2021 年 69 巻 8 号 p. 773-780
2021 年 69 巻 8 号 p. 773-780
Multifunctional synthetic polymers can bind to target molecules and are therefore widely investigated in diagnostics, drug delivery carriers, and separation carriers. Because these polymers are synthesized from nonbiological components, purification processes (e.g., chromatography, dialysis, extraction, and centrifugation) must be conducted after the synthesis. Although several purification methods are used for polymer purification, few reports have revealed the influence of purification process on the functions of polymer. In this study, we demonstrated that the characteristics, function, and stability of synthetic polymer depend on the purification process. N-Isopropylacrylamide-based polymer nanoparticles (NPs) and melittin (i.e., honey bee venom) were used as a model of synthetic polymer and target toxic peptide, respectively. Synthesized NPs were purified by dialysis in methanol, acetone precipitation, or centrifugation. NPs purified by dialysis in ultrapure water were used as control NPs. Then, NP size, surface charge, toxin neutralization effect, and stability were determined. NP size did not considerably change by purification with centrifugation; however, it decreased by purification using dialysis in methanol and acetone precipitation compared with that of control NPs. The ζ-potential of NPs changed after each purification process compared with that of control NPs. The melittin neutralization efficiency of NPs depended on the purification process; i.e., it decreased by acetone precipitation and increased by dialysis in methanol and centrifugation compared with that of control NPs. Of note, the addition of methanol and acetone decreased NP stability. These studies implied the importance of considering the effect of the purification method on synthetic polymer function.
Protein–protein interactions (PPIs) consist of multiple non-covalent interactions such as hydrogen bond, electrostatic, and hydrophobic interactions. Because of their highly specific and precisely controlled interactions, they are important in several functions in our body (e.g., cellular growth, host defense, and enzyme reaction).1) Abnormal PPIs often induce various biological dysfunctions and diseases; therefore, protein affinity reagents that specifically inhibit PPIs have been used for disease therapy, diagnosis, detection, and purification.2) Antibodies and their fragments are attracting considerable attention as an ideal modality of specific PPI inhibitor owing to their high specificity and affinity against the target.3) Theoretically, they can target all proteins. However, antibodies still have problems, including high purification cost, and instability. In addition, because of the biological product, anaphylactic shock and serum sickness are serious concerns for the patients. Although many approaches have been studied for the inhibition of PPIs, the development of stable and inexpensive abiotic protein affinity reagents is a great challenge alternative to antibodies.
Multifunctional synthetic polymers are attracting attention as an abiotic protein affinity reagent for therapeutic application,4) drug delivery carrier,5,6) separation carrier,7,8) and cell engineering.9,10) For example, sulfate-functionalized dendrimers bind to cell adhesion molecules E-, L-, and P-selectin and treat inflammation in vivo by blocking leukocyte and endothelium interactions.11) Dendrimer “molecular glue” sticks and stabilizes microtubules owing to a multivalent salt bridge formed by sticky guanidinium ion pendants.12) The length-tuning of glycopolymers changes the degree of clustering lectins expressed in dendritic cells (DC-SIGN).13) Homogenous oligomers neutralize venom peptides by precise molecular recognition of critical domain.14) Rationally designed acrylamide-based copolymers bind to small molecules,15) peptides,16) and proteins17) with high selectivity by optimizing functional monomer structure and feed ratio.18,19) It has been also reported that polymer flexibility and length are important for the induction of high target specificity and affinity.20,21) Although these synthetic polymers can bind to target molecules with high affinity and specificity both in vitro and in vivo, they must be purified before use because they are synthesized using nonbiological monomers.
In general, polymerized (or synthesized) materials contain by-products, unreacted compounds, surfactant, salt, and/or free radicals in addition to the proposed polymers after the synthesis. These nontarget compounds often induce severe side effects, such as oxidative stress and hemolysis, and reduce polymer functionality; therefore, synthesized polymers must be purified before use. Several methods have been used for polymer purification, such as chromatography, centrifugation, dialysis, and extraction.22) Each purification method has disadvantages. For example, dialysis can be applied to higher-molecular-weight polymers, but it depends on polymer solubility.15,23,24) Centrifugation is conducted on high-density polymers, including gold or silver nanoparticles.13,25,26) Relatively low-molecular-weight polymers (e.g., dendrimers and oligomers) are usually purified by chromatography and extraction.11,12,14) Therefore, purification method was not unified. Thus, sometimes, several methods are used even for the same materials. However, there are few reports on the influence of the purification method of synthetic polymers on their functions.
