) δ: 6.79 (1H, br s), 5.89 (1H, br s), 2.27–2.19 (4H, m), 1.69–1.58 (4H, m).
4-(5-(Cyclohex-1-en-1-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (22b) was synthesized from 22a (25 mg, 115 µmol) in 98% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, CD3OD) δ: 8.16 (2H, d, J = 8.8 Hz), 7.63 (2H, d, J = 8.8 Hz), 6.67 (1H, s), 5.92–5.90 (1H, m), 2.16–2.08 (4H, m), 1.69–1.60 (4H, m).
22c was synthesized from 22b (37 mg, 110 µmol) in 70% yield using the method described for the preparation of 13c. Purity: 97.3%; tR: 34.3 min; 1H-NMR (400 MHz, CD3OD) δ: 8.16 (2H, d, J = 6.8 Hz), 7.65 (2H, d, J = 6.8 Hz), 6.67 (1H, s), 5.91–5.90 (1H, m), 3.95 (3H, s), 2.14–2.08 (4H, m), 1.69–1.60 (4H, m); HRMS (ESI-TOF) m/z [M + H]+: 351.1332 (Calcd for C18H18F3N2O2: 351.1320).
Methyl 4-(5-Cyclohexyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (23c)(Z)-4-Cyclohexyl-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (23a) was synthesized from cyclohexyl methyl ketone (150 µL, 1.09 mmol) in 58% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 5.52 (1H, s), 2.15–2.09 (1H, m), 1.73–1.71 (4H, m), 1.41–1.19 (6H, m).
4-(5-Cyclohexyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (23b) was synthesized from 23a (98 mg, 441 µmol) in 97% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.22 (1H, d, J = 8.8 Hz), 8.18 (1H, d, J = 8.8 Hz), 7.60 (2H, d, J = 8.8 Hz), 6.66 (1H, s), 2.78–2.71 (1H, m), 1.89–1.69 (4H, m), 1.57–1.24 (6H, m).
23c was synthesized from 23b (96 mg, 284 µmol) in 36% yield using the method described for the preparation of 13c. Purity: 95.8%; tR: 33.9 min; 1H-NMR (500 MHz, CDCl3) δ: 8.19 (2H, d, J = 9.0 Hz), 7.52 (2H, d, J = 8.5 Hz), 6.48 (1H, s), 3.97 (3H, s), 2.70–2.64 (1H, m), 1.86–1.70 (4H, m), 1.42–1.20 (6H, m); HRMS (ESI-TOF) m/z [M + H]+: 353.1475 (Calcd for C18H20F3N2O2: 353.1477).
Methyl 4-(5-(Pyridin-4-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (24c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(pyridin-4-yl)-3-buten-2-one (24a) was synthesized from 4-acetylpyridine (200 µL, 1.82 mmol) in 71% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.65 (2H, d, J = 5.6 Hz), 7.76 (2H, d, J = 5.6 Hz), 6.35 (1H, s).
4-Hydrazinobenzoic acid (54 mg, 355 µmol, 1.1 equiv.) was dissolved in dry EtOH (4.5 mL), added 2 N HCl (190 µL) and 24a (73 mg, 336 µmol). After stirring for 1.5 h at 75 °C, the reaction mixture was evaporated. The residue was dissolved in EtOAc and the precipitate was removed by filtration. The filtrate was evaporated to afford 4-(5-(Pyridin-4-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (24b) (69 mg, 207 µmol, 62%). 1H-NMR (400 MHz, CD3OD) δ: 8.55 (2H, d, J = 6.4 Hz), 8.12 (2H, d, J = 8.8 Hz), 7.48 (2H, d, J = 8.8 Hz), 7.34 (2H, d, J = 6.0 Hz), 7.21 (1H, s).
24c was synthesized from 24b (40 mg, 120 µmol) in 55% yield using the method described for the preparation of 13c. Purity: 95.5%; tR: 23.0 min; 1H-NMR (400 MHz, CDCl3) δ: 8.62 (2H, d, J = 5.6 Hz), 8.09 (2H, d, J = 8.8 Hz), 7.40 (2H, d, J = 8.4 Hz), 7.13 (2H, d, J = 6.0 Hz), 6.90 (1H, s), 3.95 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 348.0967 (Calcd for C17H13F3N3O2: 348.0960).
