2021 年 69 巻 8 号 p. 727-733
2021 年 69 巻 8 号 p. 727-733
Recently, a novel humidifier that sprays water fine droplets equipped with a copolymer, poly(3,4-ethylene dioxythiophene)–poly(styrene sulfonate) (PEDOT/PSS) was developed. PEDOT/PSS in the humidifier absorbs water from the environment and releases fine water droplets by heating. In the present study, the effect of hydration on the skin barrier, stratum corneum, was first determined by the application of fine water droplets using the humidifier. The skin-penetration enhancement effect of a model hydrophilic drug, caffeine, was also investigated using the humidifier and compared with a conventional water-evaporative humidifier. More prolonged skin hydration effect was observed after application of the fine water droplet release humidifier using PEDOT/PSS than that using a conventional humidifier. In addition, markedly higher skin permeation of caffeine was observed in both infinite and finite dose conditions. Furthermore, higher skin permeation of caffeine from oil/water emulsion containing caffeine was observed in finite dose conditions by pretreatment with the humidifier using PEDOT/PSS. This device can provide water droplets without replenishing water, so it is more convenient for enhancing the skin permeation of chemical compounds from topical drugs and cosmetic formulations.
Human skin is the largest organ of the human body with a total area of about 2 m2.1,2) It defines the boundary between the body and its surroundings. The skin is composed of three layers, the epidermis and dermis, and the underlying hypodermis (or subcutis). The epidermis is further subdivided, stratum corneum (SC) and viable epidermis. The SC is made up of rigid corneocytes (keratin-filled dead cells) in a specialized lipid matrix.3) The lipid-enrich extracellular is the only continuous domain in the SC and thus likely to be pivotal to its barrier function.4)
Skin is utilized as an administration site for pharmaceutical and cosmetics. Regarding to skin permeation of topically applied chemicals, the 500 Dalton rule5) is well known, where the SC is almost impermeable to drugs with molecular weights above 500 Da. In addition, it is more difficult for hydrophilic substances to pass through the SC than lipophilic substances.6) This is why most drugs that are absorbed into the systemic circulation system as a transdermal drug delivery system and exert their medicinal effects are lipophilic low-molecular-weight molecules. On the other hand, hydrophilic small molecules in pharmaceuticals and cosmeceuticals are expected to be effective in the shallow part of the skin. Typical examples are vitamin C and B derivatives, as well as hydroquinone, tranexamic acid, and kojic acid, which have a whitening effect at skin local site.7) A slight increase in the skin permeation of these hydrophilic low-molecular-weight molecules enables higher effectiveness of these products.
Chemical enhancers and physical means have been used to break the barrier function of the SC and to increase the skin permeation of chemicals. Chemical enhancers such as surfactants, terpens and their derivatives, fatty acid and ester, etc. have increased drug permeability by disrupting the SC lipid organization, by acting as anti-solvent, by changing the solvent nature of the skin membrane in order to enhance the drug partitioning into the skin.8) Physical skin permeation approaches such as microneedle and electroporation create pore routes in the SC to enhance skin permeation of drugs.9,10) Iontophoresis enhances skin permeation of drug by electrically assist of drugs diffusion in the skin.11) Although these approaches would be effective to improve skin permeation of drugs, in general, excessive damage to the SC barrier function increases the risk of skin irritation.12) Therefore, it is necessary to modify the SC barrier by particularly mild means to increase the skin permeation of the above-mentioned drugs and cosmeceuticals that are expected to be effective in the shallow part of the skin. A typical means is hydration of the SC. Hydration of the intercellular lipids in the SC using occlusive dressing therapy with wrapping using plastic film or bandages can decrease the SC barrier function, so this technique may be used at a hydration process to improve the skin permeation of hydrophilic compounds.13,14) When a moisturizer contains humectants, the water content of the skin may increase more rapidly. Humectants such as polyols and polysaccharides are expected to improve the skin permeation of chemicals of hydrophilic chemicals.15,16)
Application of micro- or nano-sized water droplets generated by steam- and ultrasonic-type humidifiers may effectively increase the moisture content in the SC. Recently, a novel type of humidifier that equipped with a copolymer, poly(3,4-ethylene dioxythiophene)–poly(styrene sulfonate (PEDOT/PSS) has been developed.17) PEDOT/PSS has water-absorbable sulfonic acids-coated pores with 1–2 nm size. The humidifier contains a PEDOT/PSS layer of 10 nm thickness on a thermo conductive metallic layer. The absorbed water molecules into PEDOT/PSS are detached smoothly by heating the PEDOT/PSS layer on the thermo conductive metallic layer. Thus, the PEDOT/PSS layers in the humidifier absorb water from the environment, and fine droplets are released by heating.18,19) The released water droplets have a small particle size (less than 0.5 µm). Nishimura et al.18) reported that the application of the smallest water particles on the skin released from a humidifier equipped with PEDOT/PSS improved skin conductance and skin elasticity compared with larger sized water droplets. Skin conductance was maintained over 360 min, although transepidermal water loss showed its lowest value compared with other conditions. Water retention in the SC by spraying fine water particles might play a positive role in enhancing the skin permeation of low-molecular-weight hydrophilic drugs.
