2022 年 70 巻 1 号 p. 82-84
2022 年 70 巻 1 号 p. 82-84
Knoevenagel condensation, an olefin-forming reaction from active methyl/methylene-containing compounds and aldehydes, is a fundamental and useful synthetic method. Benzothiazoles are, however, out of the scope of Knoevenagel condensation. Here, we report that Knoevenagel condensation between aldehydes and 2-methyl-thiazolo[4,5-b]pyrazines (MeTPy), a fused ring structure comprising pyrazine and thiazole, proceeded smoothly, despite minor structural differences from benzothiazoles. This finding will be useful for short synthesis of MeTPy-containing functional molecules, such as a tau probe analog 1.
Knoevenagel condensation is a synthetically useful olefin-forming reaction from active methyl/methylene-containing compounds and aldehydes in the presence of weak amine bases.1) In most cases, active methyl/methylene can be easily deprotonated due to the existence of strong electron-withdrawing groups (EWGs), such as cyanides, esters, nitro groups,2) and/or electron-deficient heteroaromatic rings.3,4) However, attachment of EWGs to the substrates often complicates the synthesis, limiting the structural diversity of accessible molecules through Knoevenagel condensation. For example, Knoevenagel condensation cannot construct the C=C double bond moiety of PBB3, a promising tau positron emission tomography (PET) probe,5) due to weak electron-withdrawing ability of benzothiazoles. Thus, pre-functionalization to phosphonates and Horner–Wadsworth–Emmons (HWE) reaction by use of strong bases were necessary6) (Chart 1a). Although introduction of a cyanide EWG to the substrates assisted Knoevenagel condensation7) (Chart 1b), the substitution of a hydrogen atom by a cyanide group decreased the probe performance.8–10) In such a case, olefin formation using an electron-deficient benzothiazole-based Knoevenagel condensation may be an alternative.
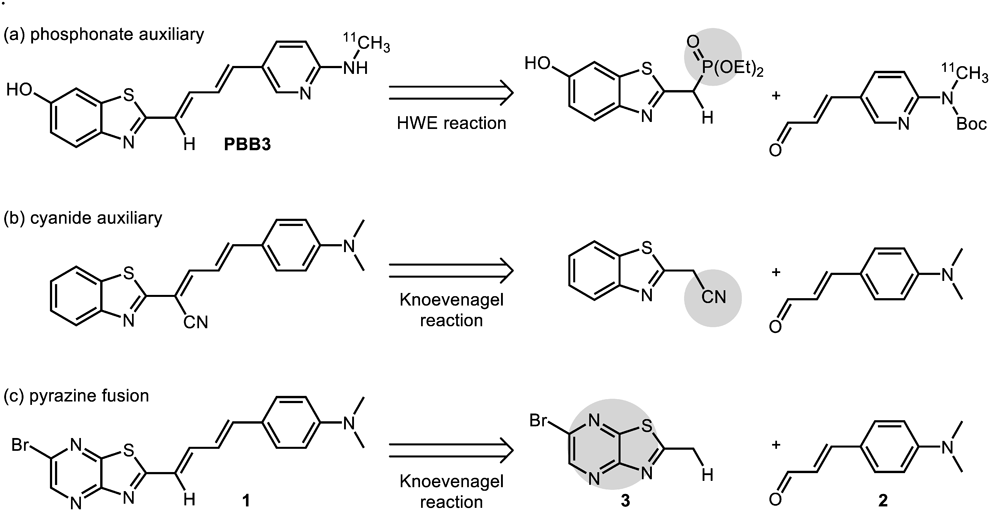
(a) Horner–Wadsworth–Emmons (HWE) reaction for the synthesis of PBB3. (b) Knoevenagel reaction of active methylene moiety connected with a cyanide auxiliary. (c) Knoevenagel reaction of 2-methyl-thiazolo[4,5-b]pyrazine, MeTPy (this work).
