2021 年 69 巻 8 号 p. 789-795
2021 年 69 巻 8 号 p. 789-795
In this study, the effect of contact time, temperature, pH, and coexistences on the adsorption of phosphate ions using the complex nickel–aluminum–zirconium hydroxide (NAZ) was evaluated. Moreover, the recovery of adsorbed phosphate ions from NAZ using desorption solution with different concentrations was demonstrated. The results showed that the quantity of phosphate ions adsorbed gradually increased with time, and the adsorption equilibrium was achieved within 24 h after adsorption. This kinetic data could be well described by the pseudo-second-order model with the correlation coefficient in the value of 0.997. Additionally, the quantity of phosphate which was adsorbed increased as temperature increased, and these results corresponded well with both the Langmuir, the correlation coefficient ranged from 0.920–0.949, and Freundlich models, the correlation coefficient ranged from 0.863–0.995. These results showed that the adsorption of phosphate ion was monolayer adsorption onto the NAZ surface. The optimal pH for removal of phosphate ions from aqueous media was during 4–8. In addition, chloride, nitrate, and sulfate ions did not significantly affect to the adsorption capability of phosphate ions in the complex solution system. Finally, the phosphate ions which were adsorbed onto NAZ could be recovered using sodium sulfate solution (recovery percentage: approx. 50% using sodium sulfate solution at 1000 mmol/L). These results highlight the potential of using NAZ as the cost-effectiveness adsorbent for phosphate ions removal from aqueous media.
Phosphorus is a critical element for DNA, RNA, and energy transfer in aquatic plants, and it is an important factor which limits nutrients in most aquatic ecosystems.1) In addition, phosphorus is also essential element for agriculture fertilizer.2) In water environments such as natural water or wastewater, phosphorus exists as orthophosphates and polyphosphates.3) Phosphorus pollution has become a global environmental issue with increasing discharges of phosphorus from human activities.4) Previous studies reported that approximately 1.3 million t/year of P is discharged into the water environment globally. Additionally, this phenomenon causes eutrophication and subsequent ecosystem degradation.5,6) Therefore, maximum permissible P concentrations ranged between 0.01–0.5 and 0.1 mg-P/L in wastewater treatment plants have been established in the U.S.A. and European Union (EU), respectively.7,8) In addition, it is also reported that concentrations <0.03 mg-P/L is considered as the lower boundaries of eutrophication in aquatic systems.9) Therefore, the removal of P from aquatic systems is of great concern to prevent or control water eutrophication.
In contrast, P is a valuable global resource. A previous study reported “The disappearing nutrient,” which indicates the world might soon run out of phosphorus. Additionally, phosphate rock is becoming a strategic material for various countries. Therefore, there is lacking of single international organization responsible for phosphate resources.10) In 2017, the European Commission added “phosphorus” to the list of critical raw materials for the EU.11) Thus, techniques for phosphorus recovery from aquatic systems must be established as soon as possible.
Various techniques have been explored to remove phosphate ions from aqueous media, involving adsorption, ion exchange, chemical precipitation, and biological processes. Among these techniques, adsorption has been extensively used in phosphate ion removal from aqueous media such as surface water and wastewater because this method is economical, convenient, and highly efficient.12)
In recent years, using zirconium-containing materials, such as zirconium oxides and zirconium hydroxides, as adsorbents to remove (or recover) phosphate ions from aqueous media has gained substantial attention.13) Previous studies reported that zirconium and zirconium hydroxide had high chemical stability and selective adsorption affinity for phosphate ions.14,15) Our study previously also reported the characterization of nickel–aluminum–zirconium complex hydroxide (NAZ) and adsorption capability of phosphate ions using samples from aqueous media.16) This study revealed the optimal molar ratio of nickel, aluminum, and zirconium content, and one of the adsorption mechanisms of phosphate ions is related to ion exchange with sulfate ions in the interlayer of NAZ. However, this was a reference study, and the obtained results were not sufficient to elucidate the mechanism of NAZ on phosphate ions adsorption. Therefore, further studies are necessary to extensively elucidate the relationship between phosphate ions and NAZ.
In this study, NAZ was used as an adsorbent for the removal of phosphate ions from aqueous media. The specific objectives of this study were to: (1) investigate the effect of initial concentration, temperature, contact time, pH, and coexistences on the adsorption of phosphate ions; (2) clarify the kinetics of adsorption and evaluate the adsorption mechanism; and (3) recover phosphate ions adsorbed onto NAZ using a desorption solution.