Thus, in the present study, the usefulness of a humidifier containing PEDOT/PSS as a skin penetration-enhancing means was investigated by comparing the skin permeation of hydrophilic drugs through pre-hydrated skin and non-hydrated skin. Caffein was used as a model drug because it is used in variety of cosmetic products.20) It has the effect on the microcirculation of blood vessels,21) antioxidant properties,22) and anti-cellulite properties.23) In addition to these biological properties, caffeine has no ideal properties for skin permeation due to its hydrophilic physicochemical properties (M.W. 194.08, log Ko/w: −0.07). In other words, it would be a good model drug to evaluate the enhancement effect of skin permeation as it is a low skin permeation.
Caffeine was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Octyldodecyl lactate and acrylates/C10–30 alkyl acrylate crosspolymer (Lubrizol Corporation, OH, U.S.A.) were supplied by the Kao Corporation (Tokyo, Japan). Caffeine conforms to 99.7% of assay specification. Octyldodecyl lactate and acrylates/C10–30 alkyl acrylate crosspolymer were of commercial cosmetic grade. All other reagents and solvents were of reagent grade or HPLC grade and used without further purification.
Experimental AnimalsHairless rats (140–200 g, 8 weeks old) were supplied by Life Science Research Center, Josai University (Sakado, Saitama, Japan) and were used in all animal experiments. The rats were housed in a room at 25 ± 2 °C, and a light cycle of 12 h on and 12 h off. The rats were allowed free access to water and diet (Oriental Yeast Co., Ltd., Tokyo, Japan). All animal feeding and experiments were approved by the Institutional Animal Care and Use Committee of Josai University.
The hairless rats were anesthetized using an intraperitoneal injection of three types of anesthesia (medetomidine hydrochloride; 0.15 mg/kg, midazolam; 2 mg/kg and butorphanol tartrate; 2.5 mg/kg) and used for in vivo experiment. The rats were sacrificed by cervical dislocation, and the abdominal region was carefully shaved and excised before the in vitro experiment.
Preparation of Applied FormulationsCaffeine-containing solution and oil/water (o/w) emulsion were prepared in the present study. Caffeine was dissolved in purified water at a concentration of 1.0% (w/v) for the solution. 2.0% (w/v) of acrylates C10–30 alkyl acrylate cross polymer gel was prepared by mixing with purified water by Labolution® (PRIMIX Corporation, Tokyo, Japan) at 500 rpm at 32 °C for 5 min. Then 1.6 g of of 2.0% acrylates/C10–30 alkyl acrylate crosspolymer gel, 14.13 g of purified water, 4.0 g of octyldodecyl lactate and 2.0 g of caffeine were well mixed by Labolution® at 4500 rpm for 3 min at 80 °C. After 0.05 g of 20% (w/v) KOH solution was added the solution, 0.02g of phenotype ethanol were mixed, (4500 rpm for 5 min at 70 °C and 4500 rpm for 3 min at 40 °C, respectively, using Labolution®), to obtain o/w emulsions.
HumidifiersA humidifier equipped with PEDOT/PSS was kindly provided by Aisin Seiki Co., Ltd. (Kariya, Aichi, Japan). The release rate of water was 30 µg/min from this humidifier were supplied in the water dispatch period. Water droplets were released from the outlet of the humidifier over 1 min of every 2 min. Because the dispatch of water droplets occurred by heating the PEDOT/PSS layers, warm air was also released during the process. In the present study, the application of each humidifier was performed for 1.0 h (total spraying period of fine droplets was 30 min). An evaporative humidifier (F-GMHK10, Panasonic Corp., Kadoma, Osaka, Japan) was used for comparison, where the evaporative humidifier supplied water droplets at 3.3 g/min.
