2021 年 69 巻 4 号 p. 391-399
2021 年 69 巻 4 号 p. 391-399
We have been interested in the reactivities of small-ring compounds and have reported reactions that proceed through cyclopropane intermediates starting from coumarin derivatives bearing an electron-withdrawing group at the 3-position or 2-oxo-2H-pyran-3-carboxylate derivatives and dimethylsulfoxonium methylide. This time, the reaction between 3-oxa-2-oxobicyclo[4.2.0]oct-4-ene-1-carboxylate and dimethylsulfoxonium methylide has been investigated. 3a,4,5,7a-Tetrahydro-7-hydroxybenzofuran-6-carboxylate and/or 2-hydroxybicyclo[4.1.0]hept-2-ene-3-carboxylate were obtained. The compounds were characterized using various spectral and X-ray crystallographic techniques. A plausible reaction mechanism has been discussed. This reaction was applied to some 3-oxa-2-oxobicyclo[4.2.0]oct-4-ene-1-carboxylate derivatives to clarify the generality.
Cycloalkanes with small ring sizes (such as cyclopropanes and cyclobutanes) constitute the basic structural framework of a wide range of natural products.1–10) Cycloalkanes play an important role in the field of organic synthesis as they can take part in various types of reactions.11–22) These compounds are highly reactive toward ring-opening reactions. The high reactivities can be attributed to the angle and torsional strains. Ring-opening reactions with cyclopropanes and cyclobutanes result in the formation of acyclic compounds. The reactions can also lead to ring enlargement. Cyclobutanes may undergo ring contraction to form cyclopropanes. These interesting properties of the small cycloalkanes have attracted immense attention in the field of organic chemistry.
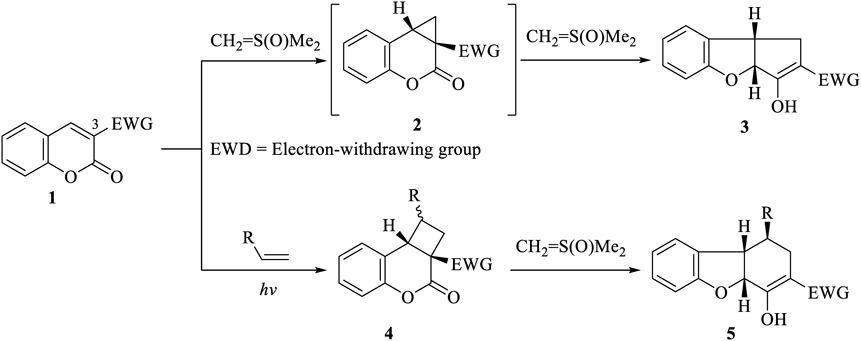
We have reported reactions that proceed through the cyclopropane intermediate 2. Coumarin derivatives bearing an electron-withdrawing group (EWG) at the 3-position 1 were used as the starting materials. The reaction of 1 with dimethylsulfoxonium methylide (CH2 = S(O)Me2)23–26) yields the cyclopenta[b]benzofuran derivative 3.27,28) The [2 + 2]photocycloaddition reaction between 1 and the alkene afforded the cyclobutane derivative 4. Compound 4, when reacted with CH2 = S(O)Me2, afforded the dibenzofuran derivative 529,30) (Chart 1).
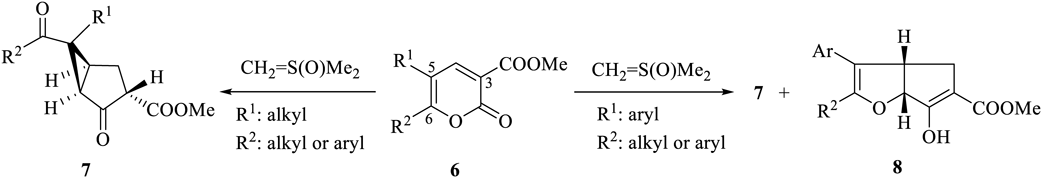
Recently, we reported the reactions between 2-oxo-2H-pyran-3-carboxylates 6, bearing an electron-withdrawing group at the 3-position, and CH2 = S(O)Me2. The reaction proceeded through a cyclopropane intermediate. The substituents at the 5-position of the substrate dictated the formation of the products. When alkyl-substituted compounds were used as the substrates, 7 was exclusively formed.31) Aryl substituted substrates yielded both 7 and 832) (Chart 2).
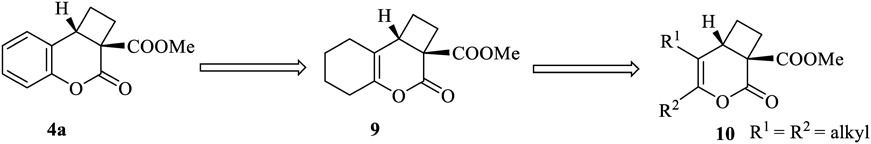
The cyclobutane derivative 9 can be regarded as a compound in which the benzene ring of 4a (4, R = H, EWG = COOMe) has lost its aromaticity (Chart 3). Furthermore, 9 can be regarded as 5,6-disubstituted 3-oxa-2-oxobicyclo[4.2.0]oct-4-ene-1-carboxylate 10.
From the results of the reaction of 1, 4, or 6 with CH2 = S(O)Me2, we were interested in the reaction of 10 with CH2 = S(O)Me2 (Chart 4). We investigated the reactions between the cyclobutane derivatives 10 and CH2 = S(O)Me2 from the point of view of whether the same reaction as 6 with CH2 = S(O)Me2 occurred, and reports its result herein.
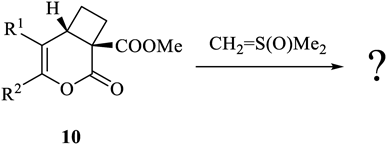
The synthesized compound 1133) was reacted with 2.4 equivalents of CH2 = S(O)Me2 in dimethyl sulfoxide (DMSO) at room temperature, following reported protocols where 5 was synthesized from 429,30) (Chart 5). The result of the reaction between 6 and CH2 = S(O)Me2 indicated that the compounds containing one carbon atom more than 7 and 8 would be obtained. The two products, 12 and 13, were obtained in 44 and 21% yields, respectively.
