2021 Volume 69 Issue 4 Pages 360-373
2021 Volume 69 Issue 4 Pages 360-373
The M3 muscarinic acetylcholine receptor (mAChR) plays an essential pharmacological role in mediating a broad range of actions of acetylcholine (ACh) released throughout the periphery and central nerve system (CNS). Nevertheless, its agonistic functions remain unclear due to the lack of available subtype-selective agonists or positive allosteric modulators (PAMs). In the course of our extended structure–activity relationships (SARs) study on 2-acylaminothiazole derivative 1, a previously reported PAM of the M3 mAChR, we successfully identified N-pyrimidyl/pyridyl-2-thiazolamine analogues as new scaffolds. The SARs study was rationalized using conformational analyses based on intramolecular interactions. A comprehensive study of a series of analogues described in this paper suggests that a unique sulfur–nitrogen nonbonding interaction in the N-pyrimidyl/pyridyl-2-thiazolamine moiety enable conformations that are essential for activity. Further, a SARs study around the N-pyrimidyl/pyridyl-2-thiazolamine core culminated in the discovery of compound 3g, which showed potent in vitro PAM activity for the M3 mAChR with excellent subtype selectivity. Compound 3g also showed a distinct pharmacological effect on isolated smooth muscle tissue from rat bladder and favorable pharmacokinetics profiles, suggesting its potential as a chemical tool for probing the M3 mAChR in further research.
Muscarinic acetylcholine receptors (mAChRs) are one of the most physiologically important targets in the pharmaceutical industry. Five receptor subtypes are known, M1, M2, M3, M4, and M5, with each subtype preferentially coupling to distinct heterotrimeric G-proteins to enable modulation of a variety of ion channels and other signaling pathways.1–4) Coupling of the M3 mAChR to Gq is known to activate the phospholipase C/inositol 1,4,5-triphosphate (IP3)/Ca2+ signaling pathway.5,6) Activation of the M3 mAChR by acetylcholine (ACh) released throughout the periphery and central nervous system (CNS) mediates contraction of various smooth muscle tissues, stimulation of glandular secretion, and regulation of a variety of cholinergic processes in the brain.7,8) Although the effects of inhibiting the M3 mAChR have been well studied and have led to the discovery of a number of important therapeutic agents,9–14) little is known about the agonistic actions of the receptor due to the lack of available subtype-selective agonists or positive allosteric modulators (PAMs).15–18) Analyses of high resolution crystal structures of mAChR subtypes have revealed the difficulty of obtaining subtype-selective ligands owing to high homology in the orthosteric binding pockets among mAChR subtypes.19–21) To this end, we have strategically focused on the discovery of novel PAMs of the M3 mAChR based on the well-established relevance of PAMs in subtype selectivity.22–25)
We previously reported novel PAMs of the M3 mAChR and an associated structure–activity relationships (SARs) study of the moieties around 2-acylaminothiazole derivative 1,26) which led to the discovery of the more potent compound 2 (Fig. 1). Compound 2 exhibited potent in vitro PAM activity towards the M3 mAChR with excellent subtype selectivity over M1, M2, and M4 mAChRs and moderate selectivity over the M5 mAChR. Moreover, compound 2 also enhanced muscle contraction when applied to isolated smooth muscle tissue from rat bladder.

(Color figure can be accessed in the online version.)
In a follow-on study on 2, we have turned our attention to replacing the 2-acylaminothiazole core of 2 to further explore SARs. We began by conducting conformational analysis of the core structure based on a high-level quantum chemical calculations assessment. The conformational isomerism of 2-acylaminothiazole, the extracted core structure of 2, was theoretically investigated based on intramolecular interactions (Fig. 2). Four possible conformations were generated from the rotation of the amide moiety in between two aromatic rings, and conformational energy differences were calculated using quantum chemical calculations at the MP 2/6-31++G**//HF/6-31G** level. As a consequence, we determined that conformation 2 was significantly more stable than the other conformations due to intramolecular hydrogen bonding and a sulfur–oxygen interaction. In contrast, the remaining conformations possessed at least one electron pair repulsion. Therefore, conformation 2 was considered the most stable conformation and is expected to play an essential role in interactions with the human M3 mAChR.

(Color figure can be accessed in the online version.)
In the meantime, Lin et al. reported the design of 2-aminothiazol-5-yl-pyrimidines to mimic 2-aminothiazole-5-carboxamide in the discovery of novel p38α mitogen-activated protein (MAP) kinase inhibitors, where a unique sulfur–nitrogen intramolecular nonbonding interaction was used to stabilize the conformation required for binding to the p38α active site.27) Similar types of sulfur–heteroatom interactions have been reported to incorporate intramolecular n→σ* nonbonding interactions.28–30)
Encouraged by our calculations and the report of the unique sulfur–nitrogen intramolecular nonbonding interaction, we designed N-pyrimidyl-2-thiazolamine derivative 3a to possess a sulfur–nitrogen interaction that mimics the sulfur–oxygen interaction for replacement of the 2-acylaminothiazole scaffold. Introduction of the sulfur–nitrogen interaction and replacement of substituents on the benzene ring at the 4-position of the thiazole culminated in the discovery of 3a, which showed a 23-fold shift in the concentration–effect curve of carbachol (CCh) with increasing concentrations of the compound (Fig. 3). This finding prompted us to further investigate SARs and favorable conformations essential for activity.

(Color figure can be accessed in the online version.)
Here, we report the extended SARs study of 2, with focus on replacing the 2-acylaminothiazole core, which was guided and rationalized by conformational analysis of intramolecular interactions around the core.
Compounds reported herein were synthesized as shown in Charts 1–3.

Reagents and conditions: (a) phenyltrimethylammonium tribromide, tetrahydrofuran (THF), room temperature (r.t.); (b) thiourea, ethanol (EtOH), 65–75 °C; (c) acetic anhydride (Ac2O), pyridine, 60 °C; (d) 36% formaldehyde (HCHO) aq., Ac2O, acetic acid (AcOH), microwave 170 °C; (e) (2R)-2-methylpyrrolidine, N,N-diisopropylethylamine (DIPEA), N,N-dimethylformamide (DMF), 100 °C; (f) 6 M sodium hydroxide (NaOH) aq., EtOH, microwave 120 °C; (g) sodium hydride (NaH), THF or N-methyl-2-pyrrolidone (NMP), r.t.; (h) ethyl 3-(piperazin-1-yl)propanoate dihydrochloride, DIPEA, NMP, 80 °C; (i) 1 M NaOH aq., EtOH, THF, 50–60 °C.

Reagents and conditions: (a) ethyl 3-(piperazin-1-yl)propanoate dihydrochloride, potassium carbonate (K2CO3), NMP, −10–80 °C; (b) 8, NaH, THF, 0 °C or tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3), di-tert-butyl(2′,4′,6′-triisopropylbiphenyl-2-yl)phosphine (di-tBuXPhos), cesium carbonate (Cs2CO3), toluene–H2O, 80–100 °C; (c) 1 M NaOH aq., EtOH, THF, 60 °C; (d) ethyl acrylate, EtOH, reflux.
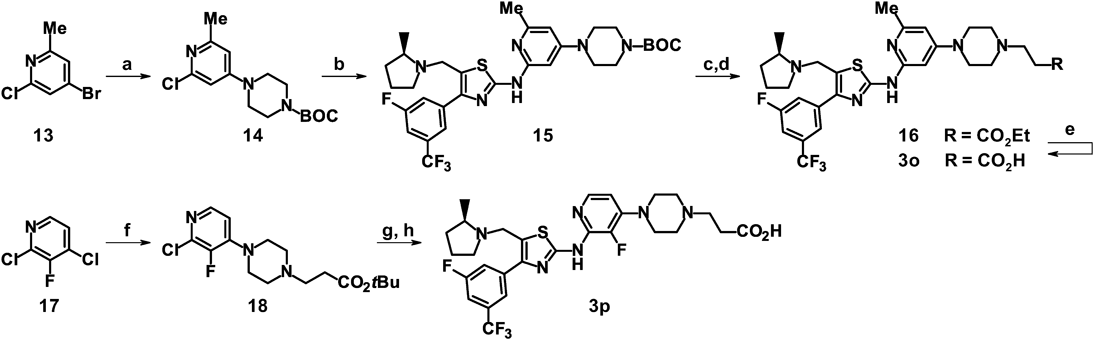
Reagents and conditions: (a) 1-boc-piperazine, Pd2(dba)3, (9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (XantPhos), potassium tert-butoxide (tBuONa), toluene, 100 °C; (b) 8, Pd2(dba)3, XantPhos, Cs2CO3, NMP, 100 °C; (c) trifluoroacetic acid (TFA), dichloromethane (CH2Cl2), r.t.; (d) ethyl acrylate, EtOH, microwave 100 °C; (e) 1 M NaOH aq., EtOH, THF, 60 °C; (f) tert-butyl 3-(piperazin-1-yl)propanoate, K2CO3, NMP, 80 °C; (g) 8, Pd2(dba)3, 1,1′-binaphthalene-2,2′-diylbis(diphenylphosphine) (BINAP), Cs2CO3, NMP, 100 °C; (h) 4 M hydrochloric acid (HCl), 1,4-dioxane, r.t.