In this study, we demonstrated that the polymer purification method drastically alters the characteristics, function, and stability of nanoparticles (NPs). Specifically, synthetic polymers were purified by three major purification methods: dialysis in methanol, acetone precipitation, and centrifugation in addition to the purification by dialysis in ultrapure water. N-Isopropylacrylamide (NIPAm)-based polymer NPs were used as a model of abiotic protein affinity reagent. Melittin (i.e., honey bee venom) was used as a model of toxic peptide for the evaluation of NP function. The size, ζ-potential, yield, and monomer incorporation ratio of NPs were determined after each purification method. The melittin neutralization effect of each NP was measured to determine the NP function.
The functionalization of lightly cross-linked NIPAm-based NPs with acrylic acid (AAc) (negatively charged monomer) and N-tert-butylacrylamide (TBAm hydrophobic monomer) enables them to bind to and neutralize honey bee venom (i.e., melittin) both in vitro and in vivo.16,18) Therefore, we used NIPAm-based NPs and melittin as a model of abiotic protein affinity reagent and toxic peptide, respectively, for the evaluation of NP function in vitro. NPs were prepared by free-radical copolymerization using NIPAm (18 mol%), Bis (2 mol%, cross-linker), TBAm (40 mol%), and AAc (40 mol%, Fig. 1). We and other groups purified synthesized synthetic polymers by dialysis against ultrapure water,23,24) and the polymers showed high affinity for the target both in vitro and in vivo.16,18) Therefore, NPs purified by dialysis against ultrapure water were used as control NPs (NP1). Synthesized NPs were added to the dialysis membrane and dialyzed against 100 volumes of ultrapure water. Water was changed every 12 h (8 times). The purified NP (NP1) size, ζ-potential, and polydispersity index (PDI) were approx. 166 nm, approx. −21 mV, and 0.012, respectively (Table 1).
Functional monomers used in this study. NPs were synthesized by free-radical copolymerization of functional monomer in the presence of SDS (0.0347 mM) in water. Following the addition of APS (2.63 mM), polymerization was performed at 65 °C for 3 h under nitrogen atmosphere.
| NP | Purification method | Size (nm) | PDI | ζ-Potential (mV) | T/N ratio | Yield of NIPAm (%) |
|---|---|---|---|---|---|---|
| 1 | Dialysis in ultrapure water | 166 ± 2 | 0.012 ± 0.008 | −21 ± 2 | 2.36 | 90 ± 1 |
| 2 | Dialysis in methanol (evaporated methanol) | 105 ± 1 | 0.147 ± 0.010 | −18 ± 1 | 2.68 | 82 ± 1 |
| 3 | Dialysis in ultrapure water (resuspended by methanol) | 114 ± 1 | 0.219 ± 0.016 | −22 ± 2 | 2.36 | 90 ± 1 |
| 4 | Acetone precipitation | 131 ± 1 | 0.106 ± 0.022 | −3 ± 1 | 2.77 | 79 ± 2 |
| 5 | Centrifugation | 171 ± 3 | 0.067 ± 0.017 | −24 ± 2 | 2.48 | 86 ± 1 |
T/N ratio, TBAm/NIPAm ratio; PDI, polydispersity index.
To determine the incorporation percentage of NIPAm, radiolabeled NPs were synthesized by adding a small amount of [3H]-labeled NIPAm. Then, radiolabeled NPs were purified by dialysis against ultrapure water (NP1). The incorporation percentage of NIPAm was 90%, which indicated that high percentage of NIPAm was incorporated in NPs. Next, the NIPAm/TBAm ratio of NP1 was determined by 1H-NMR. The NIPAm/TBAm ratio of NP1 was 1 : 2.36 (Table 1), which indicated that high percent of NIPAm and TBAm was incorporated in NPs.