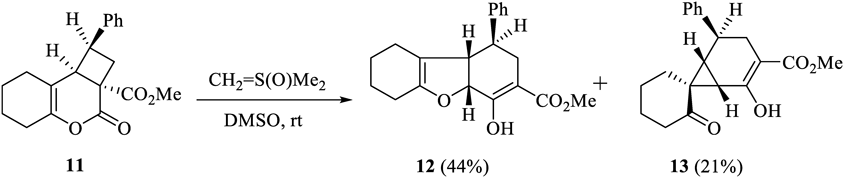
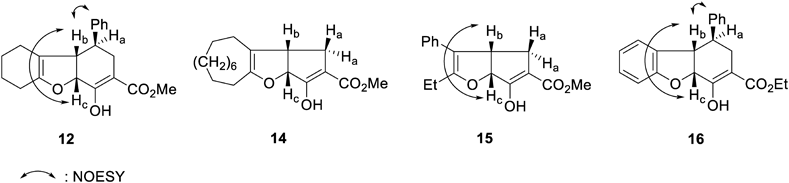
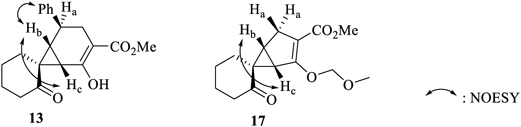
The structures of 12 and 13 were determined using spectral and X-ray crystallographic techniques. The molecular formulae of 12 and 13 (C20H22O4) were determined using the high-resolution electron impact mass spectrometric (HR-EIMS) technique. The results indicated that the number of CH2 units in the product were more than the number of CH2 units in 11. This is similar to the case where 4 is converted to 5. It was assumed that the compounds would be structurally similar to 7 and 8.31,32) The 1H-NMR spectrum of 12 was compared with the 1H-NMR spectra of previously reported compounds 14–1629,30,32) (Table 1). The 1H-NMR spectrum of 13 was compared with that of the previously reported compound 1731) (Table 2). The 1H-NMR spectra revealed nuclear Overhauser effects (NOE) between the different protons at the bridgehead positions. Characteristic chemical shifts were observed for these protons.
 | ||||
|---|---|---|---|---|
| Proton | Chemical shift (ppm) | |||
| 12 | 14 | 15 | 16 | |
| Ha | 2.63 | 2.63–2.74 | 2.45, 2.60 | 2.64 |
| Hb | 3.03 | 3.62 | 3.50 | 3.57 |
| Hc | 4.85 | 5.15 | 5.31 | 5.12 |
 | ||
|---|---|---|
| Proton | Chemical shift (ppm) | |
| 13 | 17 | |
| Ha | 2.90 | 2.37, 2.82 |
| Hb | 2.22 | 2.17 |
| Hc | 2.26 | 2.75 |
The X-ray crystallographic technique was used to determine the structures of compounds 12 and 13. The stereochemistry of the chiral centers was also determined (Fig. 1).
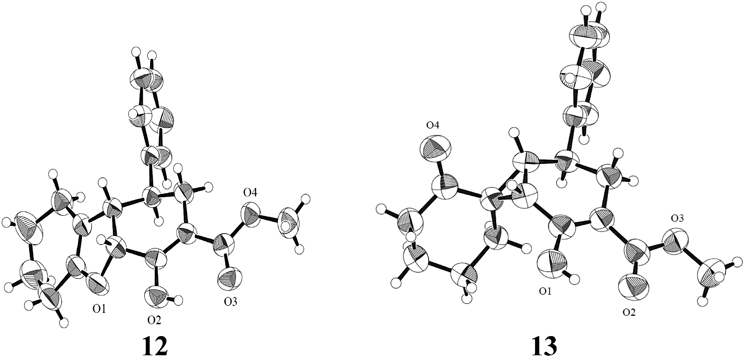
Based on the results, 12 was determined to be methyl rac-(1R,4aS,9bR)-1,2,4a,6,7,8,9,9b-octahydro-4-hydroxy-1-phenyldibenzofuran-3-carboxylate and 13 was determined to be methyl rac-(1R,5R,6S,7R)-2-hydroxy-2′-oxo-5-phenylspiro[bicyclo[4.1.0]hept-2-ene-7,1′-cyclohexane]-3-carboxylate. Despite the use of 11 as the substrate, the reaction did not produce epi-12 and epi-13 but yielded 12 and 13 (Chart 6). These observations have already been reported in literatures.29,30) The reactions of exo-18 and endo-18 with 2 equivalents of CH2 = S(O)Me2 yielded the same product 19.
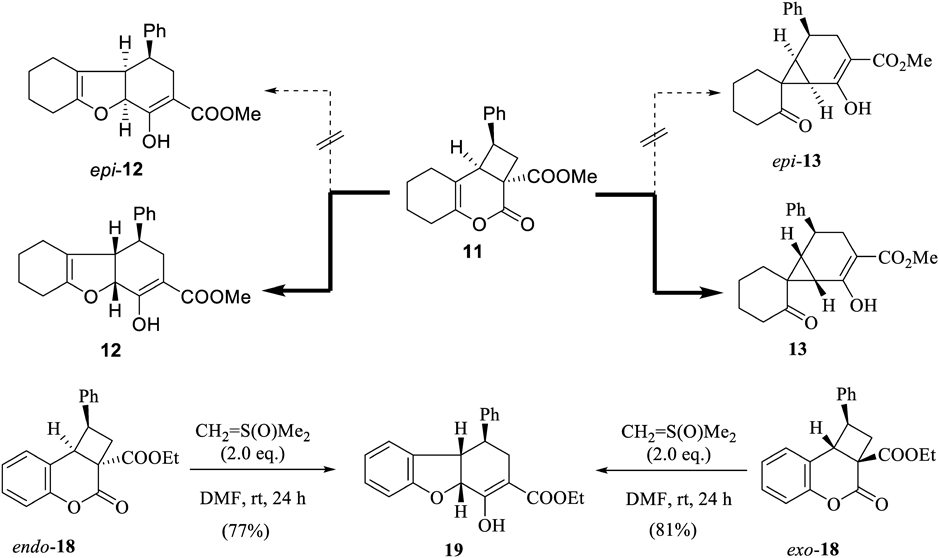
A plausible reaction mechanism is shown in Chart 7. The ylide attacks the carbonyl carbon of the lactone ring of 11. The lactone and cyclobutane rings undergo ring-opening reactions to produce 20. An extra equivalent of CH2 = S(O)Me2, used as a base, would deprotonate the dimethylsulfoxonium salt to produce the ylide 21. The cyclization of 21 via the transition state 22 would be disturbed because of instability caused by the axial cyclohexanone group. The 1–8b single bond in 21 rotates to form the intermediate 23. The cyclization of 23 to 24 would proceed easily. After the ring flip process to produce 25, 12 was formed via route a, and 13 was formed via route b. DMSO is released during the process. The cyclization via route a would proceed to avoid the steric hindrance between the axial cyclohexanone ring and the cyclohexene-carboxylate ring.