The key intermediate 8 was prepared in 6 steps from commercially available acetophenone 426) (Chart 1). Acetophenone 4 was reacted with phenyltrimethylammonium tribromide, followed by thiourea to obtain 2-aminothiazole 5. Compound 5 was then treated with acetic anhydride to yield the acetylated intermediate 6, which underwent functionalization of the 5-position of the thiazole to give (2R)-2-methylpyrrolidine, and subsequent deprotection of the acetyl group to give the key intermediate 8.
Compound 8 was coupled with the required core via ipso-substitution under basic conditions to yield 9a–g, and the subsequent introduction of the piperazine moiety followed by hydrolysis of the terminal ester gave the desired compounds 3a–g.
For the synthesis of 3h–n, the piperazine moiety was introduced into 10h–m ahead of thiazole via basic ipso-substitution or a palladium-catalyzed coupling reaction to give 11h–m (Chart 2). Meanwhile, N-bromophenyl piperazine 12 was treated with ethyl acrylate to give 11n. Final compounds 3h–n were obtained after a coupling reaction of 11h–m with 8, followed by hydrolysis as described above.
Synthesis of compounds 3o and 3p is outlined in Chart 3. Buchwald–Hartwig reaction31) of piperazine derivatives and corresponding pyridines gave adducts 14 and 18, and a subsequent coupling reaction with 8 gave the corresponding compounds. Finally, the terminal unit of the obtained intermediates were properly transformed to give the desired compounds 3o and 3p.
All analogues were screened by the Fluorometric Imaging Plate Reader (FLIPR™) assay using CHO-K1 cells stably expressing the human M3 mAChR with CCh as a ligand. The magnitude of PAM activity for the M3 mAChR was indicated by the shift toward lower concentrations on a CCh concentration-response curve after exposure to the test substance.15–18) That is, PAM activity was determined by dividing the 50% effective concentration (EC50) value of CCh in the absence of the test substance by the EC50 value in the presence of the test substance. In the tables below, values indicated for human M3 PAM (-fold shift) represent values obtained when the test substances were added at a final concentration of 1 µM.
We began our SARs study by conducting conformational analysis of 3a as shown in Fig. 4. The results revealed that an intramolecular sulfur–nitrogen interaction (conformation 1) as well as hydrogen bonding (conformation 4) contributed to a lower conformational energy. In contrast to the case of compound 2, conformations 1 and 4 had lower conformational energies than conformations 2 and 3. We also note that the conformational analyses performed in this report were based only on the four depicted conformations without any consideration for transition states. Therefore, if the energy barrier was low enough, it would be possible for conformation 4 to transition into conformation 1. For further study on SARs and favorable conformations essential for activity, we prepared an array of analogues by examining replacements of the pyrimidine ring.

(Color figure can be accessed in the online version.)
As shown in Table 1, pyrimidine 3b, 3c and 1,3,5-triazine 3h, which commonly had a nitrogen atom at the 5-position of the ring, drastically decreased activity. This result suggested that conformation 3, due to the sulfur–nitrogen interaction derived from the nitrogen atom at the 5-position of the ring, negatively affected activity. In addition, the presence of the nitrogen atom at the 5-position of the ring was thought to raise the conformational energy of conformation 4 by generating electron pair repulsion with the thiazole nitrogen. On the other hand, pyridazine 3i resulted in severe loss of activity, suggesting that its linear structure might have substantially altered the binding mode, turning the terminal carboxylic acid such that the orientation was inappropriate for interaction with the receptor. Meanwhile, pyrazine 3j, pyridine analogues 3k, 3l and benzene 3n abolished activity presumably owing to the absence of the nitrogen atom at the 3-position of the ring for interaction with the thiazole sulfur atom in conformation 1. Although pyridine analogues 3k and 3l considerably diminished activity, 3m induced activity (12-fold shift). These findings indicate that unfavorable combination with conformation 2 and 3 decreased activity. We hypothesize that conformation 2 or 3 hampered the ability of the molecule to obtain an appropriate binding mode with the receptor due to repulsion between the remaining residue of the molecule and the binding pocket, which was not found for conformations 1 or 4.
 |
a Data are expressed as the mean (n = 2). b Hydrochloride salt, c 1 µM of test substance was used.
Next, we explored compounds 3a and 3m following the addition of substituents (Table 2). While addition of a methyl group to the 2-position of the pyrimidine ring (3d) retained activity, addition of a methyl group to the 5-position (3e) decreased activity. Meanwhile, 5-fluorine-substituted pyrimidines such as 3f and 3g maintained activity. The difference in activity induced by these analogues appeared to be attributed to steric hindrance with neighboring substituents. For example, in 3e, the methyl group at the 5-position and the neighboring piperazine produced a twisted conformation, leading to steric hindrance and loss of activity. In contrast, 5-fluorine-substituted analogues retained activity because the relatively smaller fluorine atom kept conformational change within an acceptable range. In the case of pyridine, compound 3p, which had a fluorine at the 5-position enhanced activity, while 3o retained the same level of activity as 3m. The idea that 5-fluorine-substituted analogues were not expected to take conformation 4 owing to loss of the intramolecular hydrogen bonding led us to hypothesize that stable conformation 1 was the “active conformation.”
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | X | Human M3 PAM (-fold shift)b) |
| 3ac) | H | H | N | 23 |
| 3dc) | Me | H | N | 31 |
| 3ec) | H | Me | N | 4.7 |
| 3fc) | H | F | N | 35 |
| 3gc) | Me | F | N | 39 |
| 3mc) | H | H | C | 12 |
| 3od) | Me | H | C | 9.8 |
| 3pc) | H | F | C | 31 |
a) Data are expressed as the mean (n = 2 or 3). b) 1 µM of test substance was used, c) hydrochloride salt, d) sodium salt.
We further evaluated 3g as a representative compound of this chemotype to characterize its potential as a chemical probe. Compound 3g shifted a CCh concentration–response curve for human M3 mAChR to the left in a concentration-dependent manner (Table 3).
| Concentration (µM) | 0.01 | 0.03 | 0.1 | 0.3 | 1 | 3 | 10 | 30 |
| Human M3 PAM (-fold shift) | 2.2 | 4.8 | 9.2 | 35 | 57 | 96 | 180 | 219 |
a) Representative values are shown (n = 2).
In addition, the selectivity profiles of compound 3g for other subtypes of human mAChRs were also evaluated (Table 4). We found that 3g showed specific modulation of the M3 mAChR over the other human mAChR subtypes including M1, M2, and M4 mAChRs.
| Human mAChR PAM (-fold shift) | ||||
|---|---|---|---|---|
| M1 | M2 | M3 | M4 | |
| 1 µM | 1.5 | 1.1 | 57 | 0.8 |
| 10 µM | 0.8 | 1.4 | 180 | 1.2 |
a) Representative values are shown (n = 2).
The PAM activity of 3g for the rat M3 mAChR showed a 36-fold shift at 1 µM, and was comparable to that for the human M3 mAChR. To assess the pharmacological properties of 3g, we examined the effect of compound 3g on electrical field stimulation (EFS)-induced contractions of isolated rat bladder tissue. Tension of isolated bladder contractions before and after addition of 10 µM 3g are 2.40 ± 0.56 g and 3.66 ± 0.63 g (mean ± standard error of the mean (S.E.M.), n = 4), indicating that 3g is pharmacologically functional.
We also evaluated the pharmacokinetics (PK) profiles of compound 3g in rats (Table 5). After intravenous (i.v.) administration (1 mg/kg), compound 3g showed moderate total clearance (0.8 mL/min/kg), a steady-state volume of distribution (0.38 L/kg), and a half-life of 7.8 h. Compound 3g was absorbed after oral dosing at 1 mg/kg and showed favorable bioavailability (62%), therefore showing its potential as a chemical probe.
| Route | Dose (mg/kg) | Cmax (ng/mL) | Tmax (h) | AUC0–∞ (ng·h/mL) | t1/2 (h) | CLtot (mL/min/kg) | Vdss (L/kg) | F (%)b) |
|---|---|---|---|---|---|---|---|---|
| i.v.c) | 1 | — | — | 22000 | 7.8 | 0.8 | 0.38 | — |
| per os (p.o.)d) | 1 | 934.0 | 6.0 | 13600 | — | — | — | 62 |
—: not applicable. a) Pharmacokinetics parameters were determined from mean plasma concentrations at each time point. b) F = Bioavailability, c) n = 2, d) n = 3.
Novel chemotypes were discovered through conformational analysis of the previously reported analogue 2 based on the analysis of intramolecular interactions and the application of a unique sulfur–nitrogen nonbonding interaction. A series of analogues with different types of core structures were prepared and evaluated, and led to the discovery of 3a. Compound 3a contains intramolecular sulfur–nitrogen interaction in the N-pyrimidyl-2-thiazolamine core that mimics sulfur–oxygen interaction in 2-acyl-aminothiazole, demonstrating that this intramolecular interaction affect activity by dictating conformation. Further exploration of substituents on pyrazine/pyridine led to the identification of 3g, which showed potent in vitro PAM activity for the M3 mAChR with excellent subtype selectivity. Moreover, compound 3g showed a distinct pharmacological effect on isolated smooth muscle tissue from rat bladder and favorable PK profiles in rats following oral dosing, suggesting that it may be useful as a chemical probe for investigating agonistic modulation of the M3 mAChR both in vitro and in vivo. To evaluate the utilities of compound 3g as a potential pharmacological tool, further experiments are needed.