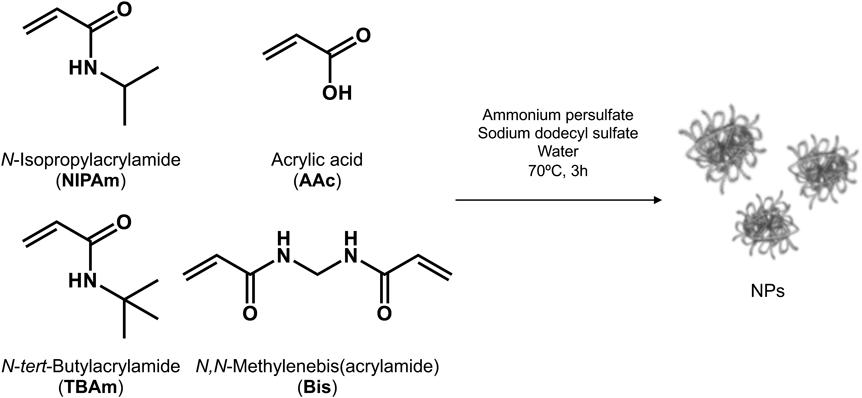
NP Purification by Dialysis against MethanolIt was reported that NIPAm-based NPs swell in methanol.27) In fact, using dynamic light scattering method we were unable to determine the size of NP dispersed in methanol. We hypothesized that dialyzing NPs in methanol effectively remove short-length polymers and oligomers, which caught into polymer chains owing to swelling in methanol. Synthesized NPs were added to the dialysis membrane and dialyzed in 100 volumes of methanol (Fig. 2a). Methanol was changed every 12 h (8 times). After purification, 100 volume of ultrapure water was added to NPs, and methanol was evaporated by rotary evaporator (NP2). The size and ζ-potential of NP2 are shown in Fig. 2b, Table 1. NP2 became smaller (approx. 100 nm), had large PDI (0.147), and slightly neutral charge compared with NP1 (control NPs). These results indicate that dialyzing NPs in methanol changed their characteristics. Since we suspected that this effect was caused by a small amount of methanol remaining in the evaporated solution, we used 1H-NMR to measure the amount of methanol in the solution (Fig. 2c). Results show traces of methanol in the solution. Although NPs swell in 100% methanol, NPs dispersed in water that contains only a small amount of methanol show different behavior. It has been reported that methanol forms hydrogen bonds against carbonyl or amine groups of the functional group in NPs when methanol molecules contact NPs.28) The methyl group in methanol easily interacts with another hydrophobic group in polymer chains (e.g., isopropyl or tert-butyl groups) by hydrophobic interaction. Therefore, NPs can be shrunk, and the particle size can be decreased by adding methanol.

(a) Schematic image of NP dialysis in methanol (left) and methanol (right). (b) Size distribution of NP1, 2, and 3. (c) Methanol remaining level NPs solution (1H-NMR). Peak around δ 3.34 (s, 3H, CH3OH) shows amount of methanol and δ 4.79 shows the peak of water. (d) Time-dependent remaining level of NIPAm purified by dialysis in ultrapure water or methanol. Radiolabeled NPs were purified by dialysis in ultrapure water or methanol. The dialysis medium was exchanged every 12 h (8 times). Then, radio activities in the dialysis medium were measured each time. (e) The inhibition of hemolysis by NPs purified by dialysis. RBCs were incubated with melittin (10 µM) and NPs (3, 10, 30, or 100 µg/mL) for 30 min at 37 °C after the preincubation of NPs and melittin for 30 min at 37 °C in PBS. Then, the amount of hemoglobin was measured. Significant difference; ** p < 0.01, *** p < 0.001. (Color figure can be accessed in the online version.)
To confirm that the NP characteristic change occurred by dialysis in methanol, NPs dialyzed in ultrapure water were lyophilized and redispersed in a small amount of methanol, and 100 volume of ultrapure water was added. Then, methanol was evaporated (NP3). As a result, NP3 size became smaller and PDI became larger, and ζ-potential did not change compared with that of NP1 (control NPs) and NP2 (Fig. 2b, Table 1). These results suggest that NP characteristics change by the addition of methanol regardless of the incubation time with methanol.
