2021 Volume 69 Issue 2 Pages 218-221
2021 Volume 69 Issue 2 Pages 218-221
A robust ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) technique was proven effective for simultaneous characterization of six flavonoids including quercetin-3-O-beta-galactoside (Q3GAL), quercetin-3-O-beta-glucoside (Q3GLU), quercetin-3-(2-galloylglucoside) (Q3GG), kaempferol-3-O-beta-galactoside (K3GAL), kaempferol-3-O-beta-glucoside (K3GLU), and kaempferol-3-(2-galloylglucoside) (K3GG) in rat eyes. By investigation of corresponding validation parameters (linearity, selectivity, precision, accuracy, matrix effect, extraction recovery, and stability), the method was verified to be within current acceptable criteria. Thereafter, the validated method enabled quantification of the six compounds successful in rat eyes after oral administration of ethanol extract Diospyros kaki (EEDK) at 0, 3, 15, 35, 60, 120 min.
Diospyros kaki (Persimmon) has been cultivated throughout Eastern Asia for centuries such as China, Korea, and Japan.1,2) This plant is of interest since it has been reported that D. kaki leaves contain a variety of active constituents, such as flavonoids, resins, polysaccharides, carotene3,4) which may have beneficial health effects related to its radical-scavenging and antioxidant properties in vitro.5,6) Ethanol extract Diospyros kaki (EEDK) is well-known to exhibit some protective effects in physical and chemical models of mouse eye degeneration.7–10) A previous study shows that degeneration of retina is related to reactive oxygen species (ROS).11) Several different compounds with antioxidative activities, such as carotenoids, vitamin C, and vitamin E, may protect against retinal degeneration by preventing the formation of free radicals in retina, and it has also been reported that intake of nutrients that contain those compounds in higher levels is effective in lowering the degeneration of retina.12,13)
Flavonoids are ubiquitously present in D. kaki leaves,14) they have been reported to be responsible for the antioxidant activity of the EEDK.15,16) The major compounds were identified as the glycoside, galactoside, or galloylated derivatives of quercetin and kaempferol in EEDK.9) Although EEDK has been associated with beneficial eye outcomes in murine models of disease, the form and amount that are present in the target tissues are unknown. Herein, we established and tested an efficient ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method to characterize six flavonoids including quercetin-3-O-beta-galactoside (Q3GAL), quercetin-3-O-beta-glucoside (Q3GLU), quercetin-3-(2-galloylglucoside) (Q3GG), kaempferol-3-O-beta-galactoside (K3GAL), kaempferol-3-O-beta-glucoside (K3GLU), and Kaempferol-3-(2-galloylglucoside) (K3GG) in rat eyes and they were orally treated with EEDK before.
Negative ion mode was applied to detect the compounds including Q3GAL, Q3GLU, Q3GG, K3GAL, K3GLU, K3GG, and phloridzin (internal standard, IS) (Fig. 1) due to their better responses. Accordingly, precursor to product ion transitions in selective reaction monitoring (SRM) mode were as follows: Q3GAL, m/z 463.1/300.2; Q3GLU, m/z 463.1/300.2; Q3GG, m/z 615.1/300.9; K3GAL, m/z 447.1/283.9; K3GLU, m/z 447.1/283.9; K3GG, m/z 599.2/284.9; IS, m/z 435.0/273.0. Their chromatographic retention time was 2.0, 4.4, 6.4, 6.5, 7.1, 7.4, and 8.4 min, respectively. The calibration curves of the six analytes were linear over ranges of 0.49–500 ng/mL (r2 > 0.99) with the lower limit of quantification (LLOQ) at 0.391, 0.391, 0.391, 0.195, 0.049, and 0.049 ng/100 mg in rat eyes, respectively. The intra- and inter-day accuracy (relative error, RE) were −13.2 to 12.1% with precision (relative standard deviation, RSD) of 0 to 13.8%. Additionally, interference peaks and significant carry-over effects were not observed under the current method. Besides, phloridzin has a similar structure with the six targeted compounds. All of them belong to the class of flavonoids. So phloridzin was chosen as an internal standard after it was confirmed to be not detected in EEDK, rat food, and rat eyes.