PAM activity of the test substances for the human and rat mAChRs were evaluated as described previously.26) Briefly, Ca2+ signal transduction induced by CCh in human and rat mAChRs receptor-expressing CHO-K1 cells in the absence or presence of the test substance was monitored using the FLIPR™ system. PAM activity was determined by dividing the EC50 value of CCh in the absence of the test substance by the EC50 value in the presence of the test substance. For example, when the EC50 value of CCh in the absence of the test substance is 0.2 µM and the EC50 value of CCh in the presence of the test substance is 0.01 µM, the PAM activity is indicated by a 20-fold shift.
Effects on Transmural Electrical Field Stimulation-Induced Contraction of Isolated Rat Bladder TissueAll experimental procedures performed on the animals were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc., which has been awarded Accreditation Status by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. All efforts were made to minimize animal numbers and suffering.
Evaluation of the pharmacological effects of 3g on EFS-induced contraction of isolated rat bladder tissue was conducted as described previously.26) Briefly, a strip of bladder tissue isolated from a Sprague-Dawley (SD) female rat was suspended in an organ bath filled with 10 mL of Krebs–Henseleit solution and bubbled with 95% O2 and 5% CO2, and maintained at 37 °C. After stabilizing at an initial tension of 1 g, contraction was induced twice with 60 mM KCl. The strip was then washed and stabilized, and contraction was induced with EFS at 20 V (stimulation frequency of 8 Hz, pulse width of 0.3 ms, and stimulation time of 10 s). The voltage was adjusted to induce a contractile amplitude that was about 50% of the contractile response at 20 V. After stabilization, 3g (final concentration 3, 10, and 30 µM) was added. Each subsequent concentration of the test substance was cumulatively administered after the contractile response obtained at the lower concentration had stabilized. The effect of 3g was evaluated using the average of the EFS-induced contractions before and after application.
Pharmacokinetics StudyPharmacokinetics characterization of compound 3g was conducted in female SD rats (SD SPF rats Crl: CD(SD), 8 weeks of age). Compound 3g was intravenously and orally administered at 1 mg/kg in a mixture of dimethyl sulfoxide (DMSO)/Cremophor/saline (10/10/10). The animals were fasted prior to administration. After each administration, blood was drawn at the assigned time points up to 24 h, and the concentration of compound 3g in plasma was determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Pharmacokinetics parameters were calculated from the plasma concentrations for each animal by noncompartmental analysis using the pharmacokinetics software Phoenix WinNonlin.
Conformational Energy CalculationThe four possible planar conformations of compounds 2 and 3a as shown in Figs. 2 and 4 respectively were prepared by MOE.32) Each conformation was optimized with the HF/6-31G** model chemistry and the potential energy of the optimized structure was calculated at the MP2/6-31++G** level of theory. All calculations were performed with the Gaussian 09 program package33) and the data were analyzed with the Gaussview 5.0 molecular visualization program.34) The potential energy difference between two conformers was calculated to identify the most stable conformer.
Chemistry1H-NMR spectra were recorded on a JEOL JNM-EX400 spectrometer and were referenced against an internal standard, tetramethylsilane. The abbreviations used for the signal patterns are as follows: s, singlet; br, broad; d, doublet; t, triplet; q, quartet; dd, double doublet; m, multiplet. Mass spectra were recorded on a JEOL LX-2000 mass spectrometer. Elemental analyses were performed using a Yanako MT-5 microanalyzer (C, H, N) and a Yokogawa IC-7000S ion chromatographic analyzer (halogens). Where analyses are indicated by symbols, the analytical results were within ±0.4% of the theoretical values. Electrospray ionization (ESI) positive high-resolution (HR)MS were obtained using a Waters LCT Premier. Column chromatography was performed using Wakogel C-200, Merck silica gel 60, or Fuji Silysia ODS-DM1020T. All reactions were performed using commercially available reagents and solvents without further purification.
4-[3-Fluoro-5-(trifluoromethyl)phenyl]-1,3-thiazol-2-amine (5)To a mixture of 1-[3-fluoro-5-(trifluoromethyl)phenyl]ethanone (4, 78 g) and tetrahydrofuran (625 mL) was added phenyltrimethylammonium tribromide (143 g), followed by stirring at room temperature for 1 h. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The obtained compound and ethanol (625 mL) were mixed, and thiourea (35 g) was added thereto, followed by stirring at 65 to 75 °C for 2 h. The reaction mixture was cooled on ice, and water (625 mL) was added. A 1 M sodium hydroxide (600 mL) solution was added, and the mixture was stirred for 30 min. The solid was collected by filtration, and ethanol (30% aqueous, 600 mL) was added to dissolve the solid at 76 °C. The obtained solution was cooled to room temperature and stirred overnight. The mixture was cooled on ice and stirred for 2 h, and the precipitated solid was collected by filtration to obtain 5 (56.9 g, 57%) as a white solid: 1H-NMR (CDCl3) δ: 5.00 (2H, s), 6.85 (1H, s), 7.20–7.26 (1H, m), 7.64–7.70 (1H, m), 7.82–7.86 (1H, m); ESI-MS m/z 263 (M + H)+.
N-{4-[3-Fluoro-5-(trifluoromethyl)phenyl]-1,3-thiazol-2-yl}acetamide (6)4-[3-Fluoro-5-(trifluoromethyl)phenyl]-1,3-thiazol-2-amine (5, 2.8 g), pyridine (10 mL), and acetic anhydride (4 mL) were mixed, followed by stirring at 60 °C for 1 h. The reaction mixture was cooled to room temperature, water was added, and the resulting solid was collected by filtration. The solid was washed with methanol and collected by filtration to obtain 6 (2.9 g, 88%) as a white solid: 1H-NMR (DMSO-d6) δ: 2.18 (3H, s), 7.62 (1H, d, J = 8.6 Hz), 7.98 (1H, s), 8.04 (1H, d, J = 10.0 Hz), 8.11 (1H, s), 12.32 (1H, s); ESI-MS m/z 305 (M + H)+.
N-(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)acetamide (7)A mixture of N-{4-[3-fluoro-5-(trifluoromethyl)phenyl]-1,3-thiazol-2-yl}acetamide (6, 2.8 g), acetic acid (20 mL), a 36% aqueous formaldehyde solution (3.6 mL), and acetic anhydride (4.4 mL) was stirred at 170 °C for 30 min under microwave irradiation. The reaction mixture was concentrated under reduced pressure, and the obtained solid was washed with methanol and collected by filtration. The obtained solid (1.8 g) was mixed with N-methylpyrrolidone (20 mL), (2R)-2-methylpyrrolidine (608 mg), and N,N-diisopropylethylamine (2.5 mL), and the mixture was stirred at 100 °C for 30 min. The reaction mixture was cooled to room temperature, water was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexaneethyl acetate) to obtain 7 (1.4 g, 37%) as a white solid: 1H-NMR (DMSO-d6) δ: 1.14 (3H, d, J = 6.0 Hz), 1.32–1.44 (1H, m), 1.60–1.72 (2H, m), 1.90–2.00 (1H, m), 2.10–2.20 (1H, m), 2.15 (3H, s), 2.42–2.54 (1H, m), 2.90–3.00 (1H, m), 3.40 (1H, d, J = 14.3 Hz), 4.20 (1H, d, J = 14.3 Hz), 7.66 (1H, d, J = 8.6 Hz), 7.99 (1H, d, J = 10.1 Hz), 8.02 (1H, s), 12.18 (1H, s); ESI-MS m/z 402 (M + H)+.
4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8)A mixture of N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)acetamide (7, 1.4 g), ethanol (10 mL), and a 6 M aqueous sodium hydroxide solution (5 mL) was stirred at 120 °C for 15 min under microwave irradiation. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexaneethyl acetate) to obtain 8 (1.0 g, 83%) as a yellow oil: 1H-NMR (DMSO-d6) δ: 1.11 (3H, d, J = 6.1 Hz), 1.31–1.42 (1H, m), 1.56–1.74 (2H, m), 1.86–2.00 (1H, m), 2.11 (1H, dd, J = 17.5, 8.8 Hz), 2.35–2.46 (1H, m), 2.95–3.03 (1H, m), 3.20 (1H, d, J = 13.9 Hz), 4.02 (1H, d, J = 13.9 Hz), 7.01 (2H, s), 7.58 (1H, d, J = 8.6 Hz), 7.93 (1H, d, J = 9.9 Hz), 7.96 (1H, s); ESI-MS m/z 360 (M + H)+.
6-Chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)pyrimidin-4-amine (9a)Under an argon gas flow, a 60% oil dispersion of sodium hydride (34 mg) was added to a solution of 4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8, 300 mg) and 4,6-dichloropyrimidine (125 mg) in tetrahydrofuran (6.0 mL) while cooling on ice/methanol, and the mixture was stirred at room temperature for 1.5 h. A 60% oil dispersion of sodium hydride (17 mg) was added, and the reaction mixture was stirred at room temperature for 5 h. Ice-cooled water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and a saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain 9a (234 mg, 59%) as a pale yellow solid: 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 6.1 Hz), 1.33–1.45 (1H, m), 1.60–1.72 (2H, m), 1.90–2.02 (1H, m), 2.16 (1H, dd, J = 17.4, 8.7 Hz), 2.42–2.54 (1H, m), 2.94–3.04 (1H, m), 3.42 (1H, d, J = 14.3 Hz), 4.23 (1H, d, J = 14.3 Hz), 7.08 (1H, s), 7.68 (1H, d, J = 8.6 Hz), 8.02 (1H, d, J = 9.8 Hz), 8.04 (1H, s), 8.75 (1H, d, J = 0.9 Hz), 12.09 (1H, s); ESI-MS m/z 472, 474 (M + H)+.