Measurement of Particle Size of Water DropletsThe particle size of water droplets was measured using a scanning mobility particle sizer (Model 3936-SMPS, measurement range: 2.5–1000 nm, Daicel Corp., Osaka, Japan). It consisted of a differential mobility analyzer model 3085 for particle sizing and a condensation particle counter model 3776. This experiment was performed under high flow conditions (1.5 L/min). The particle size spectra were obtained using a 120 s up-scan and 15 s down-scan. The average particle size of water droplets was calculated in triplicate.
Measurement of Humidity and Air Temperature during Application of the HumidifierChanges in humidity and temperature were measured with a temperature and humidity sensor (Ondorori TR-7, temperature range: −30 to 80 ± 0.5 °C, humidity range: 0–99% relative humidity (RH) ± 2.5% RH, T&D Corporation, Matsumoto, Nagano, Japan) at 3.0, 5.0, and 10 cm distances from the outlet of the humidifier. This experiment was performed at 24 ± 3 °C and 20 ± 5 RH (%). The measurement of humidity and temperature was done at the center part of the formulation application area. This experiment was done with a single measurement for each distance.
Measurement of Water Content in the Stratum CorneumMeasurement of water content in the SC was investigated with in vivo experiment. A corneometer (CM825, Courage + Khazaka electronic GmbH, Cologne, Germany) was used to measure water content in the abdominal area of hairless rat skin. A circle with an area of 1.77 cm2 was marked on the abdominal area. Water droplets released from the humidifier with PEDOT/PSS or 1.0 mL of purified water was applied over 60 min inside in the marked area. After finishing the application period, applied water and excess water were removed by gently blotting the area with a Kim-towel. Relative capacitance change was calculated by dividing the skin capacitance at each measurement time by the initial skin capacitance value, just before starting hydration. The experiment was repeated 4–6 times for this experiment. Each anesthetized rat was placed abdominal side up under the humidifier with a distance from the outlet.
In Vitro Skin Permeation ExperimentThe excised hairless rat skin was mounted in a Franz-type diffusion cell (cell volume: 6.0 mL, effective permeation area: 1.77 cm2). Saline (0.9% sodium chloride solution, pH 6.8) was used as a receiver solution, and 6.0 mL was applied to the dermis side, and the receiver solution was stirred continuously with a star-head-type magnetic stirrer at 500 rpm. The receiver solution was maintained at 37 °C by the use of circulating water to maintain skin surface temperature 32 °C. As a pretreatment experiment, a humidifier equipped with PEDOT/PSS was applied at 3.0 cm and 5.0 cm distances from the skin surface for 1 h. Then, the prepared formation (17.7 µL/1.77 cm2 or 1.0 mL/1.77 cm2) was applied to the donor side. As a comparison, hydration with 1.0 mL/1.77 cm2 of purified water was conducted for 0.5 h. When the evaporative humidifier was used to hydrate skin, the water droplet application period was set as 30 min at a 3.0 cm distance from the skin surface. Before application of the prepared formulation, the applied purified water was removed using a pipette, and excess water on the skin surface was blotted with a Kim-towel. Aliquots (0.5 mL) were withdrawn from the sampling port for 8 h to determine the drug content. After each sampling, the same amount of fresh saline was added to maintain the volume in the receiver. The experiment was repeated 4–6 times for the in vitro skin permeation experiment. The setup for the permeation experiment with a humidifier equipped with PEDOT/PSS is shown in Fig. 1.

This humidifier contains a PEDOT/PSS layer (10 nm thickness) under a fan. The PEDOT/PSS layer absorbs water from the environment, and fine droplets are released by heating. (Color figure can be accessed in the online version.)
Caffeine concentrations in the sample were detected using an ultra performance liquid chromatography (UPLC, Nexera X2, Shimadzu, Kyoto, Japan) equipped with a UV detector (SPD-20 A, Shimadzu). The samples were centrifuged at 21500 × g and 4 °C for 5 min, 20 µL of the supernatant was added to the same volume of acetonitrile, and 6 µL the mixed solution was injected into the UPLC system. Chromatographic separation was performed using an Shim-pack GIS C18 (2 µm, 75 × 3.0 mm2 i.d.; Shimadzu) at 40 °C. The mobile phase was 0.1% phosphoric acid/acetonitrile (75/25, v/v). The flow rate was adjusted to 0.2 mL/min, and detection was performed at UV 254 nm. The obtained standard calibration curve was prepared from a concentration of 1.0 to 250 µg/mL (the lower limit of quantification was 0.1 µg/mL).