The complex NAZ, which was reported in a previous study,16) was used. NAZ was synthesized by the Kansai Catalyst. Co., Ltd., Osaka, Japan. The physicochemical properties, such as morphology (particle size: approximately 10 µm), crystalline structure, 51.9 m2/g of specifics surface area, 1.08 mmol/g of hydroxyl groups, 1.45 × 10−4 µL/g of micropore, 0.20 µL/g of mesopore, and 0.06 µL/g macropore volumes were also measured.16) Potassium dihydrogen phosphate, sodium sulfate, hydrochloric acid, sodium hydroxide, sodium chloride, and sodium nitrate were purchased from FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan. A standard solution of anions (anion mixed standard solution IV) was purchased from Kanto Chemical Co., Inc., Japan.
Effect on Various Parameters on the Adsorption of Phosphate IonsFirst, to assess the contact time, 100 mg/L of phosphate ion solution in the volume of 50 mL and 0.05 g of NAZ was mixed, and then the reaction solution was shaken using a water bath shaker MM-10 (TAITEC Co., Japan) at 100 rpm and 25 °C, for 0.5, 1, 3, 6, 9, 12, 16, and 24 h. Second, to assess the initial concentration and temperature, a 50 mL phosphate ion solution of 30 to 300 mg/L was mixed with 0.05 g of NAZ, and then the reaction solution was shaken at 100 rpm for 24 h with temperatures of 5, 25, and 50 °C. Finally, to assess the pH, 100 mg/L of phosphate ion solution in the volume of 50 mL at the pH solution between 2–12 was mixed with 0.05 g of NAZ, and then the reaction solution was shaken at 100 rpm and 25 °C for 24 h. After shaking, the level of phosphate ions (before and after adsorption) of filtrated solution from a membrane filter (0.45 µm) was measured using the ascorbic-acid reduction method by absorption spectrophotometry DR/890 (Hach, U.S.A.).17) The quantity of phosphate ions adsorbed onto NAZ was calculated by measuring the different concentrations before and after adsorption.
Effect of Coexistences on the Adsorption of Phosphate Ions0.05 g of NAZ was mixed with a complex solution system containing phosphate, chloride, sulfate, and nitrate ions in the concentration of 1 mmol/L in the total volume of 50 mL, and then the mixture solution was shaken at 100 rpm and 25 °C for 24 h. After shaking, the filtrated solution from a 0.45-µm membrane filter was quantified for phosphate ions adsorbed onto NAZ and calculated using the previous-mentioned method. Additionally, the concentrations of chloride, sulfate, and nitrate ions were measured by ion chromatography (DIONEX ICS-900, Thermo Fisher Scientific Inc., Japan) according to the previously reported.17)
Adsorption/Desorption Capability of Phosphate Ions Using NAZInitially, 300 mg/L of phosphate ion solution in the volume of 100 mL was mixed with 0.3 g of NAZ, and then the mixture solution was shaken at 100 rpm and 25 °C for 24 h. The filtrated solution from a 0.45-µm membrane filter was quantified for phosphate ions adsorbed onto NAZ and calculated using the previous-mentioned method. After adsorption, NAZ was collected, dried, and then used for the desorption experiment. Briefly, 100 mL of 1, 10, 100, and 1000 mmol/L sodium sulfate solutions were mixed with 0.5 g of the collected NAZ. The reaction mixture was shaken at 100 rpm and 25 °C for 24 h. After desorption, the concentration of phosphate ions released from NAZ was measured by the ascorbic-acid reduction method using absorption spectrophotometry.18) The concentration of phosphate ions desorbed from NAZ was calculated as the difference before and after desorption. All adsorption experiments are expressed as the mean ± standard deviation (S.D.).
The time-dependent removal of phosphate ion from aqueous media using NAZ is illustrated in Fig. 1. The results demonstrated that the adsorption of phosphate ions was initially rapid from 0.5–1 h. In particular, the concentration of phosphate ions adsorbed was approximately 51.0 mg/g at 0.5 h after the adsorption commenced. Subsequently, the adsorption capacity gradually increases, and the adsorption equilibrium time of NAZ to phosphate ions from the aqueous media was approximately 24 h after the adsorption commenced.