4-Chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)pyrimidin-2-amine (9b) 2-Chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)pyrimidin-4-amine (9c)Under an argon gas flow, a 60% oil dispersion of sodium hydride (115 mg) was added to a solution of 4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8, 500 mg) and 2,4-dichloropyrimidine (210 mg) in N-methylpyrrolidone (10 mL) while cooling on ice/methanol, and the mixture was stirred at room temperature for 1 h. 2,4-Dichloropyrimidine (50 mg) and a 60% oil dispersion of sodium hydride (30 mg) were added, and the reaction mixture was stirred at room temperature for 30 min. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and a saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane–ethyl acetate) to obtain 9b (71 mg, 11%) and 9c (534 mg, 81%) both as pale yellow solids.
9b: 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 6.1 Hz), 1.33–1.45 (1H, m), 1.60–1.72 (2H, m), 1.90–2.02 (1H, m), 2.16 (1H, dd, J = 17.5, 8.7 Hz), 2.41–2.56 (1H, m), 2.94–3.03 (1H, m), 3.39 (1H, d, J = 14.1 Hz), 4.19 (1H, d, J = 14.1 Hz), 7.20 (1H, d, J = 5.2 Hz), 7.67 (1H, d, J = 8.5 Hz), 8.03 (1H, d, J = 9.8 Hz), 8.07 (1H, s), 8.64 (1H, d, J = 5.2 Hz), 12.11 (1H, s); ESI-MS m/z 472, 474 (M + H)+.
9c: 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 6.0 Hz), 1.33–1.46 (1H, m), 1.60–1.72 (2H, m), 1.89–2.03 (1H, m), 2.18 (1H, dd, J = 17.4, 8.7 Hz), 2.43–2.55 (1H, m), 2.92–3.03 (1H, m), 3.43 (1H, d, J = 14.1 Hz), 4.20 (1H, d, J = 14.1 Hz), 7.04 (1H, d, J = 5.8 Hz), 7.68 (1H, d, J = 8.5 Hz), 8.03–8.10 (2H, m), 8.37 (1H, d, J = 5.8 Hz), 12.23 (1H, s); ESI-MS m/z 472, 474 (M + H)+.
The following compounds (9d–g) were prepared using a procedure similar to that described for 9a.
6-Chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)-2-methylpyrimidin-4-amine (9d)White powder (yield 67%): 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 6.0 Hz), 1.33–1.46 (1H, m), 1.60–1.72 (2H, m), 1.90–2.03 (1H, m), 2.19 (1H, dd, J = 17.5, 8.8 Hz), 2.43–2.53 (1H, m), 2.56 (3H, s), 2.92–3.02 (1H, m), 3.40 (1H, d, J = 14.0 Hz), 4.18 (1H, d, J = 14.0 Hz), 6.90 (1H, s), 7.67 (1H, d, J = 8.6 Hz), 8.04–8.13 (2H, m), 11.99 (1H, s); atmospheric pressure chemical ionization (APCI)/ESI-MS m/z 486 (M + H)+.
6-Chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)-5-methylpyrimidin-4-amine (9e)Pale yellow foam (yield 80%): 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 6.1 Hz), 1.33–1.46 (1H, m), 1.59–1.73 (2H, m), 1.90–2.03 (1H, m), 2.16 (1H, dd, J = 17.5, 8.7 Hz), 2.39 (3H, s), 2.41–2.54 (1H, m), 2.92–3.04 (1H, m), 3.40 (1H, d, J = 14.1 Hz), 4.21 (1H, d, J = 14.1 Hz), 7.67 (1H, d, J = 8.6 Hz), 8.08 (1H, d, J = 9.9 Hz), 8.11 (1H, s), 8.60 (1H, s), 11.28 (1H, s); APCI/ESI-MS m/z 486 (M + H)+.
6-Chloro-5-fluoro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)pyrimidin-4-amine (9f)Yellow solid (yield 108%): 1H-NMR (DMSO-d6) δ: 1.16 (3H, d, J = 6.1 Hz), 1.33–1.46 (1H, m), 1.58–1.73 (2H, m), 1.88–2.02 (1H, m), 2.10–2.24 (1H, m), 2.40–2.54 (1H, m), 2.94–3.05 (1H, m), 3.44 (1H, d, J = 14.0 Hz), 4.24 (1H, d, J = 14.0 Hz), 7.70 (1H, d, J = 8.6 Hz), 8.03 (1H, d, J = 9.8 Hz), 8.07 (1H, s), 8.57 (1H, s), 12.46 (1H, s); APCI/ESI-MS m/z 490 (M + H)+.
6-Chloro-5-fluoro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)-2-methylpyrimidin-4-amine (9g)Yellow solid (yield 94%): 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 5.8 Hz), 1.32–1.46 (1H, m), 1.58–1.72 (2H, m), 1.88–2.02 (1H, m), 2.13–2.27 (1H, m), 2.40–2.54 (1H, m), 2.55 (3H, s), 2.92–3.02 (1H, m), 3.41 (1H, d, J = 14.0 Hz), 4.18 (1H, d, J = 14.0 Hz), 7.67 (1H, d, J = 8.5 Hz), 8.05–8.15 (2H, m), 12.34 (1H, s); APCI/ESI-MS m/z 504 (M + H)+.
3-(4-{6-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyrimidin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3a)To a solution of 6-chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)pyrimidin-4-amine (9a, 230 mg) in N-methylpyrrolidone (3.0 mL) were added ethyl 3-(piperazin-1-yl)propanoate dihydrochloride (380 mg) and N,N-diisopropylethylamine (1.0 mL), followed by stirring at 80 °C for 3 h. The reaction mixture was cooled to room temperature, and water and ethyl acetate were added thereto, followed by extraction with ethyl acetate. The organic layer was washed with water and a saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexanesethyl acetate) to obtain the intermediate (196 mg, 65%) as a yellow syrup. A 1 M aqueous sodium hydroxide solution (1.50 mL) was added to a solution of the obtained intermediate (190 mg) in ethanol (3.0 mL) and tetrahydrofuran (3.0 mL), and the mixture was stirred at 50 °C for 30 min. The reaction mixture was cooled to room temperature, and water and a 1 M aqueous hydrochloric acid solution (3.00 mL) were added, and the mixture was extracted with chloroform/isopropanol, and the organic layer was dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was dissolved in tetrahydrofuran (20 mL), and a 4 M hydrogen chloride/1,4-dioxane solution (2.00 mL) was added thereto. The mixture was concentrated under reduced pressure, and washed with diethyl ether/acetonitrile/water to obtain 3a (88 mg, 41%) as a pale yellow solid: 1H-NMR (DMSO-d6) δ: 1.38 (3H, d, J = 6.5 Hz), 1.59–1.75 (1H, m), 1.82–1.98 (2H, m), 2.11–2.23 (1H, m), 2.89 (2H, t, J = 7.4 Hz), 3.00–3.20 (3H, m), 3.28–3.48 (5H, m), 3.48–3.64 (3H, m), 3.60–4.50 (2H, m), 4.27–4.40 (2H, m), 4.43 (1H, dd, J = 15.0, 7.8 Hz), 4.73 (1H, dd, J = 15.0, 2.2 Hz), 6.33 (1H, s), 7.79 (1H, d, J = 8.7 Hz), 7.88–7.98 (2H, m), 8.47 (1H, d, J = 0.7 Hz), 10.67 (1H, br s), 11.42 (1H, br s), 11.85 (1H, br s); ESI-MS m/z 594 (M + H)+; HRMS (M + H)+ Calcd for C27H32O2N7F4S 594.2269. Found 594.2271. Anal. Calcd for C27H31F4N7O2S·2.9HCl·3H2O·0.1C4H10O: C, 43.25; H, 5.42; N, 12.89; S, 4.21; Cl, 13.51; F, 9.99. Found: C, 43.26; H, 5.53; N, 12.66; S, 4.25; Cl, 13.43; F, 10.00.
The following compounds (3b and 3c) were prepared using a procedure similar to that described for 3a.
3-(4-{2-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyrimidin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3b)Pale yellow solid (yield 19%): 1H-NMR (DMSO-d6) δ: 1.32–1.42 (3H, m), 1.60–1.73 (1H, m), 1.80–1.95 (2H, m), 2.06–2.22 (1H, m), 2.89 (2H, t, J = 7.5 Hz), 2.95–3.09 (1H, m), 3.09–3.26 (2H, m), 3.30–3.76 (8H, m), 3.30–4.90 (3H, m), 4.30–4.42 (1H, m), 4.50–4.90 (3H, m), 6.69 (1H, d, J = 6.6 Hz), 7.80–7.96 (1H, m), 7.85 (1H, d, J = 8.2 Hz), 7.90 (1H, s), 8.19 (1H, d, J = 6.6 Hz), 11.37 (2H, br s); ESI-MS m/z 594 (M + H)+.