Statistical AnalysisThe statistical significance of differences in data for SC water content and the skin permeation experiment were evaluated using one-way ANOVA followed by the Tukey–Kramer post-hoc test. The significance level was set at p < 0.05.
Figure 2a shows changes in air temperature at 3.0, 5.0, and 10 cm distances from the output of water particles from the humidifier with PEDOT/PSS. An enlarged x-axis scale from Fig. 2a is shown in Fig. 2b. Increasing and decreasing temperatures were observed regardless of the application distance (Figs. 2a, b). The highest temperature increased in the first three water-discharged cycles, and then a steady state of the highest temperature was obtained when the humidifier was applied at 3.0 cm (black solid line) and 5.0 cm (black dotted line) distances from the skin surface. In addition, the lowest temperature observed in every cycle was also increased in the first three cycles. However, with application of the humidifier at a 10 cm (gray solid line) distance from the skin surface, an increased temperature was observed, but the change in temperature was not obvious and not as regular compared with those obtained at 3.0 and 5.0 cm distances. When looking at the enlarged temperature profiles obtained at the 3.0 cm distance, a trapezoidal-like waveform was observed. Thus, dramatically increased temperature was confirmed for approximately the first 10 s. Next, the temperature increased gradually over 20 s, followed by a gradual decrease observed at 30 s. Then, after 60 s, the temperature sharply increased again. The highest temperature was observed when the humidifier was applied at a 3.0 cm distance from the skin surface.
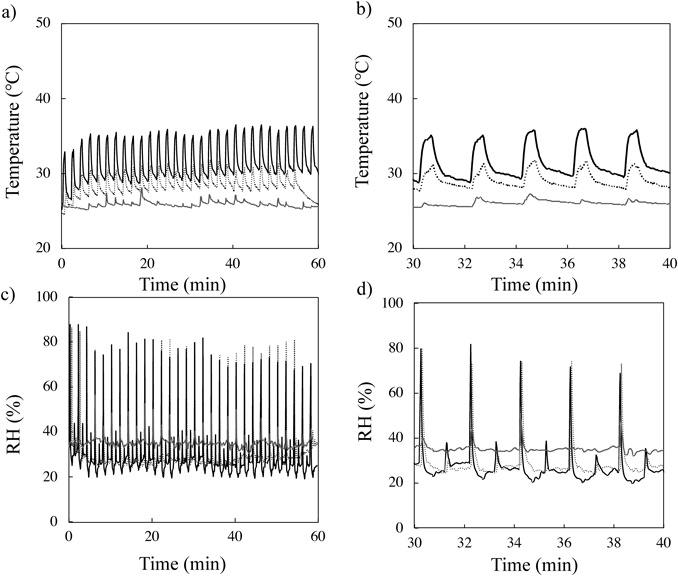
Enlargements of the x-axis ranges from (a) and (c) are shown in (b) and (d), respectively. Symbols; black solid line: 3.0 cm, black dotted line: 5.0 cm, and gray solid line: 10 cm. Each profile was obtained by single measurement.
In the case of the RH profile, unlike the temperature profile, at both 3.0 cm (black solid line) and 5.0 cm (black dotted line) distances from the skin surface showed higher RH values (Fig. 2c). Furthermore, the shape of the RH profile was different from that for the temperature. An enlarged x-axis scale from Fig. 2c is shown in Fig. 2d. The RH increased rapidly until it reached a maximum, and then it decreased to almost the same as the initial position in about 10 s. Of note, a small peak was also confirmed about 60 s after observation of the large peak. In the following experiment, application of a humidifier was evaluated at a 3.0 cm distance from the skin surface.