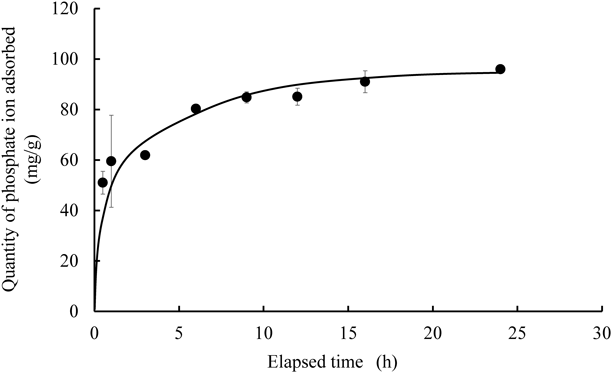
Initial concentration 100 mg/L, solvent volume 50 mL, adsorbent 0.05 g, temperature 25 °C, contact time 0.5, 1, 3, 6, 9, 12, 16, and 24 h, agitation speed 100 rpm.
The adsorption mechanism of phosphate ions was also evaluated using two kinetic models, namely the pseudo-first- and pseudo-second-order models, were applied to fit the obtained results. The two kinetic models are expressed in Eqs. (1) and (2).19,20)
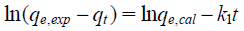 | (1) |
 | (2) |
where qe,exp and qt are the quantities of phosphate ions adsorbed at equilibrium and time t (mg/g), respectively. Additionally, qe,cal is the concentration of phosphate ions adsorbed (mg/g); k1 (1/h) and k2 (g/mg/h) are rate constants of the pseudo-first- and pseudo-second-order models, respectively.19,20)
The fitting results are shown in Fig. 2. Additionally, the kinetic parameters and correlation coefficients are also revealed in Table 1. The correlation coefficient of the pseudo-second-order model in the value of 0.997 was better fitted with the experimental data comparable to the pseudo-first-order model (r = 0.980). In addition, the value of qe,exp (96.0 mg/g) was also more consistent with the value of qe,cal (93.5 mg/g) in the pseudo-second-order model compared to that in the pseudo-first-order model (44.2 mg/g). Trends similarly were reported in previous studies.21,22) The pseudo-second-order model indicates that chemisorption occurred between NAZ and phosphate ions, involving electron exchange or valency forces because of sharing between the adsorbent and adsorbate.21)
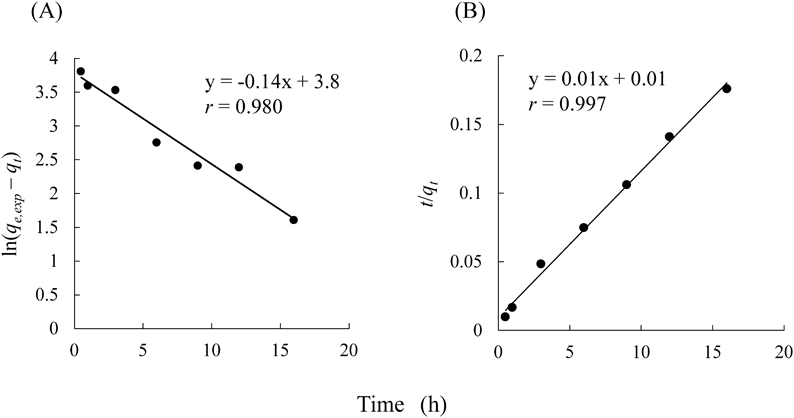
| Sample | qe,exp (mg/g) | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe,cal (mg/g) | r | k2 (g/mg/h) | qe,cal (mg/g) | r | ||
| NAZ | 96.0 | 0.14 | 44.2 | 0.980 | 1.1 × 10−6 | 93.5 | 0.997 |
A previous study reported that the adsorbate was distributed between the solid and liquid phases at a given temperature which can be indicated by the adsorption isotherm.20) Thus, the effects of different temperatures on adsorption isotherms of phosphate ions were evaluated and are shown in Fig. 3. The adsorption capacity of phosphate ions onto NAZ increased with increasing temperature. These reactions reveal that the adsorption mechanism of NAZ on phosphate ion is an endothermic process. In this study, the obtained data were evaluated using two isotherm models, namely the Langmuir (Eq. (3)) and Freundlich (Eq. (4)) models.23,24)
 | (3) |
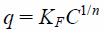 | (4) |
where q and qmax are the quantity of adsorbed phosphate ions (mg/g) and maximum quantity of adsorbed phosphate ions (mg/g), respectively; C is the equilibrium concentration (mg/L); KL is the Langmuir isotherm constant (L/mg), and KF and 1/n are the Freundlich isotherm constants. The Langmuir and Freundlich models are the theoretical model and the empirical model, respectively.23,24)
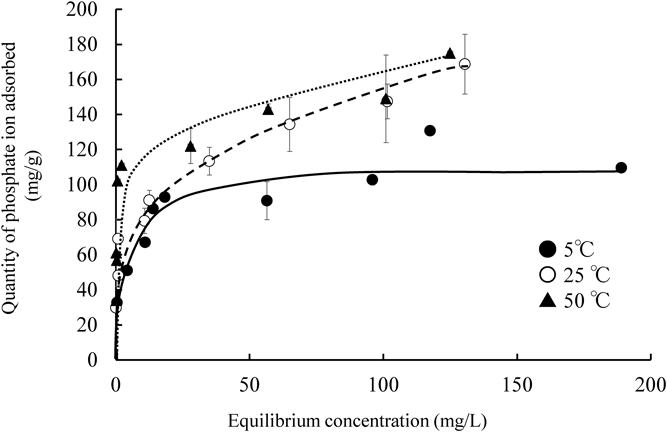
Initial concentration 30, 50, 70, 90, 100, 150, 200, and 300 mg/L, solvent volume 50 mL, adsorbent 0.05 g, contact time24 h, temperature 5, 25, and 50 °C, agitation speed 100 rpm.
