2021 年 69 巻 8 号 p. 806-810
2021 年 69 巻 8 号 p. 806-810
Effect of rubbing application on the skin permeation of a hydrophilic drug caffeine (CAF) and lipophilic drug rhododendrol (RD) from lotion and cream were investigated. Skin permeation of CAF was markedly increased by rubbing action independent of the formulation type. In addition, the skin penetration-enhancement effect was affected by the rubbing direction: rubbing application against the direction of hair growth showed the highest permeation compared with rubbing applications along the direction of hair growth and in a circular pattern on the skin. On the other hand, no enhancement effect was observed by the rubbing actions on the skin permeation of RD, regardless of formulation type. Change in the infundibula orifice size of hair follicles by the rubbing and following skin stretching may be related to the higher skin permeation for CAF. In contrast, high RD distribution into the stratum corneum may be a reason why no enhancement effect was observed by the rubbing action. These results can be helpful to predict safety and effectiveness of topically applied formulations.
The skin offers an administration site for pharmaceutical as well as cosmeceutical compounds. There are two possible permeation pathways, trans-epidermal (via the stratum corneum (SC)) and trans-appendage (via hair follicles (HFs) and sweat ducts) routes, for topically applied chemicals. The SC is known as the primary skin permeation route for transdermal drug delivery. Therefore, a diffusion model in SC can be applicable to calculate the skin permeation of chemicals.1–3) On the other hand, the HF route plays an important role for the skin permeation of hydrophilic compounds4,5) especially at an early stage for the skin permeation process,6,7) despite of a low contribution to the skin permeation at steady state. We have already developed an experimental method to separately evaluate the skin permeation of chemicals through trans-epidermal and trans-appendage routes.8,9) In addition, the effect of rubbing applications on the skin permeation of a hydrophilic compound, caffeine (CAF), was investigated,9) and the skin permeation of CAF from its solution was significantly increased by rubbing application. However, we had little experimental results for the effect of rubbing application on the chemical permeation through trans-epidermal and trans-appendage routes. Thus, effect of rubbing on the skin permeation route was investigated with two model drugs, CAF and rhododendrol (RD), in the present study. The primal skin permeation route of CAF and RD were trans-appendage and trans-epidermal routes, respectively.
Various formulations such as lotion, cream, gel, etc., have been used in the topical application of drugs. Few experiments have been performed to reveal the effect of rubbing application on the skin permeation of chemicals from various types of formulations as mentioned above, although many reports have been published on the skin-penetration enhancement effects by increasing the thermodynamic activity of chemicals in the formulation.10–13) Thus, in the present study, the effect of rubbing application on the skin permeation of chemicals from lotion and cream was investigated.
Typical formulations for cosmetic products; lotion and cream formulations were used in the present study. CAF was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). RD was provided by Kanebo Cosmetics Inc. (Tokyo, Japan). Products that were on the market in the past; lotion (product name: whitening lotion m) and cream (product name: whitening cream G) containing RD, were provided by Kanebo Cosmetics Inc.
Cosmetic grade of polyethylene glycol (PEG)-60 hydrogenated castor oil, PEG-40 stearate, glyceryl stearate, cetearyl alcohol, mineral oil, triethylhexanoate, macadamia nut oil, and dimethicone were used. Other solvents and reagents were commercially available for HPLC or special grade products. No further purification was performed.
Cellophane tape (Standard D-squame Discs) was purchased from CuDerm (Dallas, TX, U.S.A.). Aron Alpha® for general use (ethyl 2-cyanoacrylate content >95%) was purchased from Toa Gosei (Tokyo, Japan). Edible pig ear skin was purchased from National Federation of Agricultural Cooperative Associations (Tokyo, Japan) and stored at −30 °C before the experiments.
Preparation of Applied FormulationsLotion and cream containing CAF were prepared according to the formulations used in “Dose Setting Method Guideline for Human Long-duration Trials (Safety),” as published by the Japanese Cosmetic Science Society.14,15) Briefly, the preparation procedure was as follows:
Lotion: Polyethylene glycol-60 hydrogenated castor oil and ethanol were mixed at room temperature (solution A). Separately, CAF, 1–3 butylene glycol, citric acid, and sodium citrate were dissolved in water at room temperature (solution B). Lotion was prepared by gradually adding solution A into solution B while stirring.