Next, we measured the incorporation percentage of NIPAm using radiolabeled NP2 and NIPAm/TBAm ratio by 1H-NMR. The incorporation percentage of NIPAm decreased by dialyzing in methanol (82%) compared with dialyzing in ultrapure water. The NIPAm/TBAm ratio of NP2 was 1 : 2.68. These results indicate that dialyzing in methanol effectively removes short-length polymers and oligomers owing to swelling in methanol. From these results, we hypothesized that the NP dialysis speed of dialyzing in methanol was faster than that of dialyzing in ultrapure water owing to swelling NPs in methanol. To demonstrate the dialysis speed, radiolabeled NPs were dialyzed in ultrapure water or methanol, and the dialysis medium was exchanged every 12 h (8 times). Then, radioactivity in dialysis media was measured to determine the remaining level of NIPAm in NPs that rapidly decreased by dialyzing in methanol compared with dialyzing in water. The remaining level of [3H] NIPAm in NPs was almost constant after 12 h of dialyzing in water; however, that of [3H] NIPAm in NPs gradually decreased even after 12 h of dialyzing in methanol (Fig. 2d). Eventually, higher purification efficiency was obtained by dialyzing in methanol compared with dialyzing in water. These results suggested that dialyzing under swelling conditions effectively purified NPs.
To demonstrate the effect of NP purification process on the function, RBCs were incubated with NP1, NP2, or NP3 (3, 10, 30, and 100 µg/mL) and melittin (10 µM) for 1 h at 37 °C. Then, the amount of hemoglobin was measured (Fig. 2e). All tested NPs dose-dependently inhibited melittin toxicity, indicating all NPs capture and neutralize melittin. Surprisingly, NP2 (dialyzed in methanol) exhibited higher melittin neutralization efficiency compared with NP1 (dialyzed in ultrapure water). In addition, the melittin neutralization efficiency of NP3 was almost equal to that of NP2. These results suggested that the contact of NPs with methanol enhanced melittin neutralization efficiency. It has been reported that the polymer density of NPs considerably increases in the presence of methanol by NP shrinking.29) It is known that hydrophobicity is important for the binding of NPs to target melittin.30) Therefore, solvent substitution from ultrapure water to methanol should shrink and increase NP polymer density and increase hydrophobicity, which enhance the melittin neutralization efficiency of NPs.
NP Purification Using the Acetone Precipitation MethodNext, we purified NPs by the acetone precipitation method, which is a common method for polymer purification, and several groups have used this method for polymer purification.31) NPs and acetone (1 : 1 volume ratio) were vortexed for 1 min at room temperature (Fig. 3a). Then, the precipitate was lyophilized to completely dry the solvent. Because dried NPs could not redisperse in ultrapure water, we added methanol and ultrapure water (methanol/water = 1 : 100 volume ratio) for redispersion; then, methanol was evaporated (NP4). Because a small amount of methanol was added to NP4 for redispersion, we used NP3 as control NPs in the experiments. The size of NP4 was approx. 130 nm, which was smaller than that of NP1 (dialyzed in ultrapure water, Fig. 3b, Table 1). Surprisingly, the ζ-potential of NP4 was almost neutral (approx. −3 mV). The remaining acetone level in the NP4 solution was then determined using 1H-NMR (Fig. 3c). Only a small amount of acetone remained in the NP4 solution, suggesting that the NP4 size change is caused by the polymer chain interaction with acetone molecules. NPs were prepared with several functional monomers and radical initiators (ammonium persulfate (APS)). The negative charge of NPs is mainly derived from the carboxyl group of AAc and sulfate groups of APS. ζ-Potential of NP4 dramatically increased after the acetone precipitation purification; therefore, we considered that these negative charge groups may migrate to the core of NPs and other hydrophobic groups may move to NP surface after the acetone precipitation purification. The incorporation percentage of NIPAm in NP4 was 79%. The NIPAm/TBAm ratio of NP4 was 1 : 2.77. These results indicate that acetone precipitation removed NIPAm-derived polymers that could dissolve in acetone/water mixture.