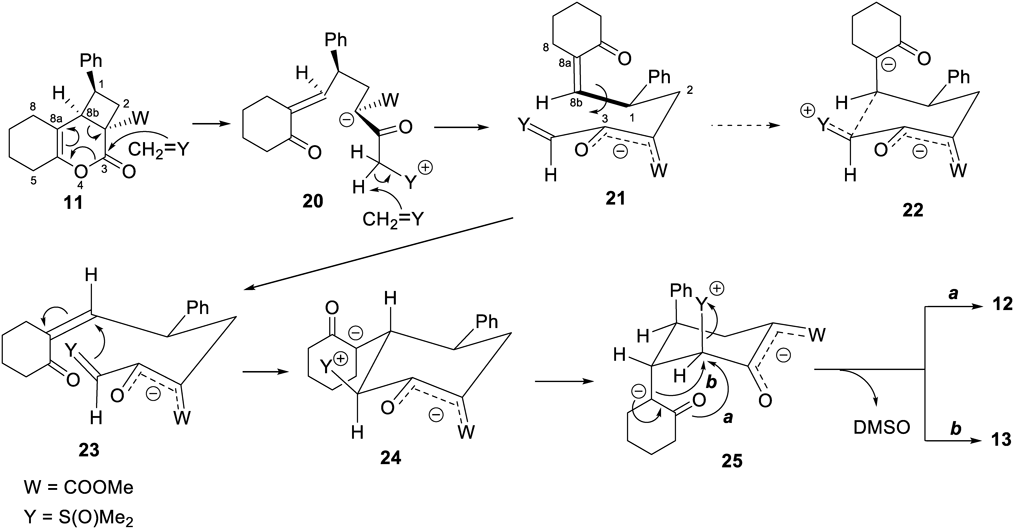
The reaction conditions were optimized to get 12 as a major product (Table 3). The optimum quantities of trimethylsulfoxonium iodide and sodium hydride (NaH) to be used for the reaction were determined. Various solvents were also screened to determine the best reaction solvent. The optimum reaction temperature was also identified. With the increase in the amount of CH2 = S(O)Me2, the yield of 12 gradually increased (entries 1, 5–8). The reaction proceeded in aprotic polar solvents such as DMSO, dimethylformamide (DMF), and dimethylacetamide (DMA) (entries 8–10). The reaction did not proceed when tetrahydrofuran (THF) was used as the reaction solvent (entry 11). The reaction temperature (between 0 and 40 °C) did not influence the yields or the ratios of 12 and 13 (entries 8–9, 12–14).
| Entry | Trimethylsulfoxonium iodide (equiv.) | NaH (equiv.) | Solvent | Temperature (°C) | Yield (%) | |
|---|---|---|---|---|---|---|
| 12 | 13 | |||||
| 1 | 1.0 | 1.0 | DMSO | r.t. | 27 | 19 |
| 2 | 1.0 | 2.0 | DMSO | r.t. | 27 | 19 |
| 3 | 1.0 | 2.0a) | DMSO | r.t. | 36 | 22 |
| 4 | 1.0 | 3.0 | DMSO | r.t. | 24 | 18 |
| 5 | 2.4 | 2.4 | DMSO | r.t. | 44 | 21 |
| 6 | 3.2 | 3.2 | DMSO | r.t. | 46 | 17 |
| 7 | 3.8 | 3.8 | DMSO | r.t. | 51 | 13 |
| 8 | 3.8 | 3.2 | DMSO | r.t. | 54 | 19 |
| 9 | 3.8 | 3.2 | DMF | r.t. | 52 | 12 |
| 10 | 3.8 | 3.2 | DMA | r.t. | 50 | 12 |
| 11 | 3.8 | 3.2 | THF | r.t. | Trace | — |
| 12 | 3.8 | 3.2 | DMF | 0 | 50 | 12 |
| 13 | 3.8 | 3.2 | DMSO | 40 | 48 | 15 |
| 14 | 3.8 | 3.2b) | DMSO | 40 | 51 | 18 |
a) NaH was added in two batches at an interval of 30 min. b) NaH was added in four batches at intervals of 30 min. Equivalent (equiv.); room temperature (r.t.).
Various cyclobutane derivatives 26a–i were reacted under the reaction conditions presented in entry 8 (Table 3). The results are shown in Table 4.
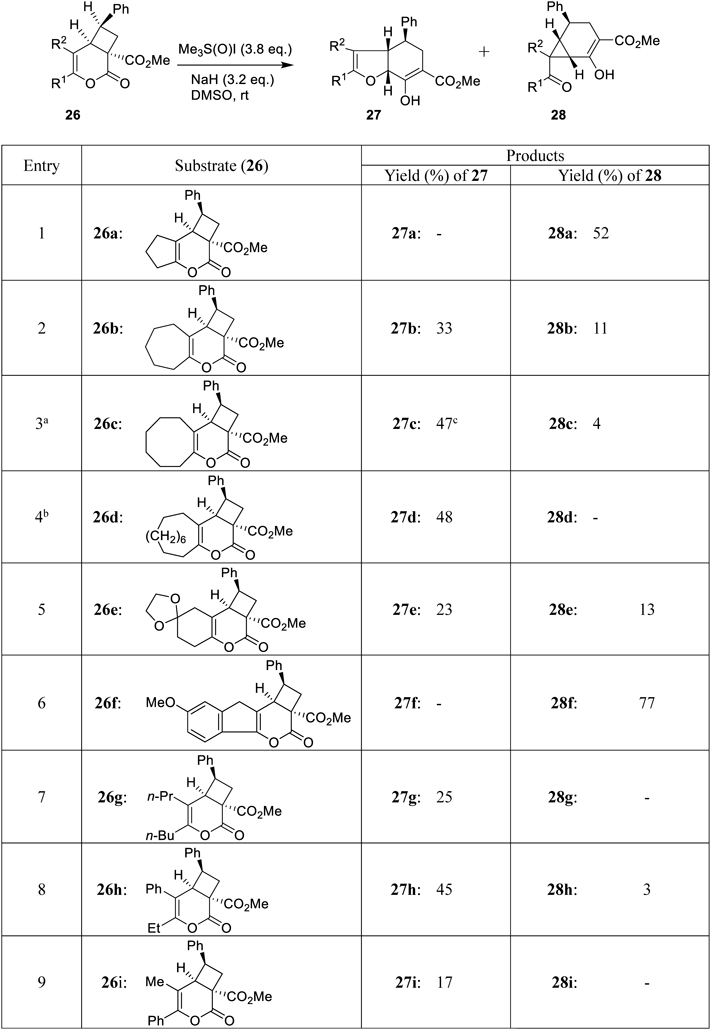 |
a: 12% of (E)-2-hydroxy-2-(2-phenylethenyl)cyclooctan-1-one was obtained. b: 8% of (E)-2-hydroxy-2-(2-phenylethenyl)cyclododecan-1-one was obtained. c: The structure was confirmed using the X-ray crystallographic technique.
The total yields of 27 and 28 were in the range of 17 to 77%. The compounds 27 and 28 were obtained in variable yields according to the substituent groups at 5- and 6-positions. The increase in the size of the left-hand ring resulted in decreased yields of the compounds 13, and 28b–28d (entry 8 in Table 3; entries 2–4 in Table 4). 28d was not formed (entry 4). With the increase in the size of the left-hand ring, the steric hindrance to the cyclohexene-carboxylate ring increases. In entries 1 and 6, the corresponding products 27a and 27f were not obtained because of the strain present in the 3,4,5,6-tetrahydro-2H-cyclopenta[b]furan framework. The compounds bearing acyclic substituents at the 5- and 6-positions primarily produced 27g–27i (entries 7–9).