The isotherm parameters and correlation coefficients are shown in Table 2. Consideration to the correlation coefficient values, the isotherms data fitted to both of Langmuir (r = 0.932–0.983) and Freundlich (r = 0.889–0.971) models. The value of maximum adsorption capacity (qmax), which was evaluated using the Langmuir model, increased as temperature increased (5 < 25 < 50 °C). These trends are supported by the adsorption isotherms presented in Fig. 3. On the other hand, the value of KL did not depend on the adsorption temperature in this study. Because the difference of quantity of phosphate ion adsorbed using NAZ between 25 and 50 °C was smaller compared to 5 °C. Moreover, it can be seen that the error bar at 25 °C is greater in the current conditions. Therefore, we have to evaluate these data in detail. Furthermore, the value of RL (a dimensionless parameter) was also calculated from Langmuir constant KL reflecting whether the adsorption is favorable.
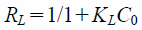 | (5) |
| Temperature (°C) | Freundlich constants | Langmuir constants | ||||
|---|---|---|---|---|---|---|
| log KF | 1/n | r | qmax (mg/g) | KL (L/mg) | r | |
| 5 | 1.6 | 0.3 | 0.927 | 105.3 | 0.26 | 0.983 |
| 25 | 1.7 | 0.3 | 0.971 | 105.3 | 2.46 | 0.932 |
| 50 | 1.9 | 0.2 | 0.889 | 153.9 | 1.71 | 0.932 |
The adsorption nature is different characteristics according to the value of RL: unfavorable (RL > 1), favorable (0 < RL < 1), and irreversible (RL = 0). According to the data (the value of RL is from 1.4 × 10−3 to 1.3 × 10−2), the adsorption nature of phosphate ion using NAZ is favorable under current experimental conditions.25,26)
Additionally, the form of the isotherm curve depends on the value of n in the Freundlich isotherm model. The adsorption occurs easily when the value of 1/n is range from 0.1 to 0.5 and difficult to adsorb phosphate ions from aqueous media if the value of 1/n is more than 2.27) In this study, the value of 1/n was approximately 0.2–0.3 which indicated that phosphate ions were easily adsorbed onto NAZ surface under our experimental conditions. These results revealed that phosphate ion adsorption using NAZ was attributed to monolayer adsorption, and one of the phosphate ion adsorption mechanisms from aqueous media was related to an endothermic process, which could have been chemical interactions rather than physical in this study. Trends similarly were reported in previous studies.28,29)
Adsorption ThermodynamicsAn adsorption thermodynamics study was demonstrated to explain the thermodynamic parameters on the adsorption of phosphate ion onto NAZ. The various thermodynamic parameters including change in enthalpy (ΔH), and change in entropy (ΔS), and change in free energy (ΔG) were calculated by the following equations:
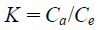 | (6) |
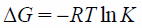 | (7) |
 | (8) |
where Ca is the quantity of adsorbate adsorbed on the adsorbent (mg/g), Ce is the equilibrium adsorbate concentration in the solution (mg/L). R and T are the gas constant (8.314 J/mol K) and the absolute temperature (K), respectively. K is the adsorption equilibrium constant (phosphate ion distribution between solid and liquid phases in this case).