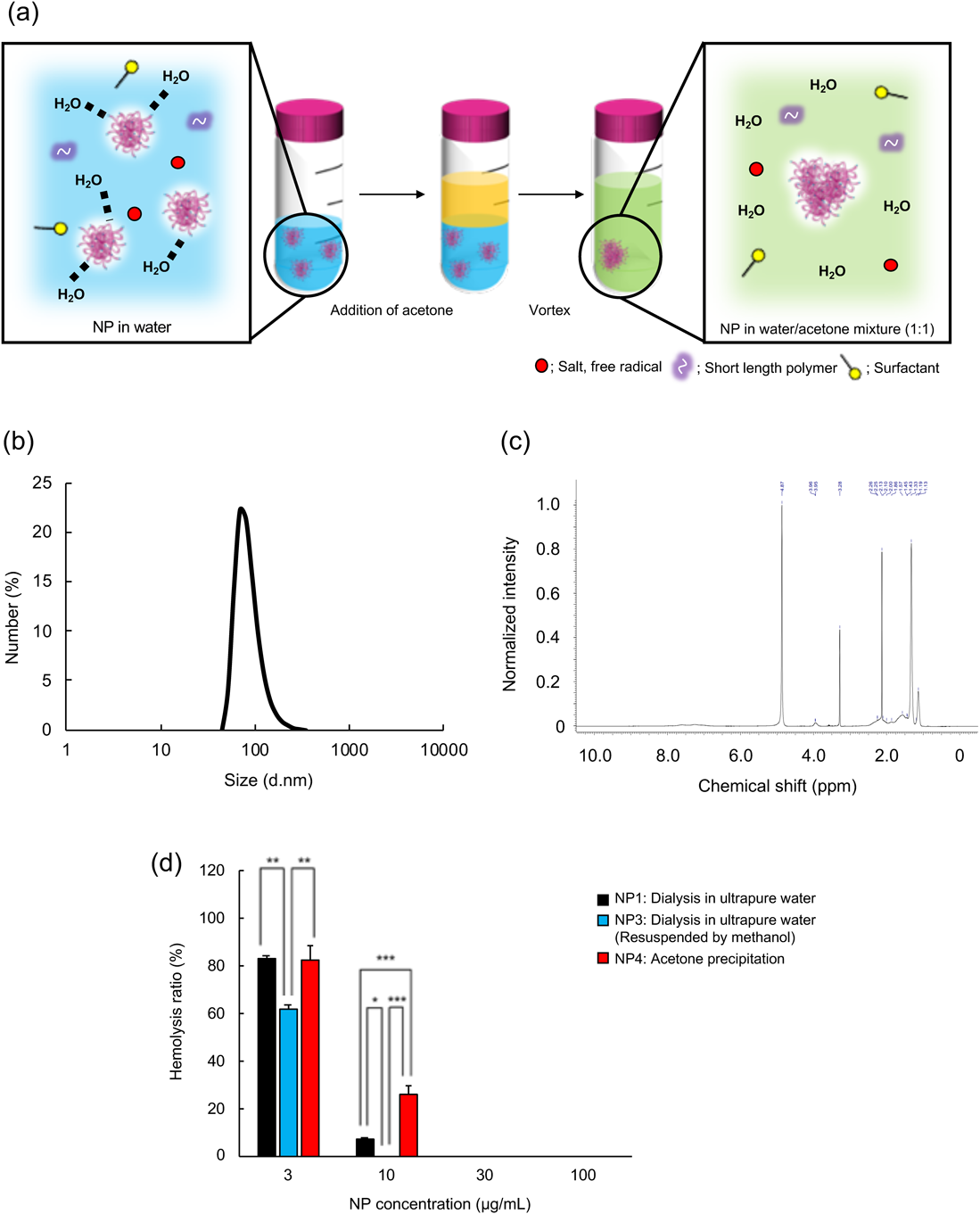
(a) Schematic diagram of NP purification by acetone precipitation. (b) Size distribution of NP4. (c) Acetone remaining level in NP4 solution (1H-NMR). Peak around δ 2.15 (s, 6H, CH3C(O)CH3) is derived from acetone. (d) The inhibition of hemolysis by NPs purified by acetone precipitation. RBCs were incubated with melittin (10 µM) and NPs (3, 10, 30, or 100 µg/mL) for 30 min at 37 °C after the preincubation of NPs and melittin for 30 min at 37 °C in PBS. Then, the amount of hemoglobin was measured. Significant difference; * p < 0.05, ** p < 0.01, *** p < 0.001. (Color figure can be accessed in the online version.)