The reaction between 3-oxa-2-oxobicyclo[4.2.0]oct-4-ene-1-carboxylate 11 and CH2 = S(O)Me2 was investigated. The reaction yielded 3a,4,5,7a-tetrahydro-7-hydroxybenzofuran-6-carboxylate 12 and/or 2-hydroxybicyclo[4.1.0]hept-2-ene-3-carboxylate 13. A plausible reaction mechanism was proposed. The reaction conditions were optimized and the optimized reaction conditions were used for carrying out reactions with 26. The 3a,4,5,7a-tetrahydro-7-hydroxybenzofuran-6-carboxylate-type compounds 27 and the 2-hydroxybicyclo[4.1.0]hept-2-ene-3-carboxylate-type compounds 28 were obtained in 0–48 and 0–77% yields, respectively. The nature of the products depended on the substituent groups at the 5- and 6-positions. Presently, the synthesis of different cyclobutane derivatives is being attempted in our laboratory. Their reactions with CH2 = S(O)Me2 are also being studied. The results will be communicated in due course of time.
Melting point was measured with a Yanaco MP micro-melting point apparatus and are uncorrected. NMR spectra were measured on Bruker Ultrashield™ 300 (1H: 300 MHz; 13C: 75 Hz), JEOL ECS-400 (1H: 400 MHz; 13C: 100 Hz), and Bruker Ascend™ 500 (1H: 500 MHz; 13C: 125 Hz) spectrometers with tetramethylsilane as the internal standard. Chemical shift are reported in parts per million. IR spectra were measured with a JASCO Fourier transform (FT)/IR-4600 spectrometer. A JEOL JMS-GC mate II spectrometer was used for low-resolution and high-resolution electron ionizations MS (LR-EIMS and HR-EIMS). X-ray crystal analyses were performed on Rigaku RAXIS RAPID II imaging plate area detector with graphite monochromated Cu-Kα or Mo-Kα radiation at 23.0 °C. All solvents were removed under reduced pressure in the usual work-up procedure. Silica gel 60 F254 (Merck, Germany) for TLC, silica gel 60N (Kanto Chemical, Tokyo, Japan) for column chromatography and silica gel packed in a glass column (Yamazen, Tokyo, Japan; 0.040 mm) for medium-pressure liquid chromatography (MPLC) were used.
Typical Procedure for the Synthesis of Methyl rac-(1R,4aS,9bR)-4-Hydroxy-1-phenyl-1,2,4a,6,7,8,9,9b-octahydrodibenzo[b,d]furan-3-carboxylate (12) and Methyl rac-(1R,5R,6S,7R)-2-Hydroxy-2′-oxo-5-phenylspiro[bicyclo[4.1.0]heptane-7,1′-cyclohexan]-2-ene-3-carboxylate (13) as a Representative ExampleTo a suspension of trimethylsulfoxonium iodide (837 mg, 3.8 mmol) in DMSO (5.0 mL), NaH (60% in mineral oil, 128 mg, 3.2 mmol) was added and the whole was stirred for 30 min at r.t. under N2 atmosphere. Methyl rac-(1R,2aS,8bS)-1,5,6,7,8,8b-hexahydro-3-oxo-1-phenyl-2H-cyclobuta[d]cyclohexa[b]pyran-2a(3H)-carboxylate 11 (312 mg, 1 mmol) was added to the reaction mixture, and the stirring was continued overnight. After acidification with 1N HCl aq., the mixture was extracted with CHCl3. The combined extracts were washed with water, dried over Na2SO4, and evaporated. The residue was purified by silica gel column chromatography with n-hexane–EtOAc (10 : 1) to give 12 (176 mg, 54%) of white powders and 13 (62 mg, 19%) of white powders.
12: Colorless plates; mp 162.8–164.6 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.04 (s, 1H), 7.30–7.28 (m, 2H), 7.24–7.21 (m, 3H), 4.85 (d, J = 9.0 Hz, 1H), 3.75 (s, 3H), 3.03 (t, J = 10.0 Hz, 1H), 2.63 (qd, J = 11.0, 4.0 Hz, 1H), 2.60 (dd, J = 16.5, 4.5 Hz, 1H), 2.26 (dd, J = 16.5, 12.0 Hz, 1H), 2.12–2.08 (m, 2H), 1.71–1.64 (m, 1H), 1.55–1.48 (m, 2H), 1.46–1.41 (m, 1H), 1.36–1.28 (m, 1H), 1.22–1.16 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ: 172.1, 165.7, 151.3, 143.9, 128.4 (2C), 127.5 (2C), 126.6, 110.9, 100.6, 77.3, 51.8, 49.5, 44.8, 31.2, 23.2, 23.0, 22.73, 22.67; FT-IR: 3027.7, 1702.8, 1661.4 cm−1; LR-EIMS m/z: 326 (M+, 37.4), 235 (100), 203 (64), 149 (29), 115 (36), 91 (29); HR-EIMS Calcd for C20H22O4: 326.1518. Found: 326.1517.
X-ray Crystal Data: Crystal Color, Habit; colorless, prism, Crystal Dimensions; 0.300 × 0.200 × 0.100 mm, Crystal System; triclinic, Lattice Type; Primitive, Lattice Parameters; a = 9.4385(9) Å, b = 9.9886(9) Å, c = 11.1944(10) Å, α = 64.187(3)°, β = 63.883(3)°, γ = 76.344(3)°, V = 851.77(13) Å3, Space Group; P-1 (#2), Z value; 2, Dcalc; 1.273 g/cm3, F000; 348.00, μ(MoKα); 0.876 cm−1, Intensity Measurement: Diffractometer; R-AXIS RAPID, Radiation; MoKα (λ = 0.71075 Å), graphite monochromated, Voltage, Current; 50 kV, 100 mA, Temperature; 23.0 °C, Detector Aperture; 460.0 × 256.0 mm, Data Images; 44 exposures, ω oscillation Range (χ = 45.0, ϕ = 0.0); 130.0–190.0°, Exposure Rate; 90.0 s./°, ω oscillation Range (χ = 45.0, ϕ = 180.0); 0.0–160.0°, Exposure Rate; 90.0 s./°, Detector Position; 127.40 mm, Pixel Size; 0.100 mm, 2θmax; 55.0°, No. of Reflections Measured; Total: 8400, Unique: 3860 (Rint = 0.0343), Corrections; Lorentz-polarization Absorption (trans. factors: 0.691–0.991), Secondary Extinction (coefficient: 2.24000e-002). Structure Solution; Direct Methods (SHELXT Version 2018/2), Refinement; Full-matrix least-squares on F2, Function Minimized; Σ w (Fo2–Fc2)2, Least Squares Weights; w = 1/[σ2(Fo2) + (0.0669·P)2 + 0.0773·P] where P = (Max(Fo2,0) + 2Fc2)/3, 2θmax cutoff; 54.9°, Anomalous Dispersion; All non-hydrogen atoms, No. Observations (All reflections); 3858, No. Variables; 237, Reflection/Parameter Ratio; 16.28, Residuals: R1 (I > 2.00σ(I)); 0.0453, Residuals: R (All reflections); 0.1068, Residuals: wR2 (All reflections); 0.1558, Goodness of Fit Indicator; 1.064, Max Shift/Error in Final Cycle; 0.000, Maximum peak in Final Diff. Map; 0.22 e−/Å3, Minimum peak in Final Diff. Map; −0.22 e−/Å3.
