2022 年 70 巻 2 号 p. 187-191
2022 年 70 巻 2 号 p. 187-191
A new pentacyclic monoterpenoid indole alkaloid glycoside named secorubenine (1) was isolated from the heartwood of Adina rubescens, collected in Indonesia. The structure was elucidated by spectroscopic analysis and chemical modification of isolated secorubenine (1). The bioinspired enantioselective total synthesis of 1 was accomplished in 12 steps, whereafter its structure was determined and the absolute stereochemistry was confirmed.
Adina rubescens Hemsl. is a species of plant belonging to the Adina genus and Rubiaceae family. This species has the following synonym names: Adina eurhyncha, Nauclea oxyphylla, Pertusadina eurhyncha, and Uncaria eurhyncha.1–3) It distributes in Sumatra, East Kalimantan, Sabah, Sarawak, Brunei, and Malay Peninsula. The main use of this species is as timber because of the good quality and durability of its wood. In South Sumatra, the stem bark has been used by the Daya tribe to treat jaundice.3)
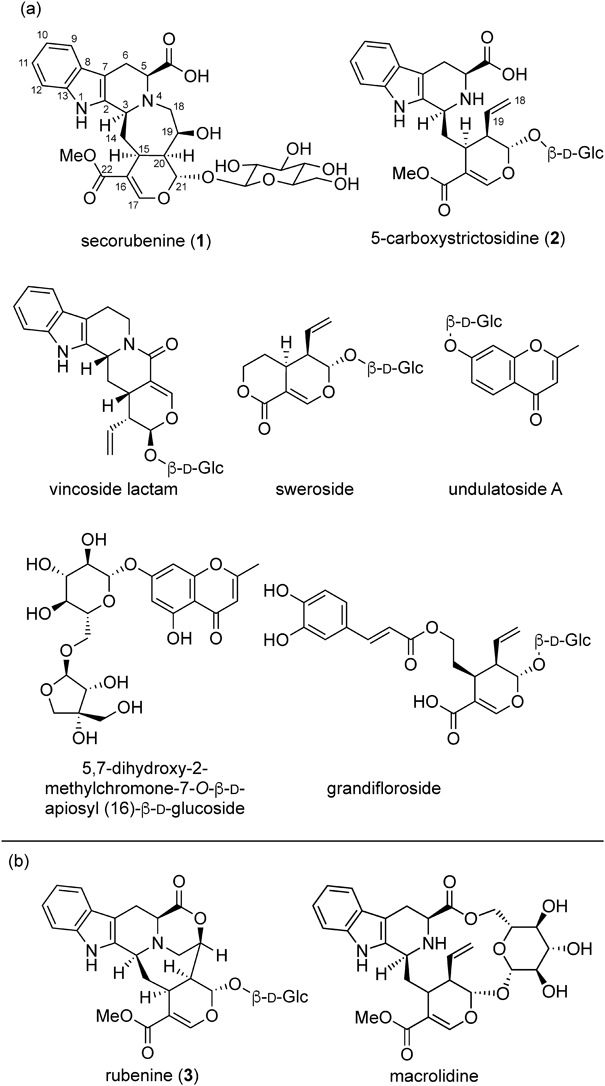
In the 1970 s, Brown et al. conducted intensive research into the components of this interesting plant and discovered monoterpenoid indole glycosides such as 5-carboxystrictosidine (2), rubenine (3), and macrolidine4–14) (Fig. 1). More recently, the divergent and bioinspired total syntheses of these alkaloids, including 5-carboxystrictosidine (2), rubenine (3), and others, have been achieved.15,16)
We carried out investigations into new alkaloid glycosides from the heartwood of Adina rubescens, followed by their rapid total synthesis. Herein, we report the discovery of a new pentacyclic indole alkaloid glycoside, secorubenine (1), and the subsequent determination of its structure, including its absolute configuration, by spectroscopic analysis and enantioselective total synthesis.