Methyl 4-(5-(1-Methyl-1H-pyrrol-3-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (25c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(1-methyl-1H-pyrrol-3-yl)-3-buten-2-one (25a) was synthesized from 3-acetyl-1-methyl pyrrole (300 µL, 2.54 mmol) in 26% yield using the method described for the preparation of 13a. 1H-NMR (500 MHz, CD3OD) δ: 7.31 (1H, br s), 6.59 (1H, s), 6.45 (1H, s), 5.97 (1H, br s), 3.66 (3H, s).
4-(5-(1-Methyl-1H-pyrrol-3-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (25b) was synthesized from 25a (49 mg, 224 µmol) in 89% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.13 (2H, d, J = 8.8 Hz), 7.54 (2H, d, J = 8.4 Hz), 6.73 (1H, s), 6.65–6.61 (2H, m), 5.85 (1H, dd, J = 2.0, 2.8 Hz), 3.60 (3H, s).
25c was synthesized from 25b (45 mg, 134 µmol) in 81% yield using the method described for the preparation of 13c. Purity: 97.4%; tR: 30.3 min; 1H-NMR (400 MHz, CDCl3) δ: 8.09 (2H, d, J = 8.8 Hz), 7.55 (2H, d, J = 8.4 Hz), 6.60 (1H, s), 6.53–6.47 (2H, m), 5.89 (1H, dd, J = 1.6, 2.8 Hz), 3.95 (3H, s), 3.61 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 350.1131 (Calcd for C17H15F3N3O2: 350.1116).
Methyl 4-(5-(Furan-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (26c)(Z)-1,1,1-Trifluoro-4-(furan-2-yl)-4-hydroxy-3-buten-2-one (26a) was synthesized from 2-acetylfuran (200 µL, 1.99 mmol) in 51% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.64 (1H, d, J = 0.8 Hz), 7.10 (1H, d, J = 2.8 Hz), 6.54–6.53 (1H, m), 6.19 (1H, s).
4-(5-(Furan-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (26b) was synthesized from 26a (72 mg, 349 µmol) in 84% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.17 (2H, d, J = 8.0 Hz), 7.57 (1H, d, J = 2.0 Hz), 7.54 (2H, d, J = 8.4 Hz), 7.05 (1H, s), 6.49–6.48 (1H, m), 6.31 (1H, d, J = 3.6 Hz).
26c was synthesized from 26b (41 mg, 127 µmol) in 59% yield using the method described for the preparation of 13c. Purity: 96.1%; tR: 30.9 min; 1H-NMR (400 MHz, CDCl3) δ: 8.15 (2H, d, J = 8.8 Hz), 7.52 (2H, d, J = 8.8 Hz), 7.43 (1H, d, J = 1.6 Hz), 6.91 (1H, s), 6.40–6.39 (1H, m), 6.18 (J = 3.6 Hz, 1H, d), 3.96 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 337.0779 (Calcd for C16H12F3N2O3: 337.0800).
Methyl 4-(5-(Thiophen-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (27c)(Z)-1,1,1-Trifluoro-4-hydroxy-4-(thiophen-2-yl)-3-buten-2-one (27a) was synthesized from 2-acetylyhiophene (200 µL, 1.85 mmol) in 91% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.55 (1H, d, J = 3.6 Hz), 7.53 (1H, d, J = 5.2 Hz), 7.06 (1H, d, J = 4.4 Hz), 5.95 (1H, s).
4-(5-(Thiophen-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (27b) was synthesized from 27a (102 mg, 459 µmol) in 93% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.13 (2H, d, J = 8.0 Hz), 7.54–7.52 (3H, m), 7.06–7.04 (2H, m), 7.01 (1H, s).
27c was synthesized from 27b (47 mg, 139 µmol) in 59% yield using the method described for the preparation of 13c. Purity: 97.7%; tR: 31.7 min; 1H-NMR (400 MHz, CDCl3) δ: 8.10 (2H, d, J = 7.2 Hz), 7.49 (2H, d, J = 8.8 Hz), 7.38 (1H, d, J = 4.8 Hz), 6.99 (1H, t, J = 4.0 Hz), 6.88 (1H, d, J = 3.6 Hz), 6.82 (1H, s), 3.95 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 353.0571 (Calcd for C16H12F3N2O2S: 353.0572).