3-(4-{4-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyrimidin-2-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3c)White solid (yield 57%): 1H-NMR (DMSO-d6) δ: 1.39 (3H, d, J = 6.5 Hz), 1.61–1.75 (1H, m), 1.80–1.96 (2H, m), 2.07–2.20 (1H, m), 2.87 (2H, t, J = 7.4 Hz), 2.95–3.08 (1H, m), 3.08–3.24 (2H, m), 3.30–3.74 (8H, m), 3.70–5.10 (2H, m), 4.45 (1H, dd, J = 14.9, 7.3 Hz), 4.77 (1H, dd, J = 14.9, 3.3 Hz), 4.77–4.96 (2H, m), 6.45 (1H, d, J = 5.8 Hz), 7.81 (1H, d, J = 8.8 Hz), 7.85–7.94 (2H, m), 8.18 (1H, d, J = 5.8 Hz), 11.18 (1H, br s), 11.54 (1H, br s), 12.22 (1H, br s); ESI-MS m/z 594 (M + H)+; HRMS (M + H)+ Calcd for C27H32O2N7F4S 594.2269. Found 594.2275. Anal. Calcd for C27H31F4N7O2S·3HCl·2H2O: C, 43.88; H, 5.18; N, 13.27; S, 4.34; Cl, 14.39; F, 10.28. Found: C, 43.70; H, 5.41; N, 13.09; S, 4.35; Cl, 14.32; F, 10.42.
3-(4-{6-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-2-methylpyrimidin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3d)6-Chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)-2-methylpyrimidin-4-amine (9d, 453 mg), N,N-diisopropylethylamine (2.00 mL), ethyl 3-(piperazin-1-yl)propanoate dihydrochloride (750 mg), and N-methylpyrrolidone (10 mL) were mixed, followed by stirring at 80 °C for 1 h. N,N-Diisopropylethylamine (2.00 mL) and ethyl 3-(piperazin-1-yl)propanoate dihydrochloride (750 mg) were added, and the mixture was stirred at 80 °C overnight. The reaction mixture was cooled to room temperature, and water and ethyl acetate were added thereto, followed by extraction with ethyl acetate. The organic layer was washed with water and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexanesethyl acetate).
To a solution of the obtained residue in ethanol (5.0 mL) and tetrahydrofuran (5.0 mL) was added a 1 M aqueous sodium hydroxide solution (5.00 mL), followed by stirring at 60 °C for 1 h. The reaction mixture was cooled to room temperature, and a 1 M aqueous hydrochloric acid solution (5.00 mL) was added thereto, followed by extraction with chloroform/methanol. The organic layer was dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (chloroform-methanol).
The obtained residue was mixed with ethyl acetate, a 4 M hydrogen chloride/1,4-dioxane solution (700 µL) was added, and the mixture was concentrated under reduced pressure, and washed with ethyl acetate to obtain 3d (525 mg, 79%) as a white powder: 1H-NMR (DMSO-d6) δ: 1.38 (3H, d, J = 6.4 Hz), 1.59–1.75 (1H, m), 1.82–1.98 (2H, m), 2.10–2.23 (1H, m), 2.48 (3H, s), 2.88 (2H, t, J = 7.3 Hz), 2.98–3.18 (3H, m), 3.26–3.47 (5H, m), 3.47–3.63 (3H, m), 3.50–4.50 (2H, m), 4.25–4.40 (2H, m), 4.43 (1H, dd, J = 14.9, 7.7 Hz), 4.74 (1H, dd, J = 14.9, 2.4 Hz), 6.14 (1H, s), 7.79 (1H, d, J = 8.7 Hz), 7.87–7.97 (2H, m), 10.65 (1H, br s), 11.26 (1H, br s), 11.75 (1H, br s); ESI-MS m/z 608 (M + H)+; HRMS (M + H)+ Calcd for C28H34O2N7F4S 608.2425. Found 608.2419. Anal. Calcd for C28H33F4N7O2S·2.8HCl·3H2O: C, 44.03; H, 5.52; N, 12.84; S, 4.20; Cl, 13.00; F, 9.95. Found: C, 44.34; H, 5.58; N, 12.63; S, 4.19; Cl, 13.05; F, 9.96.
The following compound (3e) was prepared using a procedure similar to that described for 3d.
3-(4-{6-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-5-methylpyrimidin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3e)Off-white powder (yield 9.2%): 1H-NMR (DMSO-d6) δ: 1.37 (3H, d, J = 6.4 Hz), 1.58–1.75 (1H, m), 1.83–1.97 (2H, m), 2.11–2.24 (1H, m), 2.19 (3H, s), 2.89 (2H, t, J = 7.4 Hz), 2.97–3.25 (3H, m), 3.29–3.47 (5H, m), 3.47–3.59 (3H, m), 3.60–4.80 (2H, m), 3.74–3.88 (2H, m), 4.44 (1H, dd, J = 15.1, 7.9 Hz), 4.75 (1H, dd, J = 15.1, 2.3 Hz), 7.80 (1H, d, J = 8.7 Hz), 7.91–8.02 (2H, m), 8.51 (1H, s), 10.60 (1H, br s), 11.16–11.38 (2H, m); ESI-MS m/z 608 (M + H)+; HRMS (M + H)+ Calcd for C28H34O2N7F4S 608.2425. Found 608.2423. Anal. Calcd for C28H33F4N7O2S·2.8HCl·3H2O: C, 44.03; H, 5.52; N, 12.84; S, 4.20; Cl, 13.00; F, 9.95. Found: C, 44.15; H, 5.59; N, 12.47; S, 4.15; Cl, 13.09; F, 9.86.
The following compounds (3f and 3g) were prepared using a procedure similar to that described for 3a.
3-(4-{5-Fluoro-6-[(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyrimidin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3f)White powder (yield 34%): 1H-NMR (DMSO-d6) δ: 1.38 (3H, d, J = 6.5 Hz), 1.59–1.76 (1H, m), 1.82–1.99 (2H, m), 2.10–2.25 (1H, m), 2.88 (2H, t, J = 7.3 Hz), 3.07–3.24 (3H, m), 3.26–3.36 (2H, m), 3.36–3.48 (1H, m), 3.48–3.66 (5H, m), 3.70–4.70 (2H, m), 4.36–4.54 (3H, m), 4.75 (1H, dd, J = 14.8, 2.0 Hz), 7.80 (1H, d, J = 8.7 Hz), 7.91–8.02 (2H, m), 8.31 (1H, d, J = 1.4 Hz), 10.73 (1H, br s), 11.45 (1H, br s), 12.01 (1H, br s); ESI-MS m/z 612 (M + H)+; HRMS (M + H)+ Calcd for C27H31O2N7F5S 612.2175. Found 612.2175. Anal. Calcd for C27H30F5N7O2S·3HCl·2H2O: C, 42.84; H, 4.93; N, 12.95; S, 4.24; Cl, 14.05; F, 12.55. Found: C, 42.77; H, 4.88; N, 12.91; S, 4.22; Cl, 14.09; F, 12.69.
3-(4-{5-Fluoro-6-[(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-2-methylpyrimidin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3g)White powder (yield 31%): 1H-NMR (DMSO-d6) δ: 1.39 (3H, d, J = 6.5 Hz), 1.61–1.75 (1H, m), 1.84–1.98 (2H, m), 2.10–2.23 (1H, m), 2.48 (3H, s), 2.88 (2H, t, J = 7.3 Hz), 3.05–3.21 (3H, m), 3.26–3.35 (2H, m), 3.35–3.47 (1H, m), 3.46–3.62 (5H, m), 3.90–4.90 (2H, m), 4.35–4.50 (3H, m), 4.74 (1H, dd, J = 14.9, 2.3 Hz), 7.79 (1H, d, J = 8.7 Hz), 7.89–7.99 (2H, m), 10.91 (1H, br s), 11.44 (1H, br s), 11.90 (1H, br s); ESI-MS m/z 626 (M + H)+; HRMS (M + H)+ Calcd for C28H33O2N7F5S 626.2331. Found 626.2330. Anal. Calcd for C28H32F5N7O2S·3HCl·1.5H2O: C, 44.13; H, 5.03; N, 12.87; S, 4.21; Cl, 13.96; F, 12.47. Found: C, 44.00; H, 5.01; N, 12.76; S, 4.19; Cl, 13.95; F, 12.49.
Ethyl 3-[4-(4-Chloro-1,3,5-triazin-2-yl)piperazin-1-yl]propanoate (11h)To a solution of 2,4-dichloro-1,3,5-triazine (10h, 1.00 g) in N-methylpyrrolidone (20 mL) was added dipotassium carbonate (2.77 g), and ethyl 3-(piperazin-1-yl)propanoate dihydrochloride (1.73 g) while cooling on ice/methanol, followed by stirring at the same temperature for 1 h. The reaction mixture was warmed to room temperature and stirred for 1.5 h. Water and ethyl acetate were added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with a saturated aqueous sodium chloride solution and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain 11h (1.32 g, 66%) as a colorless oil: 1H-NMR (DMSO-d6) δ: 1.19 (3H, d, J = 7.1 Hz), 2.42–2.52 (6H, m), 2.62 (2H, t, J = 6.9 Hz), 3.67–3.74 (2H, m), 3.74–3.82 (2H, m), 4.07 (2H, q, J = 7.1 Hz), 8.47 (1H, s); ESI-MS m/z 300, 302 (M + H)+.