From the methanolic extract of the heartwood of A. rubescens collected in Indonesia, a new monoterpenoid indole alkaloid glycoside, named secorubenine (1), was isolated as a minor component together with known glycosylated indoles, iridoids, and chromones: 5-carboxystrictosidine (2), vincoside lactam,17) sweroside,18) grandifloroside,19) undulatoside A,20) and 5,7-dihydroxy-2-methylchromone-7-O-β-D-apiosyl (16)-β-D-glucoside.21)
Secorubenine (1) was obtained as a pale yellow amorphous powder. High-resolution electrospray ionization MS (HR ESI-MS) analysis gave m/z 591.2172 [M + H]+ (Δ–1.8 mmu) and established the molecular formula as C28H34N2O12. The presence of an indole ring was suggested by the four aromatic protons in the 1H-NMR spectrum (Table 1), which resonated at δH 7.36 (d, J = 7.5 Hz), 7.29 (d, J = 7.5 Hz), 7.01 (dd, J = 7.5, 7.5 Hz), 6.94 (dd, J = 7.5, 7.5 Hz), and UV absorptions at 290.5, 281.5, 223.5 nm. 1H- and 13C-NMR spectra showed signals assignable to one methyl β-alkoxy acrylic ester residue [δH 7.37 (s, H-17), 3.68 (3H, s, CO2Me); δC 167.1 (CO2Me), 151.8 (C-17), 111.7 (C-16), 51.4 (CO2Me)], one acetal group [δH 5.69 (br d, J = 4.5 Hz, H-21), δC 96.9 (C-21)], and one β-linked glucose unit [δH 4.53 (d, J = 10.0 Hz, H-1′), 3.60–2.90 (6H, H-2′ to 6′); δC 98.8 (C-1′), 77.4 (C-5′), 76.6 (C-3′), 73.1 (C-2′), 70.0 (C-4′), 61.0 (C-6′)], revealing that 1 contained a secologanin unit—a biosynthetic precursor for monoterpenoid indole alkaloids. In addition, a carboxyl carbon signal at δC 174.8 (CO2H) was observed in the 13C-NMR spectrum. The data suggested that 1 was a derivative of 5-carboxystrictosidine (2). In the NMR spectra, the obvious difference between 1 and 2 was the absence of signals assignable to a double bond between the 18 and 19 positions of the secologanin part in 1. The presence of a signal assignable to an additional oxymethine carbon at δC 69.5 in the 13C-NMR spectrum and the molecular formula predicted that the double bond of the secologanin part was oxidized in 1. The two dimensional (2D) NMR data supported that 1 was a derivative of 2 (Fig. 2). The heteronuclear multiple bond connectivity (HMBC) correlations between the proton at δH 2.90 (H-18) and carbons at δC 58.4 (C-3) and 69.5 (C-19) revealed the presence of a D-ring that consisted of the seven-membered ring structure containing a hydroxy group at C-19. From the above data, the planar structure of secorubenine was deduced to be that shown as formula 1.
| Position | 1 | |
|---|---|---|
| δH | δC | |
| 2 | — | 134.9 |
| 3 | 4.15 (m) | 58.4 |
| 5 | 3.59 (overlapped) | 63.9 |
| 6a | 2.81 (overlapped) | 22.7 |
| 6b | 2.81 (overlapped) | |
| 7 | — | 106.4 |
| 8 | — | 126.6 |
| 9 | 7.36 (d, 7.5) | 117.8 |
| 10 | 6.94 (dd, 7.5, 7.5) | 118.7 |
| 11 | 7.01 (dd, 7.5, 7.5) | 120.9 |
| 12 | 7.29 (d, 7.5) | 111.4 |
| 13 | — | 136.3 |
| 14a | 1.75 (ddd, 12.0, 12.0 12.0) | 34.4 |
| 14b | 2.55 (overlapped) | |
| 15 | 3.08–2.90 (overlapped) | 29.9 |
| 16 | — | 111.7 |
| 17 | 7.37 (s) | 151.8 |
| 18a | 2.81 (overlapped) | 53.0 |
| 18b | 2.90 (m) | |
| 19 | 4.20 (m) | 69.5 |
| 20 | 2.55 (overlapped) | 41.9 |
| 21 | 5.69 (br d, 4.5) | 96.9 |
| CO2H | — | 174.8 |
| CO2Me | — | 167.1 |
| CO2Me | 3.68 (3H, s) | 51.4 |
| 1′ | 4.53 (d, 10.0) | 98.8 |
| 2′ | 3.08–2.90 (overlapped) | 73.1 |
| 3′ | 3.18–3.15 (overlapped) | 76.6 |
| 4′ | 3.08–2.90 (overlapped) | 70.0 |
| 5′ | 3.18–3.15 (overlapped) | 77.4 |
| 6′a | 3.45 (m) | 61.0 |
| 6′b | 3.59 (overlapped) |

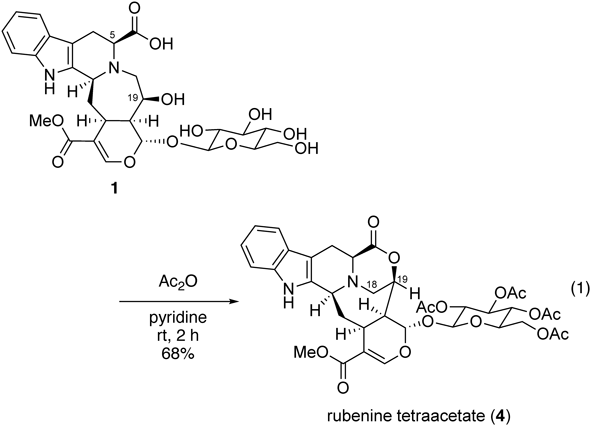
Next, we turned our attention to determining the stereochemistry of 1 by nuclear Overhauser effect (NOE) experiments. The hydroxy groups of 1 were acetylated to improve the solubility in organic solvents. Thus, 1 was treated with acetic anhydride in pyridine (2.7 mg of 1, room temperature (r.t.), 2 h). Unexpectedly, only four of the five hydroxy groups were acetylated. The resulting compound was a rubenine tetraacetate (4), which we had previously synthesized (Eq. 1).15) Hence, as this product 4 might be formed by intramolecular lactonization via a carboxylic anhydride, the stereochemistry of 1, including that of the hydroxy group at C-19, was estimated at this stage.