Change in Water Content in the Stratum CorneumFigure 3 shows the capacitance profiles in the SC after application of water droplets using the humidifier with PEDOT/PSS. As a control, SC hydration with 1.0 mL/1.77 cm2 of purified water and no hydration treatment was also evaluated. Purified water or water droplets were applied for first 60 min. Markedly higher capacitance was observed just after removing purified water from the skin (shown as ●). Significantly higher capacitance was observed immediately after removal of water from the skin surface (p < 0.05) compared with the other treatments, but no significant difference was confirmed for the other measurement points. On the other hand, although the change in capacitance value was slightly higher than that with the non-hydration process (○), significantly higher values were observed at 0, 10, 20, and 30 min after finishing the hydration process with the humidifier at a 3.0 cm distance from the skin surface (■). Of note, at 30 min after the end of the hydration process, the skin capacitance obtained using the humidifier at a distance of 3.0 cm was significantly different from that obtained by applying 1.0 mL of purified water.
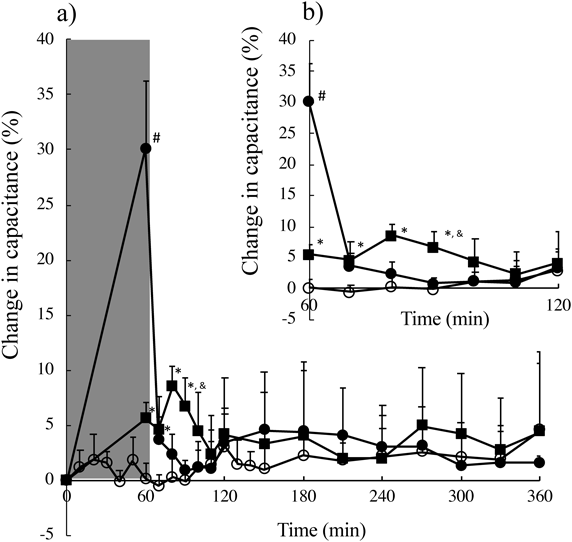
Enlarged Fig. 3(a) was shown in Fig. 3 (b). Symbols; ○: without hydration, ●: hydration with 1.0 mL purified water, and ■: hydration with a humidifier at a 3.0 cm distance from the skin surface. Gray rectangle area shows hydration period. # p < 0.05: Significant difference between hydration with 1.0 mL purified water and the other treatments: * p < 0.05: Significant difference between hydration with a humidifier at a 3.0 cm distance from the skin surface and without hydration. & p < 0.05: Significant difference between hydration with a humidifier at a 3.0 cm distance from the skin surface and hydration with 1.0 mL purified water. Error bars denote standard deviation (S.D.) (n = 4–6).
The water particles released from the humidifier equipped with PEDOT/PSS were measured. However, the particle size was not detected with the scanning mobility-particle sizer. On the other hand, the evaporative humidifier released water droplets of 2.5 nm.
In Vitro Skin PermeationFigure 4 shows the effect of the SC hydration process on the skin permeation profiles of caffeine when its solution was applied at an infinite dose (1.0 mL/1.77 cm2). SC hydration with 1.0 mL of purified water (●) showed approximately 1.35-fold higher permeation of caffeine compared with non-hydrated skin (○), but the skin permeation enhancement effect obtained by the application of a humidifier at a 3.0 cm distance (■) was higher (1.74-fold) than that with non-hydrated skin (○). Significantly higher permeation of caffeine (p < 0.05) was observed in the SC hydration with a humidifier with PEDOT/PSS at a 3.0 cm distance compared with non-hydrated skin. On the other hand, no skin permeation effect was observed when the SC was hydrated at a 5.0 cm distance (▲).
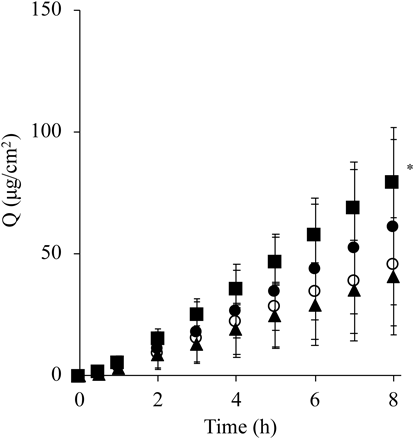
Symbols; ○: without hydration,: ● hydration with 1.0 mL purified water, ■: hydration with a humidifier at a 3.0 cm distance from the skin surface, and ▲: hydration with a humidifier at a 5.0 cm distance from the skin surface. Mean ± S.D. (n = 4–6). * p < 0.05: Significant difference between hydration with a humidifier at a 3.0 cm distance and without hydration.