Thiazolo[4,5-b]pyrazines (TPy) are fused ring structures of pyrazines and thiazoles, and more electron deficient than benzothiazols.11–14) Therefore, we envisioned that 2-methyl-TPy (MeTPy: 3) could be used as a nucleophile for Knoevenagel condensation, affording PBB3 derivatives such as 1, containing a diene linker between two aromatic groups (Chart 1c). Here, we report the synthesis of compounds 1, 6, 7, and 8 by Knoevenagel condensation between MeTPy and electron-donating aldehydes.
Synthesis of 1 is outlined in Fig. 1. First, 2-amino-3,5-dibromopyrazine (4) was acetylated using acetyl chloride in the presence of 4-dimethylaminopyridine (DMAP) in acetonitrile, affording 5 in 63% yield. After 5 was converted to the corresponding thioamide using P4S10, intramolecular cyclization simultaneously took place to afford MeTPy 3 in 74% yield.13) In this reaction, use of Lawesson’s reagent instead of P4S10 provided an inseparable by-product with 3. Subsequently, 3 and 4-dimethylaminocinnamaldehyde (2) were subjected to typical Knoevenagel condensation conditions using piperidine as a base.2) The desired reaction proceeded to afford 1 in 13% yield (Fig. 1, entry 1, NMR yield) but 3 was recovered in 47%. While extension of the reaction time enhanced the conversion of 3 (15% recovery), yield of 1 was comparable to a shorter reaction time because 1 was unstable under the heating conditions (entry 2 vs. entry 1). Increasing the amount of piperidine to 10 equivalents improved yield to 33% (entry 3). Considering that protonated piperidine also catalyzed Knoevenagel reactions,15–17) 10 equivalents acetic acid was added to the reaction mixture (entry 4). As a result, yield of 1 markedly improved to 56%. Isolated yield of 1 (14%) was significantly lower than NMR yield, because product 1 was unstable, as was also reported for PBB318) and its derivatives.19)
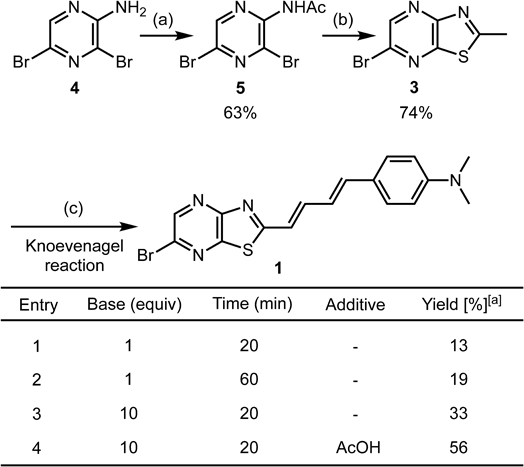
Reagents and conditions: (a) acetyl chloride (3 equivalents (equiv.)), DMAP (1 equiv.), acetonitrile, 70 °C. (b) P4S10 (0.3 equiv.), toluene, 110 °C. (c) 4-Dimethylaminocinnamaldehyde (2: 1 equiv.), piperidine (1 or 10 equiv.), additive (10 equiv.), toluene (0.2 M), 110 °C. [a] Yield was determined by 1H-NMR in the presence of nitromethane as an internal standard.
To explore the reaction mechanism, we next investigated Knoevenagel condensation using other benzothiazole analogs under the optimized conditions (Table 1). When the pyrazine moiety was changed to a pyridine or benzene ring, the reaction did not proceed at all (entries 2–4). According to 1H-NMR experiments, chemical shift values of C2-methyl protons dramatically shifted to lower magnetic field when the second nitrogen atom was in the six-membered aromatic ring (Table 1). The results suggest that electron withdrawing effects of nitrogen atoms in MeTPy 3 sufficiently increased the acidity of the C2-methyl group in 3 for the Knoevenagel condensation to proceed.