Methyl 4-(5-(Naphthalen-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (28c)Sixty percent NaH (237 mg, 5.93 mmol, 2.1 equiv.) and 2′-acetonaphtone (490 mg, 2.88 mmol) were dissolved in dry THF (10 mL) under ice-cold. After stirring for 1 h under 0 °C, ethyl trifluoroacetate (515 µL, 4.31 mmol, 1.5 equiv.) was added, and the reaction mixture was stirred for 29 h at room temperature. The reaction mixture was acidified to pH 6 with 1 N HCl, extracted two times with EtOAc, and washed with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The residue was reprecipitated with EtOAc and n-hexane, purified by silica gel flash column chromatography (n-hexane/EtOAc = 2 : 1) to afford (Z)-1,1,1-trifluoro-4-hydroxy-4-(naphthalen-2-yl)-3-buten-2-one (28a) (286 mg, 1.07 mmol, 37%). 1H-NMR (500 MHz, CD3OD) δ: 8.54 (1H, s), 8.07 (1H, d, J = 8.5 Hz), 7.99–7.88 (3H, m), 7.56–7.52 (2H, m), 6.56 (1H, s).
4-(5-(Naphthalen-2-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (28b) was synthesized from 28a (51 mg, 192 µmol) in 76% yield using the method described for the preparation of 2. 1H-NMR (400 MHz, (CD3)2CO) δ: 8.09 (2H, d, J = 8.8 Hz), 8.03 (1H, s), 7.95–7.89 (3H, m), 7.60–7.55 (4H, m), 7.37 (1H, d, J = 6.8 Hz), 7.16 (1H, s).
28c was synthesized from 28b (38 mg, 99.4 µmol) in 79% yield using the method described for the preparation of 13c. Purity: 97.9%; tR: 34.4 min; 1H-NMR (400 MHz, CDCl3) δ: 8.01 (2H, d, J = 6.8 Hz), 7.85–7.77 (4H, m), 7.55–7.51 (2H, m), 7.43 (2H, d, J = 7.2 Hz), 7.19 (1H, d, J = 8.8 Hz), 6.87 (1H, s), 3.91 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 397.1163 (Calcd for C22H16F3N2O2: 397.1164).
Methyl 4-(5-(2,3-Dihydro-1H-inden-5-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (29c)(Z)-4-(2,3-Dihydro-1H-inden-5-yl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (29a) was synthesized from 5-acetylindane (150 µL, 999 µmol) in 29% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, CD3OD) δ: 7.78–7.72 (2H, br), 7.23 (1H, d, J = 7.2 Hz), 6.32 (1H, br s), 2.94–2.91 (4H, m), 2.13–2.06 (2H, m).
4-(5-(2,3-Dihydro-1H-inden-5-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (29b) was synthesized from 29a (50 mg, 195 µmol) in 96% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.04 (2H, d, J = 9.2 Hz), 7.42 (2H, d, J = 8.8 Hz), 7.20 (1H, d, J = 8.0 Hz), 7.15 (1H, s), 6.99 (1H, d, J = 8.0 Hz), 6.88 (1H, s), 2.93–2.84 (4H, m), 2.11–2.04 (2H, m).
29c was synthesized from 29b (44 mg, 118 µmol) in 42% yield using the method described for the preparation of 13c. Purity: 98.3%; tR: 35.1 min; 1H-NMR (500 MHz, CDCl3) δ: 8.03 (2H, d, J = 8.5 Hz), 7.41 (2H, d, J = 8.5 Hz), 7.16 (1H, d, J = 7.5 Hz), 7.11 (1H, s), 6.93 (1H, d, J = 7.5 Hz), 6.72 (1H, s), 3.92 (3H, s), 2.93–2.85 (4H, m), 2.12–2.06 (2H, m); HRMS (ESI-TOF) m/z [M + H]+: 387.1334 (Calcd for C21H18F3N2O2: 387.1320).
Methyl 4-(5-(Benzo[d][1,3]dioxol-5-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoate (30c)(Z)-4-(Benzo[d][1,3]dioxol-5-yl)-1,1,1-trifluoro-4-hydroxy-3-buten-2-one (30a) was synthesized from 3′,4′-(methylenedioxy)acetophenone (199 mg, 1.21 mmol) in 80% yield using the method described for the preparation of 13a. 1H-NMR (400 MHz, (CD3)2CO) δ: 7.55–7.35 (2H, m), 6.83 (2H, d, J = 8.4 Hz), 6.22 (1H, s), 6.02 (2H, s).