Ethyl 3-[4-(6-Chloropyridazin-3-yl)piperazin-1-yl]propanoate (11i)3,6-Dichloropyridazine (10i, 1.50 g), ethyl 3-(piperazin-1-yl)propanoate dihydrochloride (5.00 g), dipotassium carbonate (8.50 g), and N-methylpyrrolidone (30 mL) were mixed, followed by stirring at 80 °C overnight. The reaction mixture was cooled to room temperature, water was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and a saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The insoluble materials were then separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (chloroform-ethyl acetate), and washed with hexanes/diethyl ether to obtain 11i (2.04 g, 68%) as a light yellow solid: 1H-NMR (DMSO-d6) δ: 1.19 (3H, d, J = 7.1 Hz), 2.46–2.60 (6H, m), 2.62–2.74 (2H, m), 3.51–3.64 (4H, m), 4.07 (2H, q, J = 7.1 Hz), 7.38 (1H, d, J = 9.7 Hz), 7.52 (1H, d, J = 9.7 Hz); ESI-MS m/z 299 (M + H)+.
The following compounds (11j and 11k–m) were prepared using a procedure similar to that described for 11i.
Ethyl 3-[4-(6-Chloropyrazin-2-yl)piperazin-1-yl]propanoate (11j)Colorless oil (yield 69%): 1H-NMR (DMSO-d6) δ: 1.19 (3H, d, J = 7.1 Hz), 2.44–2.53 (6H, m), 2.62 (2H, t, J = 6.8 Hz), 3.51–3.58 (4H, m), 4.07 (2H, q, J = 7.1 Hz), 7.84 (1H, s), 8.27 (1H, s); ESI-MS m/z 299 (M + H)+.
Ethyl 3-[4-(6-Bromopyridin-2-yl)piperazin-1-yl]propanoate (11k)Colorless oil (yield 87%): 1H-NMR (DMSO-d6) δ: 1.19 (3H, d, J = 7.1 Hz), 2.42–2.48 (4H, m), 2.48–2.53 (2H, m), 2.57–2.64 (2H, m), 3.40–3.48 (4H, m), 4.07 (2H, q, J = 7.1 Hz), 6.64 (1H, d, J = 7.3 Hz), 6.76 (1H, d, J = 8.4 Hz), 7.53 (1H, dd, J = 8.4, 7.3 Hz); ESI-MS m/z 342, 344 (M + H)+.
Ethyl 3-[4-(4-Chloropyridin-2-yl)piperazin-1-yl]propanoate (11l)Colorless oil (yield 17%): 1H-NMR (DMSO-d6) δ: 1.18 (3H, d, J = 7.1 Hz), 2.41–2.46 (4H, m), 2.46–2.52 (2H, m), 2.57–2.64 (2H, m), 3.44–3.51 (4H, m), 4.07 (2H, q, J = 7.1 Hz), 6.69 (1H, dd, J = 5.3, 1.6 Hz), 6.90 (1H, d, J = 1.6 Hz), 8.05 (1H, d, J = 5.3 Hz); ESI-MS m/z 298, 300 (M + H)+.
Ethyl 3-[4-(2-Chloropyridin-4-yl)piperazin-1-yl]propanoate (11m)Colorless oil (yield 72%): 1H-NMR (DMSO-d6) δ: 1.18 (3H, d, J = 7.1 Hz), 2.42–2.52 (6H, m), 2.57–2.64 (2H, m), 3.30–3.36 (4H, m), 4.07 (2H, q, J = 7.1 Hz), 6.80–6.86 (2H, m), 7.91–7.96 (1H, m); ESI-MS m/z 298, 300 (M + H)+.
Ethyl 3-[4-(3-Bromophenyl)piperazin-1-yl]propanoate (11n)1-(3-Bromophenyl)piperazine (12, 2.00 g), ethyl acrylate (2.70 mL), and ethanol (6.0 mL) were mixed, followed by heating to reflux for 6 h. The mixture was cooled to room temperature and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain 11n (3.14 g, 111%) as a colorless syrup: 1H-NMR (DMSO-d6) δ: 1.18 (3H, d, J = 7.1 Hz), 2.45–2.54 (6H, m), 2.61 (2H, t, J = 7.4 Hz), 3.08–3.18 (4H, m), 4.07 (2H, q, J = 7.1 Hz), 6.87–6.95 (2H, m), 7.03–7.07 (1H, m), 7.13 (1H, t, d, J = 8.1 Hz); APCI/ESI-MS m/z 341,343 (M + H)+.
3-(4-{4-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-1,3,5-triazin-2-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3h)Under an argon gas flow, a 60% oil dispersion of sodium hydride (110 mg) was added to a solution of 4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8, 335 mg) and ethyl 3-[4-(4-chloro-1,3,5-triazin-2-yl)piperazin-1-yl]propanoate (11h, 335 mg) in tetrahydrofuran (7.0 mL) while cooling on ice/methanol, and the mixture was stirred at 0 °C for 1.5 h. Water was added to the reaction mixture and the mixture was extracted with ethyl acetate. The organic layer was washed with a saturated aqueous sodium chloride solution and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (hexane-ethyl acetate) to obtain the intermediate (396 mg, 68%) as a white solid.
To a suspension of the obtained intermediate (374 mg) in ethanol (5.0 mL) and tetrahydrofuran (5.0 mL) was added a 1 M aqueous sodium hydroxide solution (4.00 mL), followed by stirring at 50 °C for 30 min. The reaction mixture was cooled to room temperature, water (20 mL) and a 1 M aqueous hydrochloric acid solution (4.00 mL) were added, and the mixture was extracted with chloroform/isopropanol, and the organic layer was dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure.
The residue was dissolved in tetrahydrofuran (10 mL), and a 4 M hydrogen chloride/1,4-dioxane solution (2.0 mL) was added thereto. The mixture was concentrated under reduced pressure, and washed with acetonitrile and water to obtain 3h (324 mg, 77%) as a pale yellow solid: 1H-NMR (DMSO-d6) δ: 1.39 (3H, s), 1.60–1.76 (1H, m), 1.78–1.98 (2H, m), 2.04–2.22 (1H, m), 2.80–2.96 (2H, m), 2.96–3.26 (3H, m), 3.26–3.85 (8H, m), 4.00–5.50 (2H, m), 4.34–4.51 (1H, m), 4.64–4.85 (2H, m), 4.89–5.13 (1H, m), 7.81 (1H, d, J = 8.7 Hz), 7.82–7.95 (2H, m), 8.48 (1H, s), 11.62 (1H, br s), 11.78 (1H, br s), 12.16 (1H, br s); ESI-MS m/z 595 (M + H)+; HRMS (M + H)+ Calcd for C26H31O2N8F4S 595.2221. Found 595.2218. Anal. Calcd for C26H30F4N8O2S·2.8HCl·1.75H2O: C, 42.88; H, 5.02; N, 15.39; S, 4.40; Cl, 13.63; F, 10.44. Found: C, 42.82; H, 5.25; N, 15.25; S, 4.26; Cl, 13.89; F, 10.52.
3-(4-{6-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyridazin-3-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3i)4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8, 500 mg), ethyl 3-[4-(6-chloropyridazin-3-yl)piperazin-1-yl]propanoate (11i, 520 mg), tris(dibenzylideneacetone)dipalladium(0) (91 mg), di-tert-butyl(2′,4′,6′-triisopropylbiphenyl-2-yl)phosphine (96 mg), cesium carbonate (970 mg), toluene (10 mL), and water (1.0 mL) were mixed, followed by stirring at 80 °C for 7 h. Tris(dibenzylideneacetone)dipalladium(0) (100 mg), di-tert-butyl(2′,4′,6′-triisopropylbiphenyl-2-yl)phosphine (100 mg), and cesium carbonate (1.00 g) were added, and the mixture was stirred at 100 °C for 6 h. The reaction mixture was cooled to room temperature, diluted with water and ethyl acetate, and filtered through a pad of Celite™. The filtrate was separated, and the organic layer was concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (hexanes-ethyl acetate) and then by silica gel column chromatography (chloroform-ethyl acetate) to obtain the intermediate (172 mg, 20%) as a yellow oil.
To a solution of the obtained intermediate (172 mg) in ethanol (3.0 mL) and tetrahydrofuran (3.0 mL) was added a 1 M aqueous sodium hydroxide solution (1.60 mL), followed by stirring at 80 °C for 2 h. The reaction mixture was cooled to room temperature, and a 1 M aqueous hydrochloric acid solution (1.60 mL) was added thereto, followed by extraction with chloroform/methanol. The organic layer was dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was mixed with ethyl acetate, and a 4 M hydrogen chloride/1,4-dioxane solution (250 µL) was added thereto. The mixture was concentrated under reduced pressure, and washed with ethyl acetate to obtain 3i (97 mg, 50%) as a yellow powder: 1H-NMR (DMSO-d6) δ: 1.39 (3H, d, J = 6.4 Hz), 1.60–1.76 (1H, m), 1.84–1.98 (2H, m), 2.11–2.24 (1H, m), 2.92 (2H, t, J = 7.4 Hz), 3.06–3.25 (3H, m), 3.28–3.66 (8H, m), 4.00–5.50 (2H, m), 4.27–4.40 (2H, m), 4.45 (1H, dd, J = 15.0, 7.7 Hz), 4.74 (1H, dd, J = 15.0, 1.9 Hz), 7.40 (1H, d, J = 9.7 Hz), 7.66 (1H, d, J = 9.7 Hz), 7.79 (1H, d, J = 8.6 Hz), 7.90–8.00 (2H, m), 10.78 (1H, br s), 11.49 (1H, br s), 11.85 (1H, br s); ESI-MS m/z 594 (M + H)+; HRMS (M + H)+ Calcd for C27H32O2N7F4S 594.2269. Found 594.2276. Anal. Calcd for C27H31F4N7O2S·3HCl·3H2O: C, 42.83; H, 5.33; N, 12.95; S, 4.24; Cl, 14.05; F, 10.04. Found: C, 43.42; H, 5.41; N, 12.05; S, 4.02; Cl, 13.58; F, 9.63.