To then confirm the structure, including the absolute stereochemistry, we attempted the enantioselective total synthesis of secorubenine (1) (Chart 1).

In 2019, our group accomplished the biogenetically inspired total synthesis of monoterpenoid indole alkaloid glycosides, including rubenine.15) Following the protocols, secorubenine (1) was synthesized. Thus, the secologanin derivative 5, which was synthesized in 10 steps from commercially available materials, and the L-tryptophan methyl ester 6, were subjected to the Pictet–Spengler reaction in the presence of trifluoroacetic acid, to achieve stereoselective construction of the C-ring. This was followed by D-ring construction, with ring-opening of an epoxide, by treatment with silica gel, to obtain the pentacyclic compound 8 (60% yield over two steps, details in ref. 15). The structure of 8, including the unique seven-membered ring, was determined by 2D-NMR analysis, including HMBC and correlation spectroscopy (COSY) spectra. This seven-membered ring structure also occurs in dihydrocadambines,22,23) which belong to the same class of monoterpenoid indole alkaloids. Finally, the hydrolysis of 8 proceeded smoothly to afford secorubenine (1) in 74% yield. All analytical data, including NMR spectra and the optical rotation of 1 obtained by synthesis, were in full agreement with data of 1 obtained from plant source. The structure and the absolute configuration of 1 was successfully determined.

In conclusion, we isolated secorubenine (1), a new pentacyclic alkaloid, from the heartwood of Adina rubescens, a plant used as a traditional medicine in Southeast Asia. The structure of secorubenine (1) was determined by various spectral analyses. Its complete structure, including the absolute stereochemistry, was determined by derivatization from isolated 1 and enantioselective total synthesis. We consider this work as serving as a good example that the chemical supply through biogenetically inspired total syntheses greatly facilitates the structure determination of isolated natural products. Biological evaluation of the secorubenine (1) and other isolated compounds, and further exploratory studies on new alkaloids from other Adina plants, are currently in progress.
All reactions were monitored by TLC using Merck 60 F254 precoated silica gel plates (0.25 mm thickness). UV spectra were recorded in MeOH on a JASCO V-560 instrument. Specific optical rotations were measured using a JASCO P-2200 and P-1020 polarimeter. Circular dichroism (CD) spectra were recorded on a JASCO J-1100 spectrometer. Fourier transform (FT)IR spectra were recorded on a JASCO FT/IR-4700 and SHIMADZU IR Affinity-IS. 1H- and 13C-NMR spectra were recorded on an ECX 500 (500 MHz for 1H-NMR, 125 MHz for 13C-NMR) and an ECZ 600 (600 MHz for 1H-NMR, 150 MHz for 13C-NMR) FT-NMR spectrometer. Data for 1H-NMR are reported as chemical shifts (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, dd = double doublet, ddd = double double doublet, m = multiplet, br = broad), coupling constant (Hz), integration, and assignment. Data for 13C-NMR are reported as chemical shifts. The HR mass spectra were recorded on a JEOL AccuTOF LC-plus JMS-T100LP and BRUKER impact II. HPLC was performed on a Shimadzu LC-20AT system coupled with an SPD M20A photodiode array detector (Shimadzu Co., Kyoto, Japan), using a CAPCELLPAK C18 (5 µm, 250 × 20.0 mm i.d., SHISEIDO Inc., Tokyo, Japan). Recycle HPLC was performed on a Japan Analytical Industry LC-9225 NEXT SERIES system coupled with a UV-600 NEXT detector and RI-700 NEXT detector (Japan Analytical Industry Co., Tokyo, Japan), using an Asahipak GS-510 20G and Asahipak GS-310 20G column (500 × 20.0 mm i.d., Shodex Inc., Tokyo, Japan). Preparative thin layer chromatography (PTLC) was performed using Merck 60 F254 precoated silica gel plates (0.25 mm thickness).
