2021 年 69 巻 8 号 p. 802-805
2021 年 69 巻 8 号 p. 802-805
A new rearranged nitrogenous bisabolone-type sesquiterpene, halichonic acid B (1), was isolated from a marine sponge Axinyssa sp. together with halichonic acid (2) and (6R,7S)-7-amino-7,8-dihydro-α-bisabolene (3). The structure of 1 was determined by extensive NMR and MS analyses, revealing an unprecedented carbon framework, and its absolute configuration was elucidated by time-dependent density-functional theory (TDDFT)-based electronic circular dichroism (ECD) spectrum calculation. We propose that 1 and 2 may be biosynthesized in the same pathway, involving the reaction between farnesyl pyrophosphate and glycine, followed by cyclization.
Marine sponges belonging to the family Halichondriidae are rich sources of nitrogenous bisabolene-type sesquiterpenoids. Sesquiterpenes with amine and isocyanate substituents were isolated from marine sponges Halichondria sp.1) and Ciocalypta sp.,2) and other nitrogenous sesquiterpenes with formamide,3–5) isonitrile,6) isothiocyanate,5,7) and urea7) have been isolated from marine sponges of the genus Axinyssa. Recently, we also isolated a new rearranged nitrogenous bisabolene-type sesquiterpene, halichonic acid (2), with a novel 3-azabicyclo[3.3.1]nonene skeleton from Halichondria sp.8) These nitrogenous bisabolene-type sesquiterpenoids have been reported to exhibit various biological functions, including antimicrobial,1–4) cytotoxic,1,6,8) and protein tyrosine phosphatase 1B inhibitory activities.7)
In our current research on bioactive secondary metabolites from marine sponges, a new rearranged nitrogenous bisabolene-type sesquiterpene, halichonic acid B (1), together with two known biosynthetically related sesquiterpenes [28) and (6R,7S)-7-amino-7,8-dihydro-α-bisabolene1) (3)] were isolated from Axinyssa sp. collected in Indonesia (Fig. 1). Here, we describe the structural elucidation of 1 and the antimicrobial activities of 1–3.

Halichonic acid B (1) was obtained as a yellowish amorphous solid with a molecular formula of C17H29NO3 as established by high resolution-electrospray ionization-time-of-flight (HR-ESI-TOF)-MS. 1H- and 13C-NMR spectra (methanol-d4) displayed characteristic signals reminiscent of bisabolene-type sesquiterpenes, including four methyl singlets [δH 1.24/δC 28.9 (C-12); δH 1.31/δC 25.9 (C-13); δH 1.26/δC 20.7 (C-14); δH 1.67/δC 23.3 (C-15)], a trisubstituted double bond [δH 5.43/δC 120.1 (C-2); δC 135.4 (C-3)], two quaternary carbons [δC 61.0 (C-7); δC 72.6 (C-11)], three methines [δH 2.02 (m)/δC 36.4 (C-6); δH 2.02 (m)/δC 47.8 (C-10); δH 3.59 (d, J = 10.1 Hz)/δC 58.8 (C-2′)], a carbonyl carbon [δC 174.2 (C-1′)], and five methylenes (Table 1). Correlation spectroscopy (COSY) correlations showed two spin systems: H-2/H2-1/H-6/H2-5/H2-4 and H2-8/H2-9/H-10/H-2′ (Fig. 2). Heteronuclear multiple bond correlations (HMBCs) from H3-15 to C-2, C-3, and C-4 (δC 31.6), from H-2 to C-4 and C-6, and from H2-5 to C-1 (δC 25.5) and C-3 established substructure A (Fig. 2). HMBCs from H-2′ to C-7, C-9 (δC 22.2), C-11, and C-1′, from H3-14 to C-7 and C-8, from H3-12 and H3-13 to C-10 and C-11, and from H-10 to C-1′ established substructure B. The molecular formula and chemical shifts of C-7 (δC 61.0) and C-2′ (δC 58.8) suggest that these carbons are linked via a nitrogen atom, and the chemical shift of C-11 (δC 72.6) indicates that this carbon is oxygenated. For the three remaining hydrogen atoms, HMBCs between H-1β and H-5β/C-7 and H3-14 and H2-8/C-6 showed the connection of C-6 and C-7 and the tetrasubstituted piperidine structure with a carboxylic acid at C-2′ and hydroxy group at C-11 for substructure B. Thus, the planar structure of 1 was determined.