The following compounds (3j, and 3k–n) were prepared using a procedure similar to that described for 3i.
3-(4-{6-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyrazin-2-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3j)Yellow solid (yield 6.5%): 1H-NMR (DMSO-d6) δ: 1.39 (3H, d, J = 6.4 Hz), 1.61–1.74 (1H, m), 1.80–1.95 (2H, m), 2.06–2.20 (1H, m), 2.88 (2H, t, J = 7.4 Hz), 2.97–3.10 (1H, m), 3.10–3.26 (2H, m), 3.30–3.56 (6H, m), 3.40–4.20 (2H, m), 3.62–3.72 (2H, m), 4.44 (1H, dd, J = 14.8, 7.4 Hz), 4.52–4.64 (2H, m), 4.75 (1H, dd, J = 14.9, 2.9 Hz), 7.77–7.84 (2H, m), 7.85–7.92 (2H, m), 7.95 (1H, s), 11.07 (1H, br s), 11.31 (1H, br s), 11.90 (1H, br s); ESI-MS m/z 594 (M + H)+; HRMS (M + H)+ Calcd for C27H32O2N7F4S 594.2269. Found 594.2273. Anal. Calcd for C27H31F4N7O2S·2.6HCl·3.5H2O: C, 43.15; H, 5.45; N, 13.05; S, 4.27; Cl, 12.27; F, 10.11. Found: C, 43.20; H, 5.40; N, 12.65; S, 4.21; Cl, 12.55; F, 9.83.
3-(4-{6-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyridin-2-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3k)White solid (yield 33%): 1H-NMR (DMSO-d6) δ: 1.40 (3H, d, J = 6.5 Hz), 1.62–1.76 (1H, m), 1.81–1.99 (2H, m), 2.08–2.22 (1H, m), 2.91 (2H, t, J = 8.2 Hz), 3.02–3.23 (3H, m), 3.29–3.56 (6H, m), 3.57–3.73 (2H, m), 4.20–6.00 (2H, m), 4.43 (1H, dd, J = 15.0, 7.3 Hz), 4.43–4.59 (2H, m), 4.71 (1H, dd, J = 15.0, 3.0 Hz), 6.45 (1H, d, J = 7.8 Hz), 6.50 (1H, d, J = 8.3 Hz), 7.54–7.60 (1H, m), 7.78 (1H, d, J = 8.7 Hz), 7.86–7.95 (2H, m), 11.43 (2H, br s), 11.53 (1H, br s); ESI-MS m/z 593 (M + H)+; HRMS (M + H)+ Calcd for C28H33O2N6F4S 593.2316. Found 593.2319. Anal. Calcd for C28H32F4N6O2S·2.5HCl·2H2O: C, 46.72; H, 5.39; N, 11.67 S, 4.45 Cl, 12.31; F, 10.56. Found: C, 46.90; H, 5.51; N, 11.40; S, 4.34; Cl, 12.51; F, 10.48.
3-(4-{4-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyridin-2-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3l)Light brown solid (yield 78%): 1H-NMR (DMSO-d6) δ: 1.41 (3H, d, J = 6.4 Hz), 1.61–1.76 (1H, m), 1.82–1.98 (2H, m), 2.10–2.23 (1H, m), 2.88 (2H, t, J = 7.4 Hz), 3.00–4.50 (12H, m), 3.01–3.15 (1H, m), 4.15–4.40 (2H, m), 4.46 (1H, dd, J = 14.7, 7.1 Hz), 4.78 (1H, d, J = 14.7 Hz), 7.13–7.27 (1H, m), 7.76–7.89 (2H, m), 7.97–8.08 (3H, m), 11.23 (1H, br s), 11.53 (1H, br s), 12.68 (1H, br s); ESI-MS m/z 593 (M + H)+; HRMS (M + H)+ Calcd for C28H33O2N6F4S 593.2316. Found 593.2323. Anal. Calcd for C28H32F4N6O2S·3HCl·2H2O: C, 45.57; H, 5.33; N, 11.39; S, 4.34 Cl, 14.41; F, 10.30. Found: C, 45.33; H, 5.51; N, 11.37; S, 4.36; Cl, 14.58; F, 10.22.
3-(4-{2-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyridin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3m)White solid (yield 26%): 1H-NMR (DMSO-d6) δ: 1.38 (3H, d, J = 6.4 Hz), 1.60–1.75 (1H, m), 1.83–1.98 (2H, m), 2.11–2.23 (1H, m), 2.90 (2H, t, J = 7.4 Hz), 3.04–3.27 (3H, m), 3.30–3.70 (8H, m), 3.80–5.10 (3H, m), 3.95–4.18 (2H, m), 4.41 (1H, dd, J = 14.9, 7.7 Hz), 4.71 (1H, dd, J = 14.9, 1.8 Hz), 6.56–7.00 (2H, m), 7.80 (1H, d, J = 8.6 Hz), 7.92–8.04 (2H, m), 8.07 (1H, d, J = 6.8 Hz), 10.90 (1H, br s), 11.59 (1H, br s); ESI-MS m/z 593 (M + H)+; HRMS (M + H)+ Calcd for C28H33O2N6F4S 593.2316. Found 593.2314. Anal. Calcd for C28H32F4N6O2S·3.1HCl·2.5H2O: C, 44.80; H, 5.38; N, 11.19; S, 4.27; Cl, 14.64; F, 10.12. Found: C, 44.67; H, 5.53; N, 11.32; S, 4.35; Cl, 14.45; F, 10.24.
3-(4-{3-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]phenyl}piperazin-1-yl)propanoic Acid Trihydrochloride (3n)Pale yellow powder (yield 33%): 1H-NMR (DMSO-d6) δ: 1.38 (3H, d, J = 6.4 Hz), 1.60–1.73 (1H, m), 1.82–1.96 (2H, m), 2.10–2.22 (1H, m), 2.88 (2H, t, J = 7.4 Hz), 3.00–3.25 (5H, m), 3.30–3.45 (3H, m), 3.45–3.60 (3H, m), 3.60–4.30 (2H, m), 3.70–3.84 (2H, m), 4.35 (1H, dd, J = 15.0, 7.7 Hz), 4.69 (1H, dd, J = 15.0, 2.4 Hz), 6.68 (1H, dd, J = 8.1, 1.9 Hz), 7.08 (1H, dd, J = 8.1, 1.4 Hz), 7.22 (1H, t, J = 8.1 Hz), 7.35–7.42 (1H, m), 7.79 (1H, d, J = 8.6 Hz), 7.87–7.95 (2H, m), 10.73 (1H, br s), 10.87 (1H, br s), 11.03 (1H, br s); ESI-MS m/z 592 (M + H)+; HRMS (M + H)+ Calcd for C29H34O2N5F4S 592.2364. Found 592.2360. Anal. Calcd for C29H33F4N5O2S·2.5HCl·3H2O: C, 47.27; H, 5.68; N, 9.50; S, 4.35; Cl, 12.03; F, 10.31. Found: C, 47.97; H, 5.59; N, 9.29; S, 4.35; Cl, 11.65; F, 10.15.
tert-Butyl 4-(2-Chloro-6-methylpyridin-4-yl)piperazine-1-carboxylate (14)4-Bromo-2-chloro-6-methylpyridine (13, 1.70 g), tert-butyl piperazine-1-carboxylate (1.30 g), tris(dibenzylideneacetone)dipalladium(0) (150 mg), (9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (285 mg), sodium 2-methylpropan-2-olate (1.00 g), and toluene (30 mL) were mixed, followed by stirring at 100 °C for 2 h. The reaction mixture was cooled to room temperature, diluted with water and ethyl acetate, and filtered through a pad of Celite™. The filtrate was separated, and the organic layer was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexanes-ethyl acetate) to obtain 14 (1.89 g, 87%) as a yellow solid: 1H-NMR (DMSO-d6) δ: 1.42 (9H, s), 2.29 (3H, s), 3.33–3.38 (4H, m), 3.38–3.44 (4H, m), 6.68 (1H, d, J = 2.0 Hz), 6.71 (1H, d, J = 2.0 Hz); ESI-MS m/z 312, 314 (M + H)+.
tert-Butyl 4-{2-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-6-methylpyridin-4-yl}piperazine-1-carboxylate (15)tert-Butyl 4-(2-Chloro-6-methylpyridin-4-yl)piperazine-1-carboxylate (14, 180 mg), 4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8, 179 mg), tris(dibenzylideneacetone)dipalladium(0) (230 mg), 1,1′-binaphthalene-2,2′-diylbis(diphenylphosphine) (330 mg), cesium carbonate (660 mg), and N-methylpyrrolidone (6.0 mL) were mixed, followed by stirring at 100 °C for 6 h. The reaction mixture was cooled to room temperature, diluted with water and ethyl acetate, and filtered through a pad of Celite™. The filtrate was separated, and the organic layer was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (chloroform-ethyl acetate), and purified by silica gel column chromatography (hexanes-ethyl acetate) to obtain 15 (156 mg, 49%) as a pale yellow solid: 1H-NMR (DMSO-d6) δ: 1.16 (3H, d, J = 6.0 Hz), 1.33–1.46 (1H, m), 1.43 (9H, s), 1.59–1.71 (2H, m), 1.90–2.02 (1H, m), 2.18 (1H, dd, J = 17.6, 8.9 Hz), 2.35 (3H, s), 2.40–2.53 (1H, m), 2.92–3.02 (1H, m), 3.21–3.40 (5H, m), 3.40–3.50 (4H, m), 4.11 (1H, d, J = 13.8 Hz), 6.30 (1H, d, J = 1.6 Hz), 6.43 (1H, d, J = 1.6 Hz), 7.62 (1H, d, J = 8.5 Hz), 8.07–8.15 (2H, m), 10.93 (1H, s); APCI/ESI-MS m/z 635 (M + H)+.