Cream: PEG-40 stearate, glyceryl stearate, cetearyl alcohol, mineral oil, triethylhexanoate, macadamia nut oil, and dimethicone were heated and mixed (solution C). CAF, 1–3 butylene glycol, and water were mixed and heated (solution D). The solution C was gradually added into solution D while stirring for emulsification, and then cooled. Finally, air bubbles were removed under reduced pressure to make cream.
The formulations containing RD (concentration; 1.0%) were commercial products. CAF and RD were completely dissolved in the applied formulation. The concentration of CAF in the formulation was fixed to be 1.0%.
In Vitro Skin Permeation ExperimentFrozen pig ear skin thawed in a warm bath at 32 °C was sliced to 1.5 mm from the skin surface which is a length of growth hair, and a glass cap with an effective skin permeation area of 1.77 cm2 was attached to the sliced skin with Aron Alpha®. After hydration treatment was done to the skin with 1.0 mL of purified water for 1 h, the applied purified water was removed and excess water was blotted with Kim-wipe. Each formulation (20 µL) was applied onto the effective permeation area of skin. When rubbing application was performed, rubbing with a pressure of 1.87–3.12 N/cm2 using a rubber-enclosed finger was conducted for 30 s to apply the formulation. Three different types of rubbing were treated: rubbing application in a circular pattern on the skin (drHF), that along the direction of hair growth (alHF), and that application against the direction of hair growth (agHF). Hair growth directions were confirmed before application visually with a digital microscope (VHX-5000, Keyence Co., Ltd., Osaka, Japan). Details of the rubbing procedure was described in our previous report.9) Additionally, the applied formulation was also spread over the effective skin permeation area with a spatula as the control application (without rubbing application). After applied the formulation on the skin with or without rubbing application, the skin was then mounted in the Franz-type diffusion cell. During the experiment, the temperature of the receiver solution was maintained at 37 °C, and the solution was agitated continuously with a star-head-type magnetic stirrer. At predetermined times, the receiver solution (0.5 mL) was sampled, and the same volume of purified water was added to keep the volume constant.
CAF or RD Distribution in the Stratum Corneum and Hair FolliclesCAF or RD distribution in the SC and HFs was investigated 5 min after application. After finishing the application period, the skin was thoroughly washed three times with 1 mL of purified water. Distributed CAF and RD were collected from the SC and HFs by tape stripping (TS) and cyanoacrylate biopsy (CB) methods, respectively.8,9) Briefly, TS was repeated 20 times to remove the SC layers. Then, CAF or RD was extracted in 1.0 mL of ethanol in a glass vial for 1 h from the SC layers. Cured cyanoacrylate was collected from the skin using a sheet of cellophane tape after applied one drop of cyanoacrylate liquid onto a TS-treated area. CAF or RD was extracted from the HF replica in 1.0 mL of methanol in a glass vial. CAF and RD concentrations in the extracted solution were detected using HPLC.
Measurement of Skin Concentration in the Viable Epidermis/DermisWeighed CB-treated skin was minced with scissors followed by homogenizing (12000 rpm, 5 min, 4 °C) using a Polytron PT-MR 3000 (Kinematica Inc., Littau, Switzerland) with 2.0 mL of methanol. After centrifugation (21200 × g, 4 °C, 15 min) of the obtained sample, the supernatant (200 µL) was mixed with the same volume of acetonitrile and centrifuged again using the same conditions.
Detection Using HPLCCAF and RD concentrations in the samples were detected using an HPLC system (Prominence; Shimadzu, Kyoto, Japan). The sample solution was injected into the HPLC system, and analysis was performed using an Inertsil-octadecyl silica (ODS)-3 column (5 µm, 150 × 4.6 mm2 i.d.; GL Science, Kyoto, Japan) at 40 °C using 0.1% phosphoric acid/acetonitrile (10/90, v/v) for CAF detection and water/acetonitrile (25/75, v/v) for RD detection as mobile phases (flow rate 1.0 mL/min). Detection was performed using a UV detector at 254 nm for CAF and at 280 nm for RD. CAF and RD concentrations (% dose/cm2) in HFs was corrected using the total opening HF area in the application area of formulations, whereas that in the SC was corrected using the neat SC area calculated from the subtraction of the total opening HF area from the formulation application area. The total opening HF area was observed with a digital microscope (VHX-5000 Keyence Co., Ltd., Osaka, Japan) equipped with a dimensional measurement software (VH-M100 X, Keyence Co., Ltd.). The lower limitations of CAF and RD detections were 1.0 and 0.5 µg/mL, respectively.