Deposition number CCDC-2048966 for 12. Free copies of the data can be obtained via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
13: Colorless plates; mp 110.7–112.9 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.18 (s, 1H), 7.33–7.30 (m, 2H), 7.28–7.26 (m, 2H), 7.23 (tt, J = 7.0, 1.5 Hz, 1H), 3.73 (s, 3H), 2.90 (ddd, J = 10.5, 6.5, 4.5 Hz, 1H), 2.59 (dd, J = 15.5, 6.0 Hz, 1H), 2.52 (dd, J = 15.5, 9.0 Hz, 1H), 2.45–2.42 (m, 2H), 2.26 (d, J = 9.0 Hz, 1H), 2.22 (dd, J = 9.0, 4.5 Hz, 1H), 2.00–1.95 (m, 1H), 1.93–1.86 (m, 3H), 1.85–1.80 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ: 208.3, 172.1, 168.0, 145.6, 128.7 (2C), 127.2 (2C), 126.6, 98.4, 51.6, 40.1, 39.5, 35.7, 32.3, 30.2, 28.7, 24.3, 23.6, 23.2; FT-IR: 3027.7, 1686.4, 1648.8 cm−1; LR-EIMS m/z: 326 (M+, 100), 308 (43), 294 (49), 213 (99), 211 (36), 203 (37), 163 (34), 141 (37), 115 (54); HR-EIMS Calcd for C20H22O4: 326.1518. Found: 326.1517.
X-ray Crystal Data: Crystal Color, Habit; colorless, needle, Crystal Dimensions; 0.200 × 0.100 × 0.050 mm, Crystal System; monoclinic, Lattice Type; C-centered, Lattice Parameters; a = 26.4518(14) Å, b = 6.4380(4) Å, c = 20.1809(11) Å, β = 95.176(7)°, V = 3422.7(3) Å3, Space Group; C2/c (#15), Z value; 8, Dcalc; 1.267 g/cm3, F000; 1392.00, μ(CuKα); 7.094 cm−1, Intensity Measurement: Diffractometer; R-AXIS RAPID, Radiation; CuKα (λ = 1.54187 Å), graphite monochromated, Voltage, Current; 50 kV, 100 mA, Temperature; 23.0 °C, Detector Aperture; 460.0 × 256.0 mm, Data Images; 45 exposures, ω oscillation Range (χ = 54.0, ϕ = 0.0); 80.0–260.0°, Exposure Rate; 270.0 s./°, ω oscillation Range (χ = 54.0, ϕ = 105.0); 80.0–260.0°, Exposure Rate; 270.0 s./°, ω oscillation Range (χ = 54.0, ϕ = 180.0); 80.0–260.0°, Exposure Rate; 270.0 s./°, ω oscillation Range (χ = 54.0, ϕ = 270.0); 80.0–260.0°, Exposure Rate; 270.0 s./°, ω oscillation Range (χ = 0.0, ϕ = 0.0); 80.0–260.0°, Exposure Rate; 270.0 s./°, Detector Position; 127.40 mm, Pixel Size; 0.100 mm, 2θmax; 136.2°, No. of Reflections Measured; Total: 18223, Unique: 3131 (Rint = 0.0903), Corrections; Lorentz-polarization Absorption (trans. factors: 0.648–0.965), Structure Solution; Direct Methods (SHELXT Version 2018/2), Refinement; Full-matrix least-squares on F2, Function Minimized; Σ w (Fo2–Fc2)2, Least Squares Weights; w = 1/[σ2(Fo2) + (0.0541·P)2 + 0.0000·P] where P = (Max(Fo2,0) + 2Fc2)/3, 2θmax cutoff; 136.2°, Anomalous Dispersion; All non-hydrogen atoms, No. Observations (All reflections); 3131, No. Variables; 217, Reflection/Parameter Ratio; 14.36, Residuals: R1 (I > 2.00σ(I)); 0.0637, Residuals: R (All reflections); 0.1496, Residuals: wR2 (All reflections); 0.1414, Goodness of Fit Indicator; 1.014, Max Shift/Error in Final Cycle; 0.000, Maximum peak in Final Diff. Map; 0.14 e−/Å3, Minimum peak in Final Diff. Map; −0.14 e−/Å3.
Deposition number CCDC-2048967 for 13. Free copies of the data can be obtained via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
Methyl rac-(1R,5R,6S,7R)-2-Hydroxy-2′-oxo-5-phenylspiro[bicyclo[4.1.0]heptane-7,1′-cyclopentan]-2-ene-3-carboxylate (28a)Colorless plates; mp 143.2–144.8 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.32 (s, 1H), 7.31–7.27 (m, 2H), 7.26–7.23 (m, 2H), 7.22 (tt, J = 6.5, 1.5 Hz, 1H), 3.70 (s, 3H), 3.15 (dt, J = 7.5, 3.5 Hz, 1H), 2.58 (ddd, J = 16.5, 3.5, 1.5 Hz, 1H), 2.48–2.42 (m, 1H), 2.37 (dd, J = 10.5, 8.0 Hz, 1H), 2.32 (dd, J = 8.5, 2.5 Hz, 1H), 2.26–2.14 (m, 4H), 2.03–1.96 (m, 1H), 1.95–1.89 (m, 1H); 13C-NMR (75 MHz, CDCl3) δ: 215.5, 172.3, 168.4, 145.5, 128.6 (2C), 127.1 (2C), 126.6, 94.3, 51.7, 41.9, 38.4, 34.1, 32.6, 30.5, 28.0, 25.3, 21.0; FT-IR: 3022.9, 1720.2, 1644.0 cm−1; LR-EIMS m/z: 312 (M+, 88.8), 280 (44), 252 (68), 224 (45), 199 (100), 197 (38), 196 (36), 141 (37), 115 (49), 91 (59); HR-EIMS Calcd for C19H20O4: 312.1362. Found: 312.1362.
Methyl rac-(1R,4aS,10bR)-2,4a,6,7,8,9,10,10b-Octahydro-4-hydroxy-1-phenyl-1H-cyclohepta[b]benzofuran-3-carboxylate (27b)Colorless plates; mp 150.7–152.2 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 11.99 (s, 1H), 7.31–7.27 (m, 2H), 7.24–7.20 (m, 3H), 4.76 (d, J = 8.5 Hz, 1H), 3.75 (s, 3H), 2.91 (dd, J = 11.0, 9.0 Hz, 1H), 2.67 (td, J = 11.5, 4.0 Hz, 1H), 2.59 (dd, J = 16.0, 4.0 Hz, 1H), 2.30 (dd, J = 11.0, 1.0 Hz, 1H), 2.31–2.22 (m, 2H), 1.60–1.48 (m, 5H), 1.33–1.26 (m, 2H), 1.23–1.17 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ: 172.1, 165.5, 153.7, 143.7, 128.4 (2C), 127.7 (2C), 126.6, 114.1, 100.6, 77.0, 52.6, 51.7, 45.0, 30.9, 29.7, 28.2, 28.1, 25.8, 25.7; FT-IR: 3033.5, 1659.5 cm−1; LR-EIMS m/z: 340 (M+, 16.5), 249 (100), 217 (37), 163 (20), 115 (11), 113 (17); HR-EIMS Calcd for C21H24O4: 340.1675. Found: 340.1673.