Ethyl 3-(4-{2-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-6-methylpyridin-4-yl}piperazin-1-yl)propanoate (16)tert-Butyl 4-{2-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-6-methylpyridin-4-yl}piperazine-1-carboxylate (15, 1.03 g), trifluoroacetic acid (15 mL), and dichloromethane (15 mL) were mixed, followed by stirring at room temperature for 2 h. The reaction mixture was concentrated under reduced pressure, diluted with chloroform, washed with a saturated aqueous sodium hydrogen carbonate solution, and dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (chloroform–methanol) to obtain the intermediate (600 mg, 69%) as a beige solid. The obtained intermediate (600 mg) was mixed with ethyl acrylate (250 µL) and ethanol (10 mL), and the mixture was stirred at 100 °C under microwave irradiation. The mixture was cooled to room temperature and concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (hexanes–ethyl acetate) to obtain 16 (478 mg, 67%) as a pale yellow powder: 1H-NMR (DMSO-d6) δ: 1.16 (3H, d, J = 6.0 Hz), 1.19 (3H, t, J = 7.1 Hz), 1.33–1.46 (1H, m), 1.58–1.72 (2H, m), 1.90–2.03 (1H, m), 2.18 (1H, dd, J = 17.6, 8.8 Hz), 2.34 (3H, s), 2.39–2.56 (7H, m), 2.61 (2H, t, J = 7.2 Hz), 2.92–3.02 (1H, m), 3.17–3.26 (4H, m), 3.26–3.36 (1H, m), 4.07 (2H, q, J = 7.1 Hz), 4.11 (1H, d, J = 13.8 Hz), 6.26 (1H, d, J = 1.5 Hz), 6.42 (1H, d, J = 1.5 Hz), 7.62 (1H, d, J = 8.6 Hz), 8.08–8.17 (2H, m), 10.91 (1H, s); APCI/ESI-MS m/z 635 (M + H)+.
Sodium 3-(4-{2-[(4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-6-methylpyridin-4-yl}piperazin-1-yl)propanoate (3o)A mixture of ethyl 3-(4-{2-[(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]-6-methylpyridin-4-yl}piperazin-1-yl)propanoate (16, 478 mg) and a 1 M aqueous sodium hydroxide solution (4.5 mL) in tetrahydrofuran (10 mL) and ethanol (10 mL) was stirred at 60 °C for 30 min. The reaction mixture was cooled to room temperature, and a 1 M aqueous hydrochloric acid solution (4.5 mL) was added thereto. The mixture was extracted with chloroform/isopropanol, and the organic layer was dried over anhydrous magnesium sulfate. The insoluble materials were separated by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (chloroform–methanol) to give a white solid (458 mg). The solid was mixed with ethyl acetate, and a 4 M hydrogen chloride/ethyl acetate solution (1.0 mL) was added thereto. The mixture was concentrated under reduced pressure, and washed with ethyl acetate to give a white solid (393 mg). A 1 M aqueous sodium hydroxide solution (800 µL) was added to the solid and the mixture was purified by reversed-phase column chromatography (acetonitrile–water) to give 3o (164 mg, 35%) as a pale yellow powder: 1H-NMR (DMSO-d6) δ: 1.15 (3H, d, J = 6.0 Hz), 1.33–1.46 (1H, m), 1.58–1.71 (2H, m), 1.90–2.02 (1H, m), 2.12–2.26 (3H, m), 2.34 (3H, s), 2.38–2.64 (7H, m), 2.92–3.02 (1H, m), 3.16–3.26 (4H, m), 3.30 (1H, d, J = 13.7 Hz), 4.10 (1H, d, J = 13.7 Hz), 6.26 (1H, d, J = 1.4 Hz), 6.41 (1H, d, J = 1.4 Hz), 7.61 (1H, d, J = 8.5 Hz), 8.07–8.16 (2H, m), 10.91 (1H, br s); ESI-MS m/z 607 (M + H)+; HRMS (M + H)+ Calcd for C29H35O2N6F4S 607.2473. Found 607.2480. Anal. Calcd for C29H33F4N6O2S·Na·3.5H2O: C, 50.35; H, 5.83; N, 12.15; S, 4.64; F, 10.99; Na, 3.32. Found: C, 50.99; H, 5.78; N, 11.46; S, 4.44; F, 10.44; Na, 3.24.
The following compound (18) was prepared using a procedure similar to that described for 11i.
tert-Butyl 3-[4-(2-Chloro-3-fluoropyridin-4-yl)piperazin-1-yl]propanoate (18)Pale yellow solid (yield 81%): 1H-NMR (DMSO-d6) δ: 1.40 (9H, s), 2.37 (2H, t, J = 7.2 Hz), 2.48–2.54 (4H, m), 2.58 (2H, t, J = 7.2 Hz), 3.24–3.31 (4H, m), 7.01 (1H, dd, J = 6.7, 5.6 Hz), 7.93 (1H, d, J = 5.6 Hz); ESI-MS m/z 344, 346 (M + H)+.
3-(4-{3-Fluoro-2-[(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)amino]pyridin-4-yl}piperazin-1-yl)propanoic Acid Trihydrochloride (3p)4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-amine (8, 500 mg), tert-butyl 3-[4-(2-chloro-3-fluoropyridin-4-yl)piperazin-1-yl]propanoate (18, 497 mg), tris(dibenzylideneacetone)dipalladium(0) (640 mg), 1,1′-binaphthalene-2,2′-diylbis(diphenylphosphine) (900 mg), cesium carbonate (1.90 g), and N-methylpyrrolidone (15 mL) were mixed, followed by stirring at 100 °C for 6 h. The reaction mixture was cooled to room temperature, diluted with water and ethyl acetate, and filtered through a pad of Celite™. The filtrate was separated, and the organic layer was washed with water and a saturated aqueous sodium chloride solution. The residue was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography three times (chloroform-ethyl acetate, hexanes-ethyl acetate, and then chloroform-ethyl acetate) to obtain the intermediate (387 mg, 42%) as a brown foam. The obtained intermediate (387 mg) and a 4 M hydrogen chloride/1,4-dioxane solution (18.0 mL) were mixed and then stirred at room temperature overnight. The reaction mixture was concentrated under reduced pressure, and the residue was washed with ethyl acetate to obtain 3p (394 mg, 94%) as a beige solid: 1H-NMR (DMSO-d6) δ: 1.38 (3H, d, J = 6.4 Hz), 1.59–1.75 (1H, m), 1.82–1.98 (2H, m), 2.10–2.24 (1H, m), 2.91 (2H, t, J = 7.4 Hz), 3.07–3.28 (3H, m), 3.10–4.80 (2H, m), 3.30–3.49 (5H, m), 3.49–3.63 (3H, m), 3.75–3.90 (2H, m), 4.43 (1H, dd, J = 15.0, 7.8 Hz), 4.72 (1H, dd, J = 15.0, 2.2 Hz), 6.80 (1H, t, J = 6.1 Hz), 7.79 (1H, d, J = 8.7 Hz), 7.91–8.03 (3H, m), 10.70 (1H, br s), 11.30–11.80 (1H, m), 11.52 (1H, br s); ESI-MS m/z 611 (M + H)+; HRMS (M + H)+ Calcd for C28H32O2N6F5S 611.2222. Found 611.2224. Anal. Calcd for C28H31F5N6O2S·2.9HCl·3H2O: C, 43.65; H, 5.22; N, 10.91; S, 4.16; Cl, 13.35; F, 12.33. Found: C, 43.71; H, 5.32; N, 10.30; S, 4.04; Cl, 13.49; F, 12.02.
The authors thank Mr. Nozomu Hamakawa and Ms. Mayuko Miyagawa for conducting the pharmacokinetic study. The authors also thank Dr. Chikashi Saitoh and Dr. Hajime Takamatsu for their support in the research. In addition, the authors are also grateful to the staff of the Division of Analytical Science Laboratories for the elemental analysis and spectral measurements.
This study was funded by Astellas Pharma Inc. All authors were employees of Astellas Pharma Inc. when the study was conducted and have no further conflicts of interest to declare.