Statistical AnalysisStatistical analysis was carried out using unpaired Student’s t-test with JMP® Pro (ver. 14.1.0, SAS Institute, Cary, NC, U.S.A.). Experimental data were tested for statistical significance (p < 0.05) using one-way ANOVA and Tukey’s honestly significant difference post hoc analysis.
Figure 1 shows the effect of rubbing application on the skin permeation of CAF from lotion (a) and cream (b) formulations. When CAF was applied on the skin with rubbing application, skin permeation was increased from that without rubbing, and this was independent of the formulation type. The skin penetration-enhancement effect was observed in the following order in both formulations: agHF > drHF > alHF > without rubbing. These results corresponded with our previous results9) when applied CAF aq. solution. When the enhancement effect by rubbing application was calculated using Q6h values (cumulative amount of drug permeated through skin over 6 h, %/cm2/dose), agHF, drHF, and alHF showed 3.1-, 2.6-, and 1.8-fold higher than without rubbing for the lotion formulation, respectively, and 2.4-, 2.0-, and 1.3-fold higher with the cream formulation, respectively.
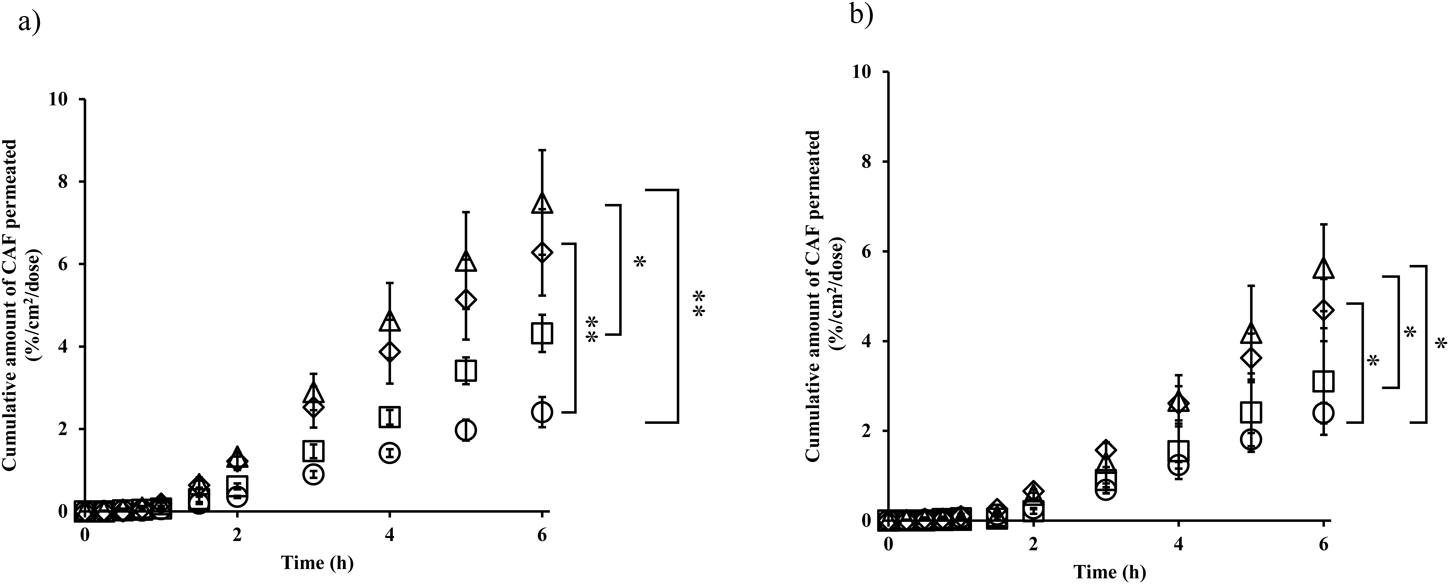
Symbols; ○: without rubbing, ◇: rubbing circular, △: against hair flow, □: toward hair flow. Each value shows the mean ± standard deviation (S.D.) (n = 3). *: p < 0.05 and **: p < 0.01 compared with no rubbing.
Figure 2 shows RD permeation from lotion and creams with or without rubbing applications. When a formulation containing RD was applied without rubbing, the Q6h from lotion was higher than that from cream. However, rubbing application provided no skin penetration–enhancement of RD regardless of rubbing direction.

Symbols; ○: without rubbing, ◇: rubbing circular, △: against hair flow, □: toward hair flow. Each value shows the mean ± S.D. (n = 3).