Figure 5a shows the permeation profile of caffeine obtained from its solution with the finite dose application (17.7 µL/cm2). In addition to pretreatment with the humidifier, simultaneous application using the humidifier was also investigated. An evaporative humidifier was used as a comparison. The skin permeation profile of caffeine through non-hydrated skin after the application of the formulation with a finite dose reached a plateau after a prolonged application period (○). Almost the same skin permeation profile was confirmed with the other profiles, although the cumulative amount of caffeine permeated over 8 h (Q8h) was different among the treatments. Among the treatments, the highest Q8h was observed by pretreatment with the humidifier equipped with PEDOT/PSS at a 3 cm distance (■), whereas no skin penetration-enhancement effect was displayed after pretreatment with the evaporative humidifier (3.0 cm distance from the skin surface, ◆). On the other hand, humidifier application after application of caffeine solution (□, ◇) showed slightly lower Q8h regardless of the type of humidifier .
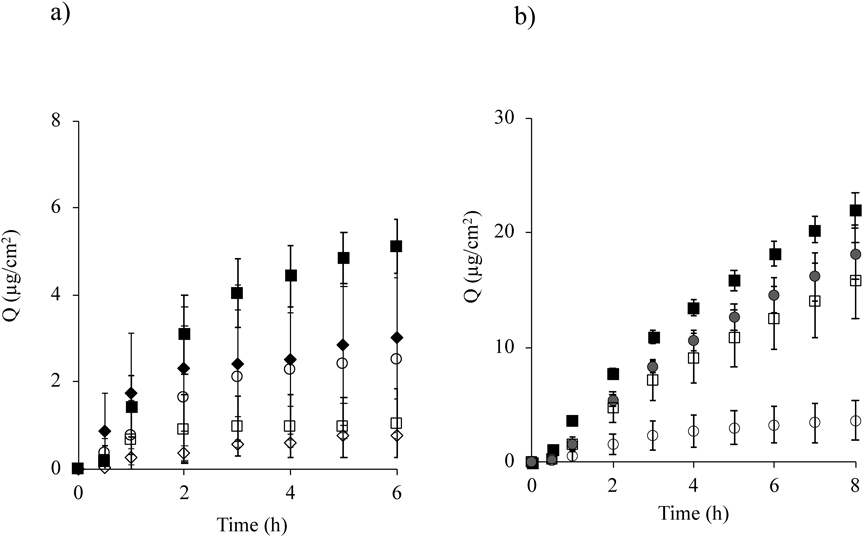
Mean ± S.D. (n = 4–6). Symbols; ○ : applied caffein solution to non-hydrated skin, ■: pretreatment with humidifier equipped with PEDOT/PSS at a 3.0 cm distance from the skin surface, ◆: pretreatment with evaporative humidifier at a 3.0 cm distance from the skin surface, □: treatment with humidifier equipped with PEDOT/PSS at a 3.0 cm distance after application of caffeine formulation, ◇: treatment with evaporative humidifier at a 3.0 cm distance after application of caffeine formulation, and ●: applied o/w emulsion to non-hydrated skin.
O/W emulsions are often used in cosmetics; therefore, in the next step, we investigated whether the enhanced skin permeation effect using the humidifier equipped with PEDOT/PSS was obtained with an o/w formulation. Figure 5b shows the skin permeation profile of caffeine from an o/w formulation. When the o/w emulsion was applied without treatment using the humidifier (●), higher skin permeation of caffeine was observed (about 5-fold higher Q8h for emulsion) compared with its solution (○). Caffeine permeation from the emulsion after pretreatment with a humidifier equipped with PEDOT/PSS at a 3 cm distance (■) was slightly higher (about 1.2-fold higher Q8h) compared with non-hydrated skin (●).
When enhancement skin permeation of caffeine that obtained by application of the humidifier with PEDOT/PSS was compared between the application distances, 3.0 cm distance showed a higher value (Fig. 4). The RH profiles obtained from 3.0 and 5.0 cm distances were almost the same, although temperature obtained from 3.0 distance was higher than 5.0 cm distance. When absolute humidity (AH) was calculated with Tetens equations shown as follows24):
 | (1) |
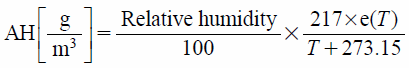 | (2) |
where e(T) is the saturation vapor pressure and T is expressed as °C. AH values obtained from 3 cm and 5 distances were about 30 and 24 g/m3, respectively, when the obtained temperature and RH in the profiles from 3 cm (35 °C, 80RH%), 5 cm (31 °C, 80RH%) were used (Fig. 2). Since higher AH would contribute to hydration of SC, 3 cm distance application showed higher caffeine permeation compared with 5 cm distance.