The thermodynamic parameters on the adsorption of phosphate ion using NAZ are presented in Table 3. The value of ΔG decreased with increasing temperatures, indicating that an increase in feasibility of adsorption at from 278 to 323 K (−5.9 from to −14.9 kJ/mol) under current experiment conditions. The negative values specify that the adsorption process is spontaneous.30) The positive value of ΔH indicates that the adsorption process using NAZ is endothermic.3) Moreover, the positive value of ΔS shows that the randomness of the solid–liquid interface increase during the adsorption process.9) The change in free energy (ΔG) was also calculated by using following equation31):
 | (9) |
| Temperature (K) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/mol) |
|---|---|---|---|
| 278 | −5.9 | 50.9 | 200.0 |
| 298 | −6.4 | ||
| 323 | −14.9 |
The calculated ΔG value at 278, 298, and 323 K was 3.1, −2.2, and −1.4 kJ/mol, respectively. These results were different compared to the results obtained from thermodynamic parameters. In general, ΔG must be negative value in significant adsorption process. Therefore, the fitting of the Langmuir constant (KL) in the thermodynamic interpretation is not suitable in the current experimental conditions.
Next, isosteric heat of adsorption (ΔHx) was evaluated in this study. It was calculated at constant surface coverage (qe = approximately 30, 50, and 110 mg/L) by means of the Clausius–Clapeyron equation.32–34)
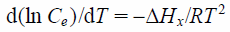 | (10) |
The equilibrium concentration (Ce) at constant quantity of adsorbate adsorbed was obtained from the adsorption isotherms data (Fig. 3). In addition, the value of ΔHx was obtained from the slop of the ln Ce versus 1/T for different quantity of phosphate ion adsorbed onto NAZ in this study. The relationship between ln Ce and 1/T were found to be linear (Fig. 4 (a)). Isosteric heats of adsorption of phosphate ions using NAZ are listed in Table 4. The positive values of ΔHx showed the endothermic adsorption process. Additionally, the relationship between isosteric heat of adsorption and surface loading in Fig. 4 (b) indicated that NAZ was having heterogeneous surfaces. A previous study reported that the value of ΔHx depended on the sorbate–sorbent interaction.35) Therefore, the sorbate–sorbent interaction in the range of lower qe values is resulting in low heats of adsorption. According to a previous study,36) the ΔHx value of below approximately 80 kJ/mol or ranges between 80 to 400 kJ/mol indicated the physical adsorption process or chemical adsorption process. Therefore, it is thought that the adsorption of phosphate ion using NAZ mainly relates to the physical adsorption process.
| qe (mg/g) | ΔHx (kJ/mol) | r |
|---|---|---|
| 30 | 19.36 | 0.993 |
| 50 | 49.2 | 0.984 |
| 110 | 92.8 | 0.741 |
The solution pH is one of the critical parameters on the adsorption of phosphate ion from aqueous media. Figure 5 exhibited the effect of pH on the adsorption of phosphate ions using NAZ. Our tested condition demonstrated that the level of adsorbed phosphate ions gradually increased as pH increased from 2 to 4. Additionally, the adsorbed quantity was maintained as pH increased to 8. Finally, the adsorbed quantity showed a slight decrease from pH 8 to 12. These results indicate that NAZ had a high adsorption performance of phosphate ions over a wide pH range. Trends similarly were reported in a previously reported.37)
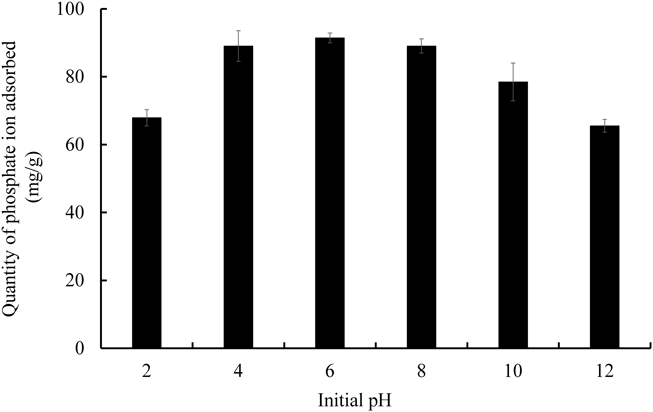
Initial concentration 100 mg/L, solvent volume 50 mL, adsorbent 0.05 g, temperature 25 °C, contact time 24 h, agitation speed 100 rpm.