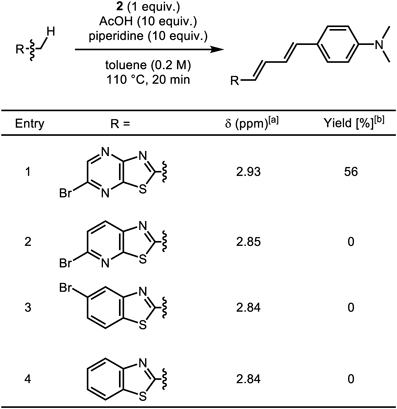 |
[a] Chemical shift values of C2-methyl protons (1H-NMR, in CDCl3). [b] Yield was determined by 1H-NMR in the presence of nitromethane as an internal standard.
Moreover, the optimized reaction conditions were applied to other pyrazines and aldehydes (Table 2). MeTPy 3 reacted with 4-dimethylaminobenzaldehyde and 9-julolidinecarboxaldehyde to give condensate mono-olefin chromophores20) 6 and 7 in 45 and 50% isolated yield, respectively. Non-brominated pyrazine also gave compound 8 in 53% isolated yield by the Knoevenagel condensation with 9-julolidinecarboxaldehyde.
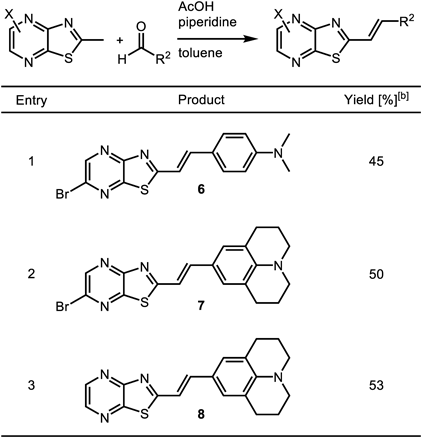 |
[a] General conditions: MeTPy, aldehyde (1 equiv.), AcOH (10 equiv.), and piperidine (10 equiv.) were stirred in toluene (0.2 M) for 20 min at 110 °C. [b] Isolated yields.
In summary, we found that Knoevenagel condensation between aldehydes and MeTPy proceeded in moderate yield in the presence of piperidine and acetic acid. The two nitrogen atoms of the pyrazine ring in MeTPy are important for accelerating the reaction. This finding is useful for the short synthesis of TPy-containing derivatives such as compound 1, a potential tau probe derived from PBB3.
NMR spectra were recorded on JEOL ECX-500 (500 MHz for 1H-NMR and 126 MHz for 13C-NMR) and JEOL ECS-400 (400 MHz for 1H-NMR and 100 MHz for 13C-NMR) spectrometers. For 1H-NMR and 13C-NMR, chemical shifts were reported in the scale relative to the residual solvent used as an internal reference (δ = 7.26 and 77.16 ppm (CDCl3)). Electronspray ionization (ESI)-MS spectra were measured on a JEOL JMS-T100LC AccuTOF spectrometer (for high-resolution (HR)MS). Melting points were recorded on ATM-01 (AS ONE Corporation, Osaka, Japan). Column chromatography was performed with silica gel 60 (spherical) 40–50 µm (Kanto Chemicals, Tokyo, Japan). Preparative HPLC was conducted using a SHIMADZU HPLC system equipped with an SPD-20A UV-VIS detector, LC-6AD pumps, a CTO-20AC column oven, an FRC-10A fraction collector, and a CBM-20A system controller (Shimadzu, Co., Kyoto, Japan). All solvents and reagents were used without purification (purchased from Aldrich, Tokyo Chemical Industry Co., Ltd. (TCI), Kanto Chemical Co., Inc., and FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). All reactions were carried out in dry solvents.