A higher temperature was observed as the application distance became shorter, and no obvious increase of RH was confirmed at 10 cm distance. Since water droplets and heat were easily diffused by air flow, no increased RH and temperature was thought to be observed at 10 cm distance condition compared with 3.0 and 5.0 cm distances.
SC hydration was performed over 1 h with the humidifier with PEDOT/PSS at in vitro skin permeation experiment. As a preliminary experiment, the skin permeation enhancement effect was investigated with a 0.5-h application period (0.25-h hydration process). Unlike the 1-h treatment, no skin permeation enhancement was observed (data not shown). Arce et al.25) and Sugibayashi et al.26) reported that 30–60 min was required to reach a steady-state permeation through the SC. The humidifier equipped with PEDOT/PSS used in this study repeats the dispatch of water droplets and filling water in the conductive polymer material every minute. Thus, a total 0.5-h application of water droplets was not enough to induce enough of a skin penetration-enhancement effect.
SC hydration may cause structural rearrangement of the intercellular lipids of corneocytes, resulting in fluidization of the lipid bilayers.27,28) Nishimura et al.18) reported that fine water particles formed using a PEDOT/PSS-equipped device showed improved skin conductance as well as skin elasticity. In addition, the water retention function remained constant over 360 min after application. They concluded that the smaller diameter of applied water particles than the intercellular spaces of the SC was key for penetration into the epidermis layer. In addition, increased drug partitioning into the skin may be obtained through increased drug solubility in the SC induced by hydration.29) Therefore, maintained higher water content in the SC might be a reason for the improved skin permeation of caffeine. Especially, humidifier with PEDOT/PSS from 3.0 cm distance showed significantly higher capacitance values at 0, 10, 20, and 30 min after finishing the hydration process. Thus, skin permeation enhancement effect might be provided at the beginning of the skin permeation of caffeine.
In the present study, particle size released from the humidifier equipped with PEDOT/PSS was not determined. Lower limitation of the scanning mobility particle sizer used in the present study was smaller than 2.5 nm. Thus, the released particle size from the humidifier equipped with PEDOT/PSS might be smaller than 2.5 nm. Further experiment should be done to confirm the released particle size.
Skin permeation of caffeine at a finite dose condition was used to evaluate the enhancement effect. When caffeine permeation through non-hydrated skin was compared between solution and o/w emulsion, the skin permeation from the emulsion was significantly higher than the solution (p < 0.05). The emulsion contained octyldodecyl lactate in the formulation. The solubility of caffeine in the ester oil was 6.17 mg/mL.30) The solubility of caffeine in purified water is much higher (solubility in purified water; 23.5 mg/mL). Therefore, the decreased solubility by adding ester oil may be related to the increased thermodynamic activity of caffeine in the formulations. On the other hand, no skin penetration-enhancement effect was observed when treatment with water droplets was done after application of the formulations. The hydration effect caused by applying water droplets was ineffective because the hydration process was hindered by the formulation. This result suggested that the penetration of fine water droplets into skin might be associated with the increased skin permeation of caffeine.
In the present study, a hydrophilic low-molecular-weight drug, caffeine, was selected as a model drug. Humidifier with PEDOT/PSS, which has skin permeation enhancement effect of caffeine without dramatically altering the barrier function of the SC, would have an advantage to increase skin permeation of a hydrophilic active ingredient from cosmetic products used on daily basis. It is necessary to investigate whether the present skin-penetration enhancing effect is observed with other drugs with different ranges of lipophilicity and molecular weight. Further experiments should be done to clarify the usefulness of this device to increase the skin permeation of topically applied chemicals.
Pretreatment with water droplets produced with a PEDOT/PSS-equipped humidifier enhanced the skin permeation of caffeine from aqueous solution and o/w emulsion compared with evaporative humidifier. The results may be related to the hydration process of the SC. Since this approach could be applied safely compared with other skin permeation means, it is considered desirable to use this PEDOT/PSS-equipped humidifier to improve the skin permeation of active ingredients from products that are used daily, such as cosmetics.
This study was funded by Aisin Seiki Co., Ltd. (Kariya, Aichi, Japan).