Plant MaterialThe heartwood of Adina rubescens was collected in Bogor Botanical Garden, Indonesia, in February 2017.
Extraction and IsolationThe powdered heartwood (1.6 kg dry weight) of Adina rubescens was macerated with MeOH (three times at room temperature for 24 h, total 12 L) to give the extract (40 g). A portion of the MeOH extract (5.3 g) was dissolved in water (1.6 L) and extracted with n-hexane (1.6 L ×3), with AcOEt (1.6 L ×3), and then with n-BuOH (1.6 L ×3) to give the n-hexane extract (49.5 mg), AcOEt extract (901.8 mg), and n-BuOH extract (2.288 g), respectively. The n-BuOH extract (2.288 g) was separated by HPLC (column: SHISEIDO CAPCELL PAK C18) with a MeCN/H2O gradient (20–60%) to give 15 fractions (fr): fr 1 (193.1 mg), fr 2 (38.8 mg), fr 3 (45.4 mg), fr 4 (22.6 mg), fr 5 (72.1 mg), fr 6 (60.4 mg), fr 7 (25.1 mg), fr 8 (31.3 mg), fr 9 (51.5 mg), fr 10 (78.1 mg), fr 11 (35.2 mg), fr 12 (61.0 mg), fr 13 (191.3 mg), fr 14 (36.6 mg), and fr 15 (87.1 mg). Fraction 11 (35.2 mg) was purified by recycling SEC-HPLC (column: Asahipak GS-510 20G and GS-310 20G) with MeOH to afford secorubenine (1, 8.9 mg). The known compounds isolated from the n-BuOH extract were 5-carboxystrictosidine (5.3 mg), vincoside lactam (0.6 mg),17) sweroside (5.6 mg),18) grandifloroside (27.5 mg),19) undulatoside A (2.0 mg),20) and 5,7-dihydroxy-2-methylchromone-7-O-β-D-apiosyl (16)-β-D-glucoside (15.3 mg).21)
Secorubenine (1): Pale yellow amorphous powder; [α]D23 –62 (c 0.34, MeOH); UV (MeOH) λmax nm 290.5, 281.5, 223.5; IR (attenuated total reflectance (ATR)) cm−1 3282, 2924, 1627, 1259; 1H-NMR (500 MHz, (CD3)2SO) δ: 7.37 (1H, s, H-17), 7.36 (1H, d, J = 7.5 Hz, H-9), 7.29 (1H, d, J = 7.5 Hz, H-12), 7.01 (1H, dd, J = 7.5, 7.5 Hz, H-11), 6.94 (1H, dd, J = 7.5, 7.5 Hz, H-10), 5.69 (1H, br d, J = 4.5 Hz, H-21), 4.53 (1H, d, J = 10.0 Hz, H-1′), 4.20 (1H, m, H-19), 4.15 (1H, m, H-3), 3.68 (3H, s, CO2Me), 3.59 (1H, overlapped, H-5), 3.59 (1H, overlapped, H-6′), 3.45 (1H, m, H-6′), 3.18–3.15 (2H, overlapped, H-3′, 5′), 3.08–2.90 (3H, overlapped, H-15, 2′, 4′), 2.90 (1H, m, H-18), 2.81 (3H, overlapped, H2-6, H-18), 2.55 (2H, overlapped, H-14, 20), 1.75 (1H, ddd, J = 12.0, 12.0, 12.0 Hz, H-14); 13C-NMR (125 MHz, (CD3)2SO) δ: 174.8 (CO2H), 167.1 (CO2Me), 151.8 (C-17), 136.3 (C-13), 134.9 (C-2), 126.6 (C-8), 120.9 (C-11), 118.7 (C-10), 117.8 (C-9), 111.7 (C-16), 111.4 (C-12), 106.4 (C-7), 98.8 (C-1′), 96.9 (C-21), 77.4 (C-5′), 76.6 (C-3′), 73.1 (C-2′), 70.0 (C-4′), 69.5 (C-19), 63.9 (C-5), 61.0 (C-6′), 58.4 (C-3), 53.0 (C-18), 51.4 (CO2Me), 41.9 (C-20), 34.4 (C-14), 29.9 (C-15), 22.7 (C-6); 1H-NMR (600 MHz, CD3OD, at 60 °C) δ: 7.56 (1H, s, H-17), 7.43 (1H, d, J = 7.5 Hz, H-9), 7.35 (1H, d, J = 7.5 