Substrates for Table 15-Bromo-2-methylbenzothiazole was purchased from FUJIFILM Wako Pure Chemical Corporation. 2-Methylbenzothiazole was purchased from Tokyo Chemical Industry Co., Ltd. (TCI). 5-Bromo-2-methylthiazolo[5,4-b]pyridine was synthesized as described in Supplementary Materials.
To a mixture of 4 (252.9 mg, 1.0 mmol) and DMAP (122.2 mg, 1.0 mmol) in acetonitrile (3 mL), acetyl chloride (214.1 µL, 3.0 mmol) was added at room temperature. After stirring at 70 °C for 1.5 h, the mixture was concentrated under reduced pressure. The residue was dissolved in EtOAc. The organic layer was washed with water and brine, dried over Na2SO4, filtered, and concentrated. The crude mixture was purified by silica gel column chromatography (dichloromethane/EtOAc = 4/1, Rf 0.5) to afford the title compound as a white solid (184.8 mg, 63%). Melting point: 150–151 °C; 1H-NMR (CDCl3) δ: 8.35 (s, 1H), 8.02 (br, 1H), 2.40 (s, 3H); 13C-NMR (CDCl3) δ: 169.9, 145.6, 143.4, 131.2 129.2, 25.1; HRMS (ESI+): m/z Calcd for C6H6ON3Br2Na+ [M + Na]+ 315.8697. Found 315.8694.
6-Bromo-2-methylthiazolo[4,5-b]pyrazine (3)A mixture of 4 (659.5 mg, 2.25 mmol) and P4S10 (300.3 mg, 0.68 mmol) in toluene (10 mL) was stirred at 110 °C for 1 h. After the mixture was cooled to room temperature, precipitates were filtered. The filtrate was concentrated. The crude mixture was purified by silica gel column chromatography (hexane/EtOAc = 1/1, Rf 0.5) to afford the title compound as white solids (380.7 mg, 74%). Melting point: 135–136 °C; 1H-NMR (CDCl3) δ: 8.71 (s, 1H), 2.93 (s, 3H); 13C-NMR (CDCl3) δ: 173.2, 156.7, 152.6, 144.7, 135.8, 22.1; HRMS (ESI+): m/z Calcd for C5H2N3SBrNa+ [M + Na]+ 251.9207. Found 251.9211.
General Procedure of Knoevenagel Condensation (1)To a mixture of 3 (45.8 mg, 0.20 mmol), acetic acid (114.5 µL, 2.0 mmol), and 4-dimethylaminocinnamaldehyde (2: 35.0 mg, 0.20 mmol) in toluene (1 mL), piperidine (197.6 µL, 2.0 mmol) was added. After stirring at 110 °C for 20 min, the solvent was removed in vacuo. The residue was purified by preparative HPLC (RT = 43.2 min, YMC-Triart C18, 20 mm I.D. × 250 mm, linear gradient; 0–100% acetonitrile in 0.1% aqueous trifluoroacetic acid (TFA) over 40 min with a flow rate of 8 mL/min) to afford the title compound as a red solid (10.7 mg, 14%). Melting point: 226–228 °C; 1H-NMR (CDCl3) δ: 8.64 (s,1H), 7.54 (dd, J = 10.9, 14.9 Hz, 1H), 7.42 (d, J = 8.6 Hz, 2H), 6.95 (d, J = 15.5 Hz, 1H), 6.87–6.82 (m, 2H), 6.73 (d, J = 8.6 Hz, 2H), 3.04 (s, 6H); 13C-NMR (CDCl3) δ: 171.5, 157.5, 151.7, 150.7, 144.4, 142.8, 142.0, 134.5, 129.6, 129.0, 122.1, 121.6, 112.0, 40.1; HRMS (ESI+): m/z Calcd for C17H15N4SBrNa+ [M + Na]+ 409.0098. Found 409.0094.
This work was supported by JSPS KAKENHI Grant Numbers JP20H00489 (MK), JP21H02602 (YS), and JP21K20727 (TS) and a JSPS Research Fellowship for Young Scientists (TS).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.