Methyl rac-(1R,5R,6S,7R)-2-Hydroxy-2′-oxo-5-phenylspiro[bicyclo[4.1.0]heptane-7,1′-cycloheptan]-2-ene-3-carboxylate (28b)Colorless oil; 1H-NMR (500 MHz, CDCl3) δ: 12.16 (s, 1H), 7.34–3.31 (m, 2H), 7.29–7.27 (m, 2H), 7.23 (tt, J = 7.0, 1.5 Hz, 1H), 3.73 (s, 3H), 2.88 (dt, J = 10.5, 6.0 Hz, 1H), 2.71 (dd, J = 9.5, 2.0 Hz, 1H), 2.67 (ddd, J = 16.5, 9.5, 2.0 Hz, 1H), 2.62 (dd, J = 15.5, 6.0 Hz, 1H), 2.52 (ddd, J = 14.5, 10.5, 1.0 Hz, 1H), 2.39 (d, J = 9.0 Hz, 1H), 2.09 (dd, J = 9.0, 5.0 Hz, 1H), 2.02 (dd, J = 14.5, 10.5 Hz, 1H), 1.89–1.84 (m, 2H), 1.82–1.70 (m, 3H), 1.67–1.59 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ: 211.7, 172.1, 168.6, 145.4, 128.7 (2C), 127.3 (2C), 126.6, 98.8, 51.6, 43.9, 43.1, 36.7, 35.8, 30.9, 30.7, 30.4, 27.8, 24.9, 24.6; FT-IR: 3025.8 1681.6, 1649.8 cm−1; LR-EIMS m/z: 340 (M+, 100), 322 (55), 308 (52), 227 (49), 225 (51), 197 (44), 177 (48), 115 (43), 91 (63); HR-EIMS Calcd for C21H24O4: 340.1675. Found: 340.1676.
Methyl rac-(1R,4aS,11bR)-1,2,4a,6,7,8,9,10,11,11b-Decahydro-4-hydroxy-1-phenylcycloocta[b]benzofuran-3-carboxylate (27c)Colorless plates; mp 116.4–118.1 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.00 (s, 1H), 7.31–7.27 (m, 2H), 7.24–7.20 (m, 3H), 4.80 (d, J = 9.0 Hz, 1H), 3.75 (s, 3H), 3.00 (dd, J = 10.5, 9.0 Hz, 1H), 2.72 (td, J = 10.5, 4.5 Hz, 1H), 2.59 (dd, J = 16.0, 4.5 Hz, 1H), 2.32–2.26 (m, 3H), 1.72–1.59 (m, 3H), 1.56–1.36 (m, 5H), 1.29–1.22 (m, 1H), 1.14–1.06 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ: 172.1, 165.7, 152.5, 143.7, 128.4 (2C), 127.6 (2C), 126.6, 111.2, 100.5, 77.0, 51.7, 50.4, 44.4, 31.0, 27.7, 26.4, 26.3, 25.6, 25.4, 23.8; FT-IR: 3030.6, 1659.5, 1626.7 cm−1; LR-EIMS m/z: 354 (M+, 9.7), 263 (56), 252, (100), 250 (64), 235 (42), 233 (27), 231 (18), 219 (30), 217 (17), 83 (26); HR-EIMS Calcd for C22H26O4: 354.1831. Found: 354.1830.
X-ray Crystal Data: Crystal Color, Habit; colorless, platelet, Crystal Dimensions; 0.700 × 0.700 × 0.050 mm, Crystal System; triclinic, Lattice Type; Primitive, Lattice Parameters; a = 9.7437(5) Å, b = 10.4931(5) Å, c = 11.2669(6) Å, α = 113.999(8)°, β = 97.462(7)°, γ = 110.914(8)°, V = 930.57(15) Å3, Space Group; P-1 (#2), Z value; 2, Dcalc; 1.265 g/cm3, F000; 380.00, μ(CuKα); 6.916 cm−1, Intensity Measurement: Diffractometer; R-AXIS RAPID, Radiation; CuKα (λ = 1.54187 Å), graphite monochromated, Voltage, Current; 40 kV, 100 mA, Temperature; 23.0 °C, Detector Aperture; 460.0 × 256.0 mm, Data Images; 45 exposures, ω oscillation Range (χ = 54.0, ϕ = 0.0); 80.0–260.0°, Exposure Rate; 100.0 s./°, ω oscillation Range (χ = 54.0, ϕ = 90.0); 80.0–260.0°, Exposure Rate; 100.0 s./°, ω oscillation Range (χ = 54.0, ϕ = 180.0); 80.0–260.0°, Exposure Rate; 100.0 s./°, ω oscillation Range (χ = 54.0, ϕ = 270.0); 80.0–260.0°, Exposure Rate; 100.0 s./°, ω oscillation Range (χ = 10.0, ϕ = 60.0); 80.0–260.0°, Exposure Rate; 100.0 s./°, Detector Position; 127.40 mm, Pixel Size; 0.100 mm, 2θmax; 136.3°, No. of Reflections Measured; Total: 10342, Unique: 3301 (Rint = 0.0562), Corrections; Lorentz-polarization Absorption (trans. factors: 0.696–0.966), Secondary Extinction (coefficient: 6.67000e-003). Structure Solution; Direct Methods (SHELXT Version 2014/5), Refinement; Full-matrix least-squares on F2, Function Minimized; Σ w (Fo2–Fc2)2, Least Squares Weights; w = 1/[σ2(Fo2) + (0.0853·P)2 + 0.0824·P] where P = (Max(Fo2,0) + 2Fc2)/3, 2θmax cutoff; 136.3°, Anomalous Dispersion; All non-hydrogen atoms, No. Observations (All reflections); 3301, No. Variables; 236, Reflection/Parameter Ratio; 13.99, Residuals: R1 (I > 2.00σ(I)); 0.0508, Residuals: R (All reflections); 0.0622, Residuals: wR2 (All reflections); 0.1575, Goodness of Fit Indicator; 1.090, Max Shift/Error in Final Cycle; 0.000, Maximum peak in Final Diff. Map; 0.50 e−/Å3, Minimum peak in Final Diff. Map; −0.18 e−/Å3.