Hz, H-12), 7.11 (1H, dd, J = 7.5, 7.5 Hz, H-11), 7.03 (1H, dd, J = 7.5, 7.5 Hz, H-10), 5.64 (1H, d, J = 8.4 Hz, H-21), 4.80 (1H, d, J = 8.4 Hz, H-1′), 4.62 (1H, overlapped, H-19), 4.59 (1H, overlapped, H-3), 4.00 (1H, m, H-5), 3.99 (1H, d, J = 12.0 Hz, H-6′), 3.86 (1H, overlapped, H-18), 3.79 (3H, s, CO2Me), 3.68 (1H, br dd, J = 12.0, 4.2 Hz, H-6′), 3.47 (1H, m, H-18), 3.36–3.25 (6H, overlapped, H2-6, H-2′, 3′, 4′, 5′), 3.24 (1H, m, H-15), 2.68 (1H, d, J = 15.0 Hz, H-14), 2.30 (1H, overlapped, H-20), 2.26 (1H, overlapped, H-14); 13C-NMR (150 MHz, CD3OD, at 60 °C) δ: 175.1 (CO2H), 169.1 (CO2Me), 153.9 (C-17), 138.5 (C-13), 131.5 (C-2), 127.6 (C-8), 123.0 (C-11), 120.4 (C-10), 119.0 (C-9), 112.4 (C-12), 111.1 (C-16), 107.7 (C-7), 101.9 (C-1′), 98.3 (C-21), 78.4 (C-5′), 78.0 (C-3′), 74.7 (C-2′), 71.4 (C-4′), 69.5 (C-5), 66.2 (C-3), 64.5 (C-19), 62.4 (C-6′), 57.0 (C-18), 52.0 (CO2Me), 44.3 (C-20), 34.9 (C-14), 33.8 (C-15), 25.9 (C-6); HRMS (ESI): found: 591.2172 [M + H]+, calcd. for C28H35N2O12: 591.2190.
Chemical Modification and Total SynthesisAcetylation of Secorubenine (1)To a solution of secorubenine (1, 2.7 mg, 0.00457 mmol) in pyridine (30 µL), an excess amount of acetic anhydride (20 µL) was added at r.t. under Ar atmosphere. The reaction mixture was stirred for 2 h at r.t. The resulting mixture was concentrated under reduced pressure. The crude materials were purified by PTLC (70% AcOEt/n-hexane) to afford rubenine tetraacetate (4, 2.3 mg, 68%) as a pale yellow amorphous powder. All spectral data were identified based on our previously synthesized 4.
Rubenine tetraacetate (4): Pale yellow amorphous powder; [α]25D –30.0 (c 0.12, CHCl3); IR (neat) νmax cm−1 1745, 1217, 1036, 744; HRMS (ESI) [M + H]+ Calcd. for [C36H41N2O15]+: 741.2507, Found: 741.2529; 1H-NMR (600 MHz, CDCl3) δ: 7.48 (1H, d, J = 6.0 Hz), 7.44 (1H, s), 7.27 (1H, d, J = 6.0 Hz), 7.13 (1H, dd, J = 6.0, 6.0 Hz), 7.07 (1H, dd, J = 6.0, 6.0 Hz), 5.34 (1H, d, J = 12.0 Hz), 5.24 (2H, overlapped), 5.14 (1H, dd, J = 12.0, 12.0 Hz), 5.08 (1H, dd, J = 12.0, 12.0 Hz), 4.98 (1H, d, J = 6.0 Hz), 4.42 (1H, d, J = 6.0 Hz), 4.28 (2H, m), 4.19 (1H, d, J = 6.0 Hz), 3.96 (1H, d, J = 6.0 Hz), 3.88 (1H, dd, J = 12.0, 6.0 Hz), 3.78 (3H, s), 3.77 (1H, overlapped), 3.64 (1H, d, J = 18.0 Hz), 3.48 (1H, d, J = 18.0 Hz), 3.24 (1H, dd, J = 9.0, 9.0 Hz), 3.04 (1H, dd, J = 12.0, 6.0 Hz), 2.07 (1H, overlapped), 2.051 (3H, s), 2.047 (3H, s), 2.011 (3H, s), 2.007 (3H, s), 1.92 (1H, m); 13C-NMR (150 MHz, CDCl3) δ: 170.9, 170.3, 169.8, 169.60, 169.59, 167.5, 152.1, 136.3, 133.2, 126.8, 122.1, 119.5, 118.7, 110.8, 109.2, 105.2, 99.1, 96.7, 72.8, 72.3, 71.2, 68.5, 62.0, 58.9, 55.8, 51.7, 50.1, 43.9, 37.9, 32.5, 29.8, 20.9, 20.8, 20.73, 20.70, 20.2.