At pH 2–4, H3PO4 is still present at >50%, which indicates a direct impact on the adsorption of phosphate ions (the interaction between NAZ and phosphate ions did not easily occur under these conditions).38,39) At pH 4–8, monovalent and divalent species of H2PO4− and HPO42− coexist in the aqueous solution. These species reacted with NAZ in electrostatic attraction, ligand exchange, and ion exchange. A detailed mechanism was reported in a previous study.16) In addition, a study previously also reported that H2PO4− possessed lower adsorption free energy compared to HPO42−,40) which indicates that NAZ could react with H2PO4− in the aqueous solution more easily. Finally, at pH >8, the surface of NAZ is protonated (pHpzc of NAZ is 6.18) and becomes positively charged. Therefore, electron repulsion occurred easily under our experimental conditions. Furthermore, hydroxyl ions competed with phosphate ions to occupy the available active adsorption sites, meaning the adsorption capability of phosphate ions onto NAZ reduced at higher pH (>8). Similar phenomena were reported in previous studies.28,40–42)
Effect of Coexistences on the Adsorption of Phosphate IonsThe selectivity is an important factor for the industrial application of NAZ. Therefore, the effect of coexistences on the adsorption of phosphate ions using NAZ was demonstrated (Fig. 6). In this study, common anions including sulfate, chloride, and nitrate ions were used, which are ubiquitous in aquatic systems and compete with phosphate ions for active sites on NAZ. The concentration of phosphate ions adsorbed onto NAZ was approximately 0.96 mmol/g (91.2 mg/g), which corresponds with the above-mentioned result. Additionally, the concentration of adsorbed phosphate ions was the same in the single and complex solutions. Chloride and nitrate ions had a limited competitive effect under our experimental conditions. This is because: (1) the attraction between zirconium material and phosphate ions is superior to chloride or nitrate ions, and (2) the interaction mechanism between each anion was the same, where the phosphate, chloride, and nitrate ions formed an inner-sphere complex with the hydroxyl groups at the NAZ surface.12,16,43,44) Therefore, chloride and nitrate ions were not adsorbed onto NAZ in this study. In contrast, the concentration of sulfate ions increased after adsorption, which indicated that ion exchange between phosphate and sulfate ions in the interlayer of NAZ occurred. These results were supported by the previous studies.16,43) Additionally, trends similarly were also reported in previous studies.9,12,28) Therefore, these results demonstrated that NAZ is a useful adsorbent for the removal of phosphate ions in complex solution systems.

Initial concentration 1 mmol/L, solvent volume 50 mL, adsorbent 0.05 g, temperature 25 °C, contact time 24 h, agitation speed 100 rpm.
The recovery of the adsorbent after adsorption was demonstrated to investigate the stability and reuse of the adsorbent in this study. Sodium sulfate solution at different concentrations was used as a desorption solution to desorb (or recover) the adsorbed phosphate ions from NAZ (Fig. 7). With the increase in concentration from 10 mmol/L to 1000 mmol/L, the concentration of phosphate ions desorbed from NAZ increased. The concentration of phosphate ions desorbed using 1000 mmol/L sodium sulfate solution was approximately 47.9 mg/g (approx. 50% recovery). Finally, these lab-scale results are fundamental in the recovery of phosphate ions from aqueous media. Therefore, field studies are required to elucidate the application of NAZ in further experiments.

Adsorption condition: initial concentration 300 mg/L, solvent volume 100 mL, adsorbent 0.3 g, temperature 25 °C, contact time 24 h, agitation speed 100 rpm. Desorption condition: 1, 10, 100, and 1000 mmol/L, solvent volume 100 mL, adsorbent 0.05 g, temperature 25 °C, contact time 24 h, agitation speed 100 rpm.
The adsorption capability of NAZ on phosphate ions from aqueous media was demonstrated. Various parameters, including contact time, temperature, and pH, significantly affected the adsorption capacity of NAZ. The concentration of adsorbed phosphate ions increased with temperatures and time. Additionally, NAZ could be candidate for the removal of phosphate ions at a wide range of pH from 4 to 8. Chloride and nitrate ions did not significantly affect the adsorption capability of phosphate ions from the aqueous media. Phosphate ions adsorbed onto NAZ could be recovered using 1000 mmol/L sodium sulfate solution. Finally, adsorption mechanisms, such as ion exchange and chemisorption, were elucidated in this study. This study proposed an efficient alternative to utilize abundant adsorbent for the removal/recovery of phosphate ions from aqueous media.
This research was supported in part by Kurita Water and Environmental Foundation (20A003).
The authors declare no conflict of interest.