To evaluate the function of NPs purified by the acetone purification method (NP4), red blood cells (RBCs) were incubated with NP4 (3, 10, 30, or 100 µg/mL) and melittin (10 µM) for 1 h at 37 °C. Then, the amount of hemoglobin was measured (Fig. 3d). NP4 dose-dependently inhibited hemolytic activity of melittin, indicating purified NP4 capture and neutralize melittin. Although NP2 (dialyzed in ultrapure water, lyophilized, and redispersed in methanol and ultrapure water) showed a higher melittin neutralization effect than NP1 at 3 and 10 µg/mL, NP4 decreased the melittin neutralization effect compared with NP1. Although we are still under the investigation to reveal the decrease of melittin neutralization effect using NPs purified by acetone precipitation purification, changing ζ-potential of NPs may be a critical factor. There was a significant difference in melittin-neutralizing efficiency at the NP concentration of 10 µg/mL between NP1 and NP4. However, at an NP concentration of 3 µg/mL, there was none. We have no exact reason; however, we believe it is because of low NP concentration that we could not observe a significant difference at the concentration of 3 µg/mL.
NP Purification by the Centrifugation MethodNext, we performed NP purification by the centrifugation method (Fig. 4a), which is commonly used for high-density nanomaterials such as liposome, polymer micelles, and polymer NPs.32–35) NPs were centrifuged (6500 × g, 60 min, 25 °C), and the pellet was redispersed by adding ultrapure water (NP5). Although NP5 exhibited an almost the same size and NIPAm/TBAm ratio, the NIPAm yield slightly decreased, and the ζ-potential slightly decreased (approx. −24 mV) compared with NP1 (dialyzed in ultrapure water, Fig. 4b, Table 1). A decrease in ζ-potential after the centrifugation purification process is due to the difference in the amount of sulfate groups derived from APS in NPs. Because centrifugation purification is a density-based separation method, low-density NPs should be removed. Therefore, NP5 contain relatively high-density polymers compared with NP1. Because NP synthesis is initiated and extended by radical initiator (in this study, APS), NP5 may contain many sulfate groups and exhibit high negative charge. Incorporation percentage of NIPAm in NP5 was 86% and NIPAm/TBAm ratio of NP5 was 1 : 2.48. These results are very similar to NP1 (control NPs).
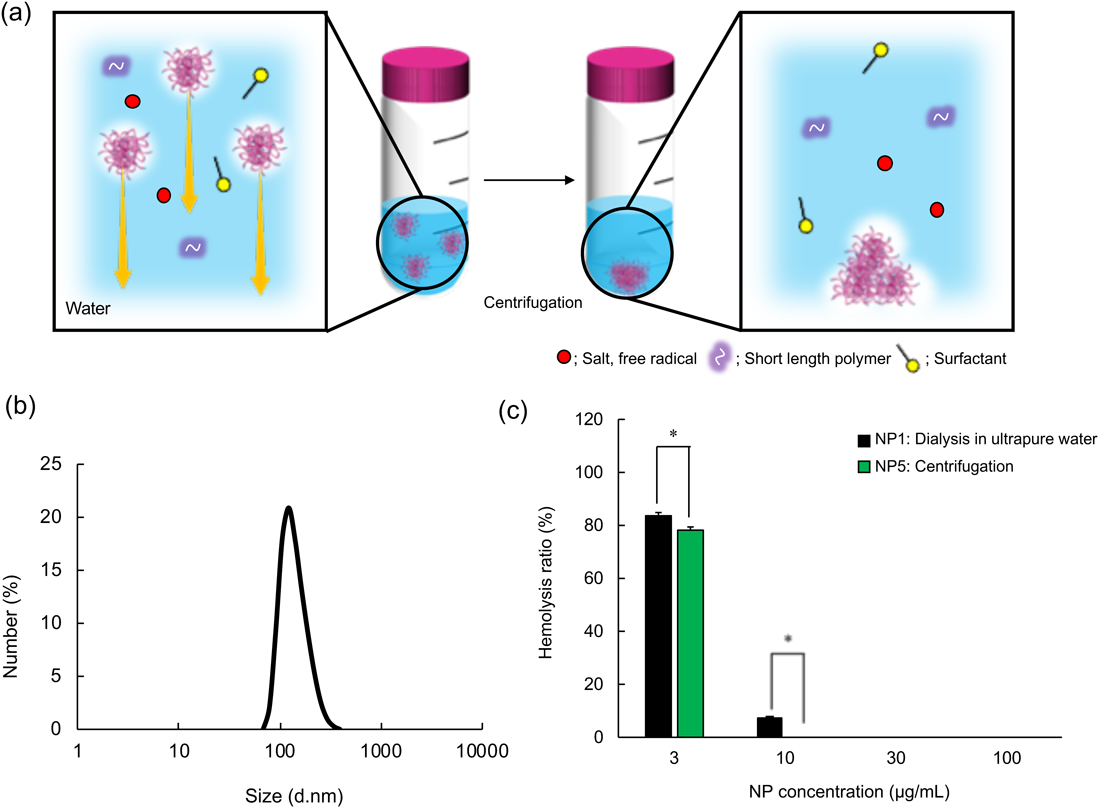
(a) Schematic image of NP purification by centrifugation. (b) Size distribution of NP5. (c) The inhibition of hemolysis by NPs purified by centrifugation. RBCs were incubated with melittin (10 µM) and NPs (3, 10, 30, or 100 µg/mL) for 30 min at 37 °C after the preincubation of NPs and melittin for 30 min at 37 °C in PBS. Then, the amount of hemoglobin was measured. Significant difference; * p < 0.05. (Color figure can be accessed in the online version.)