Generally, chemical distribution in the skin affects its skin permeation.16) CAF and RD distribution in the skin after application of the lotion and cream was also investigated. Figure 3 shows the CAF concentration in SC (a), HFs (b), and viable epidermis and dermis (VED) (c) 5 min after application of lotion (open bar) and cream (closed bar) formulations. The CAF concentration in the SC, HFs, and VED after application of lotion was higher than that of cream in all applications. As considered from the skin permeation result, rubbing application delivered high amounts of CAF into the skin. In addition, agHF showed the highest CAF concentrations in the SC, HFs, and VED in both formulations among the rubbing applications. When the distribution of polystyrene particles was observed after rubbing application using confocal microscopy with a skin section prepared with a microtome, the particles were mainly located in HFs, whereas a slight distribution of particles was observed in the SC.9) This was related to a change in the diameter of the infundibula orifices of HFs by stretching due to the rubbing action. CAF distribution seemed to be increased by the rubbing actions. However, the TS procedure recovered drugs not only from SC but also from the shallow depth in the HFs. Therefore, this might be a reason for the higher CAF concentration detected in the SC.

Each value shows the mean ± S.D. (n = 3). CAF concentration in the VED was not detected after application of cream with no rubbing action. *: p < 0.05 and **: p < 0.01
Figure 4 shows the RD concentration in skin. Unlike the CAF concentration in skin, no difference in the RD concentration was observed among the different applications, although higher RD concentrations were confirmed from lotion applications compared with those obtained from the cream formulation. RD partitions easily into the SC due to its lipophilic nature (clog P: 1.9). This may be a reason for almost no rubbing effect on the skin penetration–enhancement effect for RD. From the obtained results, different effects were thought to be related to the primal skin permeation route of drugs.
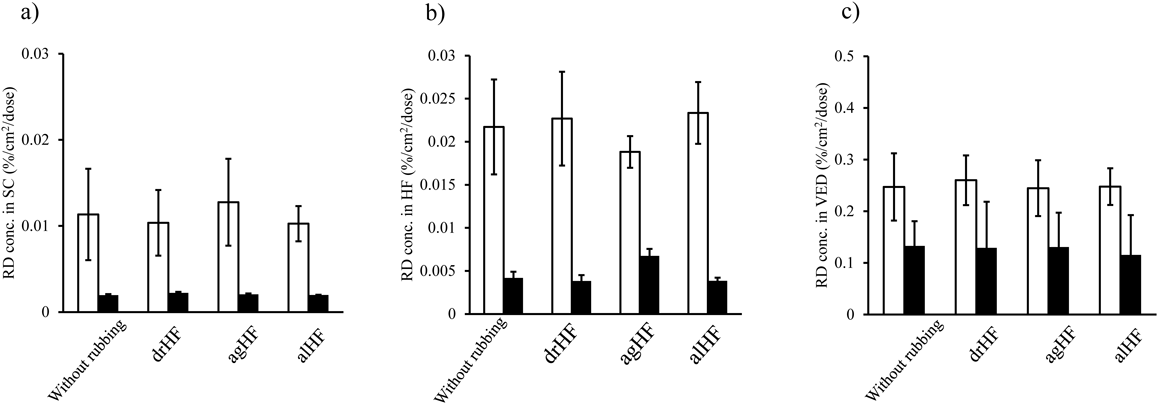
Each value shows the mean ± S.D. (n = 3).
The size and density of HFs in porcine skin used in the present study are almost the same to the thigh skin in human,17,18) with its large size and low density of HFs compared with the facial skin in human. Therefore, the enhancement ratio by rubbing application might be different to the obtained result, but a similar tendency should be observed when rubbing application was conducted on the facial area. In addition, disruption microstructure of formulation, such as emulsions, would be occurred by rubbing application. Thus, further investigation should be done to reveal the effect of rubbing application with drug characteristics, skin features, and formulation types.
In case of a hydrophilic drug CAF, the rubbing action markedly increased the skin permeation independent of formulation type. On the other hand, no effect from rubbing was observed for a lipophilic drug RD. According to a survey by Kanebo, although numerical data had been produced on the basis of inaccurate information, the incidence among patients was affected by the formulation.19) Arce et al. reported that RD permeation was influenced by the applied dose as well as type of RD-containing formulation.20) Further experiments should be done with different types of formulations and drugs with different physicochemical properties to clarify the effect of rubbing application on skin permeation. This would provide helpful information to develop topically applied formulations having enough safety and high effectiveness.
The authors declare no conflict of interest.