| Position | Methanol-d4 | Pyridine-d5 | |||
|---|---|---|---|---|---|
| δC, mult. | δH, mult. (J in Hz) | HMBC | δC, mult. | δH, mult. (J in Hz) | |
| 1 | 25.5, CH2 | α 1.91, m | 25.4, CH2 | α 2.10, m | |
| β 2.03, m | 2, 3, 5, 6, 7 | β 2.40, m | |||
| 2 | 120.1, CH | 5.43, br d (4.3) | 1, 4, 15 | 121.2, CH | 5.34, br d (4.3) |
| 3 | 135.4, C | 133.5, C | |||
| 4 | 31.6, CH2 | α 2.01, m | 15 | 31.1, CH2 | α 1.89, m |
| β 2.10, m | β 2.02, m | ||||
| 5 | 24.8, CH2 | α 1.29, m | 1, 4, 6 | 24.3, CH2 | α 1.17, m |
| β 1.75, m | 1, 3, 4, 6, 7 | β 1.61, m | |||
| 6 | 36.4, CH | 2.02, m | 35.1, CH | 2.20, m | |
| 7 | 61.0, C | 56.4, C | |||
| 8 | 32.2, CH2 | α 1.61, m | 6, 7, 10, 14 | 33.7, CH2 | α 1.65, m |
| β 2.14, d (4.1) | 6, 7, 10, 14 | β 2.05, m | |||
| 9 | 22.2, CH2 | α 1.86, m | 7, 8, 10, 11, 2′ | 22.7, CH2 | α 1.83, m |
| β 1.62, m | β 1.63, m | ||||
| 10 | 47.8, CH | 2.02, m | 48.9, CH | 2.37, m | |
| 11 | 72.6, C | 71.3, C | |||
| 12 | 28.9, CH3 | 1.24, s | 10, 11, 13 | 30.1, CH3 | 1.43, s |
| 13 | 25.9, CH3 | 1.31, s | 10, 11, 12 | 25.4, CH3 | 1.48, s |
| 14 | 20.7, CH3 | 1.26, s | 6, 7, 8 | 22.6, CH3 | 1.24, s |
| 15 | 23.3, CH3 | 1.67, s | 2, 3, 4 | 23.4, CH3 | 1.60, s |
| 1′ | 174.2, C | 175.2, C | |||
| 2′ | 58.8, CH | 3.59, d (10.1) | 7, 9, 10, 11, 1′ | 57.8, CH | 4.01, d (10.5) |

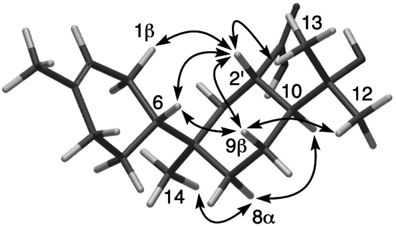
The relative configuration of 1 was determined by nuclear Overhauser effect (NOE) correlations in pyridine-d5 (Table 1) owing to the overlap of H-1β, H-4β, H-6, and H-10 in methanol-d4. NOE correlations for H-2′/H-1β, H-2′/H-6, H-6/H-9β, H-2′/H-9β, H-9β/H3-12, and H-2′/H3-13 suggest β-orientations of H-2′, H-9β, a 4-methylcyclohex-3-enyl group at C-7, and a 2-hydroxyisopropyl group at C-10 (Fig. 3). On the other hand, NOE correlations for H-8α/H3-14 and H-8α/H-10 suggest that they are α-oriented.
The absolute configuration of 1 was determined by the theoretical electronic circular dichroism (ECD) spectrum. As 3 has a 6R,7S-configuration,1) the ECD spectrum of the biosynthetically expected 6R,7S,10S,2′S-1 was calculated using a standard time-dependent density-functional theory (TDDFT) procedure (Fig. 4). The experimental and calculated spectra matched well, and thus the absolute configuration of 1 was assigned.

As shown in Chart 1, the biosynthesis of 1 may occur from farnesyl pyrophosphate and glycine in the same pathway as 2.8) First, the nitrogen atom of glycine attacks farnesyl pyrophosphate, followed by cyclization and elimination of pyrophosphate to afford 4. Subsequent oxidation and dehydration form the imine 5, followed by cyclization to afford 1. Alternatively, cyclization with the formation of a new bond between C-2 and C-2′may yield 2.

The antimicrobial activities of 1–3 were tested against Chromobacterium violaceum. Among them, 3 inhibited bacterial growth at an IC50 value of 36 µM, whereas 1 and 2 had no antimicrobial activity even at 400 µM.