To demonstrate the function of NPs purified by the centrifugation method, RBCs were incubated with NP (3, 10, 30, or 100 µg/mL) and melittin (10 µM) for 1 h at 37 °C (Fig. 4c). NP5 dose-dependently inhibited melittin toxicity, indicating NP5 capture and neutralize melittin. NP5 showed slightly higher melittin neutralization efficiency possibly because NP5 might contain a higher ratio of high-density NPs compared with NP1.
NP Stability after Each PurificationTo demonstrate the stability of NPs after each purification process, NPs were centrifuged 14 d after each purification (Fig. 5). Although precipitation was not observed in NP1 and NP5, precipitation was observed in NP2, NP3, and NP4. These results indicate that the addition of organic solvents (e.g., methanol and acetone) decrease the stability of NPs. The NP stability change may be caused by increased NP core hydrophobicity. Increased NP hydrophobicity could enhance the interaction of each NP and induce aggregation.

Each NP was centrifugated (6500 × g, 2 min, 25 °C) 14 d after purification. Then, the precipitation of NPs was observed. (Color figure can be accessed in the online version.)
We demonstrated the influence of NP purification process on the characteristics and function. We purified NPs by dialysis in water or methanol, acetone precipitation, or centrifugation, respectively. Size of NPs adding organic solvents became smaller than that of dialyzing in ultrapure water. ζ-Potential of NPs shifted to neutral by adding acetone; however, that of NPs purified by centrifugation showed higher negative charge compared with that of NPs dialyzed in ultrapure water. The melittin neutralization efficacy of NPs considerably increased by adding methanol and slightly increased after centrifugation purification; however, that of NPs decreased by the acetone purification process. These results indicate that centrifugation purification seems to be an ideal purification method for synthetic polymers. However, the centrifugation purification method has several limitations. For example, the modification of hydrophilic polymers (e.g., polyethylene glycol) decreases polymer density and makes NP precipitation difficult. In addition, centrifugation conditions should be adjusted for each polymer; therefore, choosing an optimum purification process is essential. We hope that these results are useful for the preparation of synthetic polymers.
NIPAm and AAc were purchased from Tokyo Chemical Industry, Ltd. (Tokyo, Japan). NIPAm was recrystallized from hexane before use. N,N′-Methylenebis(acrylamide) (Bis), N-tert-butylacrylamide (TBAm), sodium dodecyl sulfate (SDS), and 2-propanol were purchased from Wako Pure Chemical Corporation (Osaka, Japan). APS and melittin (obtained from honey bee venom) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, U.S.A.). RBCs were purchased from Japan Bio Serum, Corp. (Hiroshima, Japan). [3H]-labeled NIPAm was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO, U.S.A.). Hionic-Fluor was purchased from PerkinElmer Japan, Ltd. (Kanagawa, Japan). All other reagents used were of analytical grade.