Deposition number CCDC-2048968 for 27c. Free copies of the data can be obtained via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
Methyl rac-(1R,5R,6S,7R)-2-Hydroxy-2′-oxo-5-phenylspiro[bicyclo[4.1.0]heptane-7,1′-cyclooctan]-2-ene-3-carboxylate (28c)Colorless oil; 1H-NMR (500 MHz, CDCl3) δ: 12.23 (s, 1H), 7.33–7.26 (m, 4H), 7.22 (tt, J = 6.5, 1.5 Hz, 1H), 3.73 (s, 3H), 3.03 (ddd, J = 9.0, 6.5, 4.0 Hz, 1H), 2.76 (ddd, J = 11.0, 10.0, 4.0 Hz, 1H), 2.62 (dd, J = 16.0, 6.5 Hz, 1H), 2.56 (ddd, J = 16.0, 7.5, 4.0 Hz, 1H), 2.53 (dd, J = 16.0, 8.5 Hz, 1H), 2.33 (ddd, J = 16.0, 9.0, 4.0 Hz, 1H), 2.27–2.22 (m, 2H), 2.05 (ddd, J = 16.0, 7.5, 4.0 Hz, 1H), 1.83–1.67 (m, 3H), 1.64–1.50 (m, 4H), 1.48–1.40 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ: 213.3, 172.2, 168.7, 145.8, 128.6 (2C), 127.1 (2C), 126.5, 98.2, 51.6, 43.2, 39.0, 36.2, 32.7, 30.2, 29.7, 28.9, 27.6, 26.3, 25.5, 22.7; FT-IR: 3024.8, 1681.6, 1650.8 cm−1; LR-EIMS m/z: 354 (M+, 100), 322 (39), 294 (29), 263 (26), 239 (33), 197 (36), 191 (38), 141 (26), 115 (34), 91 (51); HR-EIMS Calcd for C22H26O4: 354.1831. Found: 354.1830.
Methyl rac-(1R,4aS,15bR)-1,2,4a,6,7,8,9,10,11,12,13,14,15,15b-Tetradecahydro-4-hydroxy-1-phenylcyclododeca[b]benzofuran-3-carboxylate (27d)Colorless oil; 1H-NMR (500 MHz, CDCl3) δ: 12.02 (s, 1H), 7.30–7.27 (m, 2H), 7.23–7.20 (m, 3H), 4.70 (d, J = 8.5 Hz, 1H), 3.75 (s, 3H), 3.04 (dd, J = 11.0, 8.5 Hz, 1H), 2.67 (td, J = 11.0, 4.5 Hz, 1H), 2.58 (dd, J = 16.5, 4.5 Hz, 1H), 2.34 (ddd, J = 15.5, 11.0, 5.0 Hz 1H), 2.29 (ddd, J = 16.0, 11.0, 1.0 Hz, 1H), 2.03 (dt, J = 14.5, 4.5 Hz, 1H), 1.90 (ddd, J = 14.5, 9.0, 6.0 Hz, 1H), 1.85–1.78 (m, 1H), 1.45–1.36 (m, 2H), 1.35–1.20 (m, 8H), 1.16–1.05 (m, 4H), 1.03–0.94 (m, 2H); 13C-NMR (125 MHz, CDCl3) δ: 172.2, 165.7, 151.3, 144.0, 128.4 (2C), 127.6 (2C), 126.7, 113.7, 100.5, 76.8, 51.8, 47.5, 44.2, 31.2, 25.2, 25.0, 24.5, 24.29, 24.27, 23.9, 22.7, 22.4, 21.7, 21.5; FT-IR: 3025.8, 1661.4, 1626.7 cm−1; LR-EIMS m/z: 410 (M+, 21.8), 319 (100), 306 (19), 287 (25), 233 (27), 228 (12), 115 (10), 113 (20), 91 (12), 55 (12); HR-EIMS Calcd for C26H34O4: 410.2457. Found: 410.2458.
Methyl rac-(5aR,9S,9aS)-3,4,5a,8,9,9a-Hexahydro-6-hydroxy-9-phenyl-1H-spiro[dibenzo[b,d]furan-2,2′-[1,3]dioxolane]-7-carboxylate (27e)Colorless cylindrical crystals; mp 165.3–167.1 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.03 (s, 1H), 7.31–7.28 (m, 2H), 7.24–7.21 (m, 3H), 4.96 (d, J = 9.5 Hz, 1H), 3.90–3.77 (m, 4H), 3.76 (s, 3H), 3.03 (td, J = 9.5, 1.0 Hz, 1H), 2.67 (td, J = 11.5, 4.0 Hz, 1H), 2.61 (dd, J = 16.0, 4.0 Hz, 1H), 2.38–2.28 (m, 2H), 2.24 (ddd, J = 16.0, 11.5, 1.0 Hz, 1H), 1.80–1.71 (m, 3H), 1.53 (d, J = 16.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 172.0, 165.3, 150.6, 143.5, 128.6 (2C), 127.5 (2C), 126.8, 108.8, 108.4, 100.8, 78.5, 64.4, 64.3, 51.8, 49.3, 44.7, 33.7, 31.2, 31.0, 21.3; FT-IR: 3031.6, 1702.8, 1660.4 cm−1; LR-EIMS m/z: 384 (M+, 45.2), 293 (47), 228 (71), 197 (32), 156 (32), 115 (26), 100 (30), 99 (100), 87 (35), 86 (39); HR-EIMS Calcd for C22H24O6: 384.1573. Found: 384.1576.
Methyl rac-(1R,5R,6S,7R)-2-Hydroxy-4′-oxo-5-phenyldispiro[bicyclo[4.1.0]heptane-7,3′-cyclohexane-1′,2″-[1,3]dioxolan]-2-ene-3-carboxylate (28e)Colorless oil; 1H-NMR (500 MHz, CDCl3) δ: 12.20 (s, 1H), 7.33–7.28 (m, 4H), 7.22 (tt, J = 6.5, 2.0 Hz, 1H), 4.06–3.98 (m, 4H), 3.73 (s, 3H), 3.04 (td, J = 7.5, 4.0 Hz, 1H), 2.67–2.55 (m, 4H), 2.45 (d, J = 9.0 Hz, 1H), 2.13–2.10 (m, 4H), 2.07 (dd, J = 9.0, 4.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ: 206.7, 172.1, 167.9, 145.7, 128.7 (2C), 127.1 (2C), 126.6, 107.8, 97.8, 64.8, 64.6, 51.6, 38.4, 36.7, 35.4, 34.7, 32.4, 32.2, 29.5, 28.4; FT-IR: 3025.8, 1694.2, 1650.8 cm−1; LR-EIMS m/z: 384 (M+, 83.5), 366 (20), 352 (43), 293 (21), 271 (26), 167 (22), 141 (14), 115 (20), 99 (100); HR-EIMS Calcd for C22H24O6: 384.1573. Found: 384.1573.