The extraction recovery and matrix effect were 87.4–108.7% and 86.6–98.1%, respectively (Table 1). Moreover, the accuracy under three different conditions [autosampler (4 °C, 2 d), long-term (−20 °C, 30 d), and freeze-thaw (−20 to 25 °C) cycles] were −11.0 to 9.3%, −7.7 to 9.5%, and −9.4 to 5.5% with precision of 2.3 to 13.8%, 3.1 to 14.2%, and 2.4 to 13.2%, respectively. These results indicated that the six compounds were stable for analysis over different storage conditions.
| Analytes | QCa) (ng/mL) | Matrix effect (%) | Extraction recovery (%) |
|---|---|---|---|
| Q3GALb) | 175 (High) | 93.4 ± 7.7 | 87.4 ± 8.5 |
| 21.9 (Low) | 90.0 ± 9.5 | 97.8 ± 5.9 | |
| 43.8 (Medium) | 94.2 ± 5.7 | 92.3 ± 13.7 | |
| Q3GLUc) | 175 (High) | 87.7 ± 12.5 | 98.0 ± 12.2 |
| 21.9 (Low) | 91.7 ± 10.4 | 108.7 ± 9.9 | |
| 43.8 (Medium) | 88.6 ± 7.5 | 93.9 ± 5.8 | |
| Q3GGd) | 175 (High) | 90.4 ± 5.0 | 88.4 ± 8.6 |
| 21.9 (Low) | 96.0 ± 9.8 | 92.8 ± 10.9 | |
| 43.8 (Medium) | 96.2 ± 4.7 | 105.3 ± 9.5 | |
| K3GALe) | 175 (High) | 89.7 ± 11.1 | 90.4 ± 10.1 |
| 21.9 (Low) | 92.2 ± 10.8 | 100.7 ± 8.9 | |
| 43.8 (Medium) | 86.6 ± 8.3 | 95.9 ± 7.8 | |
| K3GLUf) | 175 (High) | 91.8 ± 8.5 | 88.5 ± 5.9 |
| 21.9 (Low) | 96.2 ± 10.6 | 91.3 ± 12.3 | |
| 43.8 (Medium) | 89.7 ± 12.2 | 100.3 ± 10.8 | |
| K3GGg) | 175 (High) | 98.1 ± 11.6 | 94.2 ± 10.8 |
| 21.9 (Low) | 96.6 ± 13.3 | 102.7 ± 9.3 | |
| 43.8 (Medium) | 90.3 ± 9.1 | 93.2 ± 7.4 |
Note: a) QC, quality control; b) Q3GAL, quercetin-3-O-beta-galactoside; c) Q3GLU, quercetin-3-O-beta-glucoside; d) Q3GG, quercetin-3-(2-galloylglucoside); e) K3GAL, kaempferol-3-O-beta-galactoside; f) K3GLU, kaempferol-3-O-beta-glucoside; g) K3GG, kaempferol-3-(2-galloylglucoside).
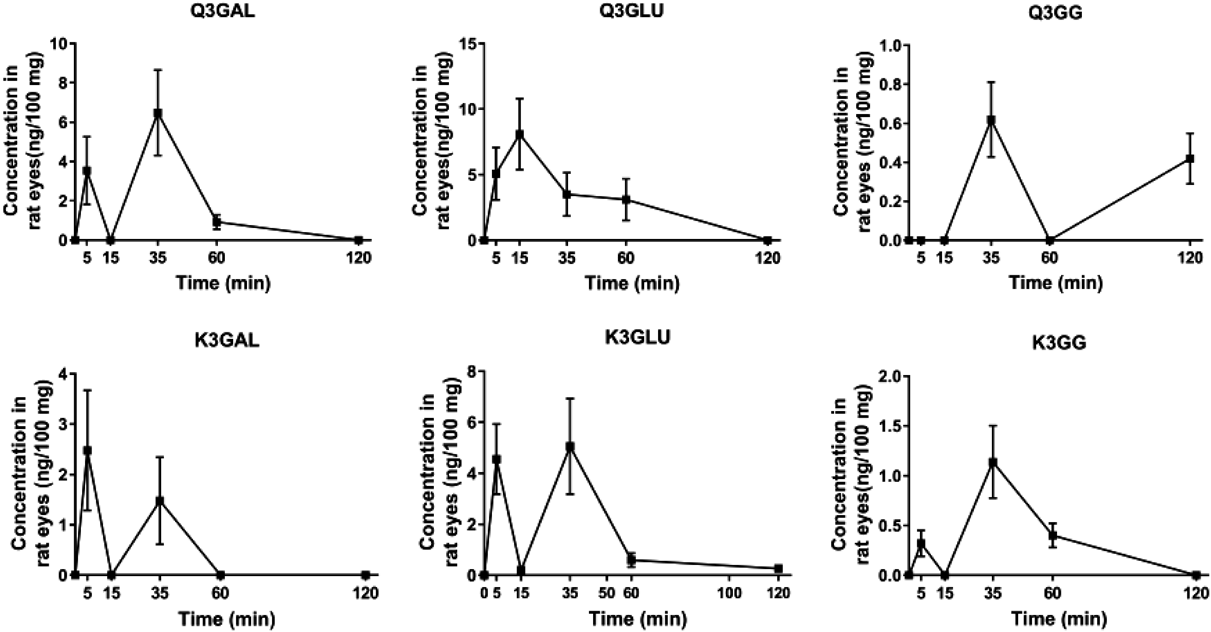
The bioavailable study showed that Q3GAL, Q3GLU, K3GAL, K3GLU, and K3GG were detectable in rat eyes at 5 min after being treated with EEDK. To be noticed, the analyte Q3GG was not bioavailable until 15 min (Fig. 2). Most of the analytes reached the maximum concentrations within 60 min and were eliminated after 120 min from rat eyes. Besides, the double-peak phenomenon was observed in the concentration–time curves, the same phenomenon was also observed in rat plasma according to the previous report.17–19) This may be attributed to many factors, such as distribution, the variability of absorption, reabsorption, enterohepatic circulation, and delayed gastric emptying.
In general, although previous studies had reported analysis of some of the six flavonoids and their analogs based on LC-MS or LC-MS/MS,20–25) most analyses were performed in the plant instead of animal samples. To the best of our knowledge, no other published method covers such six flavonoids simultaneously in rat eye tissues with a more specific and rapid UPLC-MS/MS method.