Synthesis of Polymer NPsSynthetic polymer NPs were prepared by free-radical copolymerization. Briefly, NIPAm (0.12 mmol), AAc (0.26 mmol), TBAm (0.26 mmol), and Bis (0.013 mmol) were dissolved in 10 mL of ultrapure water (final total monomer concentration: 65 mM). TBAm was added after dissolving in a small amount of ethanol. Then, SDS was added to the solution (final concentration: 0.694 mM). After nitrogen substitution for 30 min to remove oxygen in the solution, APS (final concentration: 2.63 mM) was added to initiate polymerization after sealing the flask with a rubber stopper. Polymerization reaction was conducted for 3 h at 70 °C and stopped by adding atmosphere. For the preparation of control NPs, NPs were purified by dialysis against ultrapure water at room temperature. Water was exchanged every 12 h (8 times).
NP Purification by Dialysis in MethanolNP suspension was transferred to the dialysis membrane [MWCO: 12–15 kDa, Spectrum Laboratories, Inc. (Rancho-Dominguez, CA, U.S.A.)] and dialyzed against 100 volume methanol at room temperature. Dialysis medium was exchanged every 12 h (8 times). After dialysis, methanol was removed by evaporation. NPs were dispersed in 1 mL of methanol; then, 100 volume of ultrapure water was added. Methanol in the solution was removed by evaporation.
NP Purification by Acetone PrecipitationOne volume of acetone was added to the NP suspension; then, the solution was vortexed for 1 min to precipitate NPs. The precipitate was lyophilized to remove acetone. NPs were dispersed in 1 mL of methanol; then, 100 volume of ultrapure water was added. Methanol in the solution was removed by evaporation.
NP Purification by CentrifugationNP suspension was centrifuged (6500 × g, 60 min, 25 °C). Then, ultrapure water was added to the precipitate and incubated overnight at 4 °C.
Measurement of NP Size and ζ-PotentialThe hydrodynamic diameter and ζ-potential of NPs in phosphate-buffered saline (PBS) (ionic strength: 162.7 mM36)) were measured by Zetasizer Nano ZS (Malvern, Worcestershire, U.K.) at 37 °C ±0.1 °C.”
Measurement of TBAm and NIPAm Incorporation Ratio by 1H-NMRTBAm and NIPAm incorporation ratios were determined by 1H-NMR. The peak of isopropyl group in NIPAm and tert-butyl group in TBAm was observed at δ 1.19 (s, 6H) and δ 1.38 (s, 9H), respectively.
Measurement of Remaining Level of Methanol in SolutionNPs (3 mg) were dissolved in 1 mL of methanol. 100 mL of ultrapure water was added to the NPs dispersion. Evaporation was conducted to remove the methanol until the sample volume reaches to 200 µL. Then, 400 µL of D2O was added and amount of methanol was measured by 1H-NMR.
Measurement of NIPAm Yield Using 3H-Labeled NPsEach purification step was performed using radiolabeled NPs. A total of 1 mL of the dialysis media or supernatant after vortexing in acetone or after centrifugation was mixed with 1 mL of 2-propanol and 10 mL of Hionic-Fluor. Then, radioactivity was measured by a liquid scintillation counter (LSC-7400, Hitachi Aloka Medical, Ltd., Tokyo, Japan).
Hemolysis AssayWhole bovine blood was added to PBS (pH = 7.4) and centrifuged (800 × g, 10 min, 4 °C). Purified RBCs were obtained by performing the washing step for five cycles. RBCs (3% v/v) were incubated with melittin (10 µM) and/or NPs (3, 10, 30, or 100 µg/mL) for 30 min at 37 °C after the preincubation of NPs and melittin for 30 min at 37 °C in PBS. After the incubation, the mixture was centrifuged (800 × g, 10 min, 4 °C), and the absorbance of hemoglobin in the supernatant was measured with an Infinite® M200 (Tecan Group, Männedorf, Switzerland) at a test wavelength of 413 nm.
NP Stability after PurificationEach NP was centrifuged (6500 × g, 2 min, 25 °C) 14 d after the purification. Then, we took pictures to confirm the NP aggregation.
StatisticsDifferences in groups were evaluated using ANOVA with the Tukey post hoc test.
This research was supported by Grant-in-Aid for Scientific Research B (19H04450) from the Japan Society for the Promotion of Science (JSPS).
The authors declare no conflict of interest.