Methyl rac-(1R,5R,6S,7R)-1′,3′-Dihydro-2-hydroxy-5′-methoxy-1′-oxo-5-phenylspiro[bicyclo[4.1.0]heptane-7,2′-inden]-2-ene-3-carboxylate (28f)Colorless column; mp 183.9–185.4 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.35 (s, 1H), 7.71 (dd, J = 8.0, 1.5 Hz, 1H), 7.33–7.27 (m, 4H), 7.25–7.22 (m, 1H), 6.95–6.93 (m, 2H), 3.89 (s, 3H), 3.72 (s, 3H), 3.331 (d, J = 17.5 Hz, 1H), 3.329 (dt, J = 8.0, 2.5 Hz, 1H), 3.06 (d, J = 17.0 Hz, 1H), 2.69 (ddd, J = 17.0, 2.5, 1.5 Hz, 1H), 2.51–2.43 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ: 200.1, 172.2, 169.0, 165.1, 155.1, 145.7, 129.4, 128.6 (2C), 127.0 (2C), 126.6, 125.2, 115.2, 109.7, 93.3, 55.6, 51.6, 42.3, 34.1, 32.6, 29.7, 29.5, 27.8; FT-IR: 3023.8, 1684.5, 1656.6 cm−1; LR-EIMS m/z: 390 (M+, 39.0), 358 (27), 278 (21), 277 (100), 275 (12), 215 (12), 199 (13), 162 (16), 115 (17); HR-EIMS Calcd for C24H22O5: 390.1467. Found: 390.1465.
Methyl rac-(3aR,4R,7aS)-2-Butyl-3a,4,5,7a-tetrahydro-7-hydroxy-4-phenyl-3-propylbenzofuran-6-carboxylate (27g)Colorless oil; 1H-NMR (400 MHz, CDCl3) δ: 12.01 (s, 1H), 7.31–7.27 (m, 2H), 7.25–7.20 (m, 3H), 4.70 (d, J = 8.8 Hz, 1H), 3.75 (s, 3H), 3.02 (dd, J = 10.8, 8.8 Hz, 1H), 2.67–2.56 (m, 2H), 2.33–2.26 (m, 1H), 2.17–2.04 (m, 2H), 1.70–1.61 (m, 1H), 1.48 (sext, J = 7.2 Hz, 2H), 1.30 (sext, J = 7.2 Hz, 2H), 1.17–1.05 (m, 1H), 1.04–0.94 (m, 2H), 0.89 (t, J = 7.2 Hz, 3H), 0.59 (t, J = 7.2 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ: 172.2, 165.7, 151.8, 143.9, 128.4 (2C), 127.6 (2C), 126.6, 112.6, 100.5, 76.7, 51.8, 48.0, 44.5, 31.1, 29.4, 27.0, 25.4, 22.3, 21.7, 13.9, 13.6; FT-IR: 3029.6, 1736.6 cm−1; LR-EIMS m/z: 370 (M+, 12.1), 279 (100), 247 (27), 193 (21), 179 (10), 115 (9), 113 (18), 104 (8), 91 (8), 57 (9); HR-EIMS Calcd for C23H30O4: 370.2144. Found: 370.2146.
Methyl rac-(3aR,4R,7aS)-2-Ethyl-3a,4,5,7a-tetrahydro-7-hydroxy-3,4-diphenylbenzofuran-6-carboxylate (27h)Colorless oil; 1H-NMR (500 MHz, CDCl3) δ: 12.03 (s, 1H), 7.07–6.96 (m, 8H), 6.93–6.91 (m, 2H), 5.02 (d, J = 8.5 Hz, 1H), 3.79 (t, J = 8.5 Hz, 1H), 3.75 (s, 3H), 2.92 (td, J = 8.5, 4.5 Hz, 1H), 2.54 (dd, J = 16.5, 4.5 Hz, 1H), 2.42 (dd, J = 16.5, 8.5 Hz, 1H), 2.344 (qd, J = 7.5, 1.0 Hz, 1H), 2.339 (q, J = 7.5 Hz, 1H), 1.15 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ: 172.2, 165.6, 155.5, 142.9, 134.4, 128.03 (2C), 127.95 (2C), 127.78 (2C), 127.77 (2C), 126.1, 125.4, 113.0, 100.0, 76.7, 51.8, 49.9, 42.8, 28.3, 20.0, 11.9; FT-IR: 3025.8, 1660.4, 1625.7 cm−1; LR-EIMS m/z: 376 (M+, 81.3), 285 (100), 253 (49), 199 (39), 197 (29), 172 (44), 115 (52), 113 (57), 91 (42), 57 (42); HR-EIMS Calcd for C24H24O4: 376.1675. Found: 376.1673.
Methyl rac-(1R,5R,6S,7R)-2-Hydroxy-5,7-diphenyl-7-propionylbicyclo[4.1.0]hept-2-ene-3-carboxylate (28h)Colorless column; mp 154.1–155.9 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.39 (s, 1H), 7.41–7.35 (m, 5H), 7.32–7.28 (m, 2H), 7.26–7.20 (m, 3H), 3.55 (s, 3H), 3.19 (dt, J = 6.5, 3.0 Hz, 1H), 2.65–2.61 (m, 2H), 2.22 (q, J = 7.5 Hz, 1H), 2.20 (q, J = 7.5 Hz, 1H), 2.15 (dd, J = 16.5, 3.0 Hz, 1H), 1.22 (dd, J = 16.5, 8.0 Hz, 1H), 0.88 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ: 207.9, 172.0, 168.0, 146.1, 134.7, 131.0 (2C), 128.9 (2C), 128.6 (2C), 128.1, 127.2 (2C), 126.5, 96.0, 51.4, 48.7, 35.4, 35.1, 34.7, 31.1, 27.5, 7.8; FT-IR: 3027.7, 1685.5, 1645.0 cm−1; LR-EIMS m/z: 376 (M+, 100), 344 (27), 288 (30), 287 (44), 259 (22), 115 (37), 91 (72), 85 (28), 83 (40), 57 (35); HR-EIMS Calcd for C24H24O4: 376.1675. Found: 376.1674.
Methyl rac-(3aR,4R,7aS)-3a,4,5,7a-Tetrahydro-7-hydroxy-3-methyl-2,4-diphenylbenzofuran-6-carboxylate (27i)Colorless square column crystals; mp 203.4–205.6 °C (Diisopropyl ether); 1H-NMR (500 MHz, CDCl3) δ: 12.05 (s, 1H), 7.54–7.52 (m, 2H), 7.35–7.32 (m, 4H), 7.29–7.24 (m, 4H), 4.94 (d, J = 8.5 Hz, 1H), 3.77 (s, 3H), 3.09 (dd, J = 11.0, 8.5 Hz, 1H), 2.84 (td, J = 11.0, 4.5 Hz, 1H), 2.65 (dd, J = 16.0, 4.5 Hz, 1H), 2.38 (ddd, J = 16.0, 11.0, 1.0 Hz, 1H), 1.35 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ: 172.1, 165.4, 148.6, 143.6, 131.2, 128.6 (2C), 128.04 (2C), 128.02, 127.8 (2C), 127.5 (2C), 126.8, 110.7, 100.7, 76.5, 53.3, 51.8, 44.4, 30.9, 12.9; FT-IR: 3018.1, 1656.6, 1628.6 cm−1; LR-EIMS m/z: 362 (M+, 24.9), 272 (30), 271 (100), 239 (52), 185 (45), 158 (47), 115 (28), 113 (48), 105 (59), 77 (29); HR-EIMS Calcd for C23H22O4: 362.1518. Found: 362.1520.
This research was supported in part by the Strategic Research Foundation Grant aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (S1511024L).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.