4-(5-(Benzo[d][1,3]dioxol-5-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzoic acid (30b) was synthesized from 30a (103 mg, 396 µmol) in 91% yield using the method described for the preparation of 13b. 1H-NMR (400 MHz, CD3OD) δ: 8.07 (2H, d, J = 8.0 Hz), 7.44 (2H, d, J = 8.4 Hz), 6.88 (1H, s), 6.84–6.77 (2H, m), 6.74 (1H, s), 5.99 (2H, s).
30c was synthesized from 30b (101 mg, 268 µmol) in 82% yield using the method described for the preparation of 13c. Purity: 99.5%; tR: 31.4 min; 1H-NMR (400 MHz, CDCl3) δ: 8.05 (2H, d, J = 8.4 Hz), 7.41 (2H, d, J = 8.8 Hz), 6.79–6.64 (4H, m), 6.00 (2H, s), 3.93 (3H, s); HRMS (ESI-TOF) m/z [M + H]+: 391.0894 (Calcd for C19H14F3N2O4: 391.0906).
Cell CultureHT-1080 human sarcoma cells (purchased from American Type Culture Collection, Manassas, VA, U.S.A.) were cultured in Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Corporation, Osaka, Japan). The culture medium was supplemented with 10% fetal bovine serum, and the cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C.
In Vitro WST-8 AssayIn vitro cytotoxicity was examined using a colorimetric assay with the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer’s instructions. In the WST-8 assay, triplicate wells were used for each concentration, and all assays were performed at three times. Briefly, HT-1080 cells were seeded at a density of 4 × 103 cells/well in a 96-well plate and cultured for 24 h. Serial dilutions of each compound, dissolved in dimethyl sulfoxide, were added to the culture medium at concentrations of 3.125–100 µM. After 48 h of incubation, the medium was replaced with fresh medium containing the WST-8 reagent. After 1 h, the absorbance in each well was measured at 460 nm using a microplate spectrophotometer, ImmunoMini NJ-2300 (BioTec, Tokyo, Japan).
The percentage of cell growth inhibition was calculated by applying the following formula: percentage of cell growth inhibition = (1 − [T/C]) × 100, where C and T are the mean absorbance values of the control and treated groups, respectively. The IC50 value was measured graphically from the dose-response curve with at least three drug concentration points.
Gelatin ZymographyHT-1080 cells (1 × 105 cells/well) were seeded in a 6-well tissue culture plate and cultured in 10% fetal bovine serum/Dulbecco’s modified Eagle medium (FBS/DMEM) overnight. After washing twice with phosphate-buffered saline (PBS), the cells were serum-starved with 0.5% FBS/DMEM for 6 h, and then treated with the inhibitors (25 µM) for 24 h. The conditioned medium (CM) was analyzed by gelatin zymography, as previously described.19) MMP-2 and MMP-9 levels in CM were measured by adding an equal volume of sample buffer. The samples were separated by electrophoresis on an sodium dodecyl sulfate (SDS)-polyacrylamide gel containing gelatin (Difco, Sparks, MD, U.S.A.) labeled with Alexa Fluor 680 (Molecular Probes). The gels were processed and monitored using an Odyssey infrared imaging system (LI-COR, Lincoln, NE, U.S.A.) against the normalized density of the target band in the control sample. These assays were performed at three times.
Batimastat (BB94) acts as a direct inhibitor of various MMPs such as MMP-1, MMP-2, MMP-9, MMP-7, and MMP-3. BB94 can bind the zinc ion in the active site of MMPs.22) To evaluate the inhibitory activity of the derivatives upon MMP-2/9 production, BB94 was used as the positive control.
COX-2 Inhibition AssayCOX-2 inhibitory activity was assessed using the COX-2 (human) Inhibitor Screening Assay Kit (Cayman Chemical, Ann Arbor, MI, U.S.A.) according to the manufacturer’s instructions. In brief, COX-2 was inactivated by placing it in boiling water for 3 min. Compounds (10 µL; final concentration: 200 µM) dissolved in DMSO were added to each test tube, and 10 µL of DMSO was added to the control and background tubes. All tubes were incubated for 10 min at 37 °C in a heat block. Arachidonic acid (10 µL) was added to the reaction tubes to initiate the reaction. The tubes were quickly mixed and incubated for 2 min at 37 °C. Saturated stannous chloride solution (30 µL) was added to each reaction tube to stop enzyme catalysis. All tubes were removed from the heat block, vortexed, and incubated for 5 min at room temperature. The amount of prostaglandin in the tubes was evaluated by enzyme-linked immunosorbent assay (ELISA).19) The COX-2 inhibitory assay for each compound was carried out in triplicate.
This work was supported partly by Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.