An efficient UPLC-MS/MS method was established and validated to simultaneously characterize six flavonoids in rat eyes after oral treatment with. Most of the compounds were eliminated from the eye tissue after 120 min. However, further studies are necessary to obtain a complete characterization profile of the six flavonoids at longer time points.
The analysis was conducted on an Agilent 1290 LC system (Agilent Technologies, CA, U.S.A.) coupled to an API 4000 triple quadrupole system interfaced with electrospray ionization source (SCIEX, Foster City, CA, U.S.A.). Compound separation was applied using a Waters ACQUITY BEH C18 (2.1 × 100 mm, 1.7 µm) column at 40 °C with a flow rate of 0.6 mL/min. The mobile phase system consisted of water/acetonitrile (ACN) [95/5, v/v with 0.1% formic acid (FA)] as solvent A and ACN/water (95/5, v/v with 0.1% FA) as solvent B. A gradient elution procedure was set as follows: 0–10 min, 5–100% B; then, the system rebalance was applied at 5% B for 3 min.
Preparation EEDK and Animal SamplesEEDK was prepared according to the previous report.9) Briefly, 7 L of EtOH was added to 800 g of the dried D. kaki leaves for sonication extraction for 3 h at room temperature, extraction was repeated for 3 times. The combined filtrate was concentrated to dryness by rotary evaporation at 40 °C to obtain 31.5 g (yield = 3.94%) of EEDK. Eight-week-old male rats (Sprague Dawley, 300 ± 20 g) were purchased from Central Laboratory Animal Inc. (Seoul, Korea). Rats were maintained according to the report.9)
All animal studies were performed in a pathogen-free barrier zone at the Korea Institute of Science and Technology (KIST) Gangneung branch in accordance with the procedure outlined in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The procedures used in this study were approved by the Animal Care and Use Committee of the KIST Gangneung branch (No. 2014-011). All the rats were fed with EEDK for two d (250 mg/kg/d). The rat eyes (around 50 mg/L sample) were collected at 0, 5, 15, 35, 60, and 120 min after EEDK administration. The connective tissues around the eyeball, including blood vessels, were trimmed completely, followed by homogenizing with 100 µL of physiological saline for 1 min (4 strokes of 15 s at 5 s intervals, 2–4 °C) using an autoclaved mortar and pestle. The homogenized rat eye sample was extracted with 400 µL of acetonitrile containing IS (12.5 ng/mL). Then the sample was centrifuged at 10000 × g for 10 min at 4 °C after being vortexed for 1 min, the supernatant was collected followed by another twice extraction. Finally, all combined supernatants were moved to dryness followed by reconstitution with 100 µL of 70% aqueous methanol, and 3 µL was injected into LC-MS/MS for analysis.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.