Optical rotation was measured on a JASCO DIP-1000 polarimeter in MeOH. UV spectrum was measured on a JASCO V-550 spectrophotometer in CH3CN. ECD spectrum was measured on a JASCO J-820 spectropolarimeter in CH3CN. IR spectrum was recorded on a PerkinElmer, Inc. Frontier FT-IR spectrophotometer. NMR spectra were recorded on a Bruker Avance III 600 NMR spectrometer (1H-NMR: 600 MHz, 13C-NMR: 150 MHz). Chemical shifts were referenced to the residual solvent peaks (δH 3.31 and δC 49.0 for methanol-d4; δH 7.58 and δC 135.5 for pyridine-d5). Mass spectrum was measured on a Waters Xevo G2-XS Qtof mass spectrometer. The preparative medium-pressure liquid chromatography (MPLC) was performed on a Biotage Isolera I. The HPLC system comprised a Waters 515 HPLC pump, Waters 2489 UV/visible detector, and Pantos Unicorder U-228.
Animal MaterialMarine sponge Axinyssa sp. was collected by scuba at a depth of 10 m in North Sulawesi, Indonesia, in September 2014, and immediately soaked in EtOH. The sponge was identified by one of the authors (Y.I.). A voucher specimen (14M051) has been deposited at the Department of Natural Medicines, Graduate School of Pharmaceutical Sciences, Kumamoto University, Japan.
Extraction and IsolationThe sponge specimen (116 g, wet weight) was extracted with EtOH. After evaporation, the residual aqueous solution was extracted with EtOAc and then n-BuOH. The n-BuOH fraction (1.23 g) was subjected to MPLC (Purif SI silica gel, 30 g; Biotage Japan Ltd., Tokyo, Japan) eluted with a gradient system (0–15% n-Hexane–EtOAc for 0–16 min; 0–30% CH2Cl2–MeOH for 16–47 min) to yield five fractions (Frs. A1–A5). Fraction A3 was subjected to MPLC (SNAP Ultra C18, 10 g; Biotage Japan Ltd.) with a gradient system (10–100% MeOH–H2O for 0–12 min) to yield three fractions (Frs. B1–B3). Fraction B2 was subjected to HPLC (COSMOSIL 5C18-MS-II, 20 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) eluted with 20–30% CH3CN–H2O containing 1% formic acid for 0–30 min to afford 1 (2.52 mg). Fraction B3 was subjected to HPLC (COSMOSIL 5C18-MS-II, 20 × 250 mm; Nacalai Tesque Inc.) eluted with 30% CH3CN–H2O containing 1% formic acid to afford 2 (3.60 mg). Fraction A3 was subjected to MPLC (SNAP Ultra C18, 10 g) with a gradient system (10–100% MeOH–H2O for 0–11 min) to yield four fractions (Frs. C1–C4). Fraction C3 was subjected to HPLC (Asahipack GS-310P column, 21.5 × 500 mm; Asahi Chemical Industry Co., Ltd., Tokyo, Japan) eluted with MeOH to afford 3 (9.68 mg).
Halichonic Acid B (1)Yellowish amorphous solid, [α]21D +47 (c 0.40, MeOH); UV (CH3CN) no absorption maximum above 195 nm; ECD (CH3CN) λmax (Δε) 201 (+1.9) and 226 (−0.8) nm; IR (film) νmax 3367, 2966, 2924, 2856, 1628, 1602, 1441, and 1386 cm−1; 1H- and 13C-NMR data, see Table 1; HR-ESI-TOF-MS m/z 294.2049 [M–H]− (Calcd for C17H28NO3, 294.2075).
Conformational Analysis and ECD Calculation for 1Conformational analysis and ECD calculations were performed as previously described9) using Spartan’18 instead of Spartan’16. ECD calculations were performed at the BHandHLYP/TZVP level.
Antimicrobial TestThe antimicrobial activities of 1–3 against C. violaceum were evaluated using a broth microdilution method. C. violaceum was cultured in a 96-well plate in Luria–Bertani (LB) (Lennox) broth containing 1% dimethyl sulfoxide (DMSO) and the tested compounds. After 1-d incubation at 27 °C, bacterial growth was assessed using the resazurin assay. To each well of bacterial suspension, 50 µL of resazurin sodium salt in distilled water (60 µM) was added, and the plate was shaken at 27 °C for 40 min. The fluorescence of resorufin, which was generated by microbial reduction of resazurin, was measured (EX: 520 nm/EM: 590 nm) to evaluate bacterial growth. The IC50 value of chloramphenicol (positive control) was 7.8 µM.
This work was supported by JSPS KAKENHI Grant Numbers JP26305005 (S.T.), JP20H03396 (S.T.), JP20K16026 (Y.H.), and JP21K15282 (A.H.H.E).
The authors declare no conflict of interest.