Preparation of Compound 8To a solution of L-tryptophan methyl ester (6, 25.3 mg, 0.116 mmol) and secologanin derivative 515) (33.2 mg, 0.0580 mmol) in CH2Cl2 (966 µL), powdered MS 4 Å (66.4 mg) was added at r.t. under Ar atmosphere. The resulting mixture was stirred for 1 h at r.t., followed by the addition of trifluoroacetic acid (22.2 µL, 0.290 mmol) at −20 °C. The reaction mixture was allowed to warm to 0 °C and then stirred for 1 h at this temperature. The resulting mixture was quenched with Et3N (81.0 µL) at 0 °C and filtered through a Celite pad, with CHCl3. The filtrate was concentrated under reduced pressure. The Pictet–Spengler reaction afforded the desired 3S-isomer 7, predominantly in quantitative conversion (C3S:C3R = 2.5 : 1), as determined by the crude NMR. The crude materials of 7 were directly loaded on PTLC (Wakogel® B-5F), where any remaining solvent was removed by drying with a blower. After 6 h, the silica gel was eluted with 10% MeOH/CHCl3. The resulting mixture was concentrated under reduced pressure and the resulting residue was purified by PTLC (SiO2, 60% AcOEt/n-hexane) to afford the desired product 8 (27.1 mg, 60% yield over two steps).
Compound 8: Pale yellow amorphous powder; [α]24D –44.3 (c 0.25, CHCl3); IR (neat) νmax cm−1 2922, 1744, 1705, 1638, 1435, 1368, 1219, 1034, 772, 743; HRMS (ESI) [M + H]+ Calcd. for [C37H45N2O16]+: 773.2764, Found: 773.2728; 1H-NMR (500 MHz, benzene-d6) δ: 7.53 (1H, s, H-17), 7.48–7.03 (4H, m, H-9, 10, 11, 12), 6.23 (1H, d, J = 8.5 Hz, H-21), 5.52 (1H, dd, J = 9.5, 9.5 Hz, H-3′), 5.41 (1H, dd, J = 9.5, 8.0 Hz, H-2′), 5.31 (1H, dd, J = 9.5, 9.5 Hz, H-4′), 5.13 (1H, d, J = 8.0 Hz, H-1′), 4.31 (1H, dd, J = 12.0, 5.0 Hz, H-6′), 4.28 (1H, br s, H-19), 4.08 (1H, dd, J = 12.0, 2.5 Hz, H-6′), 3.58 (1H, d, J = 10.5 Hz, H-3), 3.53–3.50 (1H, m, H-5′), 3.46 (3H, s, 22-CO2Me), 3.44 (1H, dd, J = 10.0, 4.5 Hz, H-5), 3.30 (3H, s, 5-CO2Me), 3.01–2.93 (3H, m, H-6, 15 and 18), 2.86 (1H, dd, J = 15.0, 3.5 Hz, H-6), 2.69 (1H, dd, J = 12.5, 4.5 Hz, H-18), 2.40 (1H, d, J = 11.0 Hz, H-14), 2.33–2.30 (1H, m, H-20), 1.95–1.87 (1H, m, H-14), 1.80 (3H, s, 6′-OCOMe), 1.73 (3H, s, 4′-OCOMe), 1.71 (3H, s, 3′-OCOMe), 1.66 (3H, s, 2′-OCOMe); 13C-NMR (125 MHz, benzene-d6) δ: 173.6 (CO2Me), 170.1 (6′-OCOMe), 169.9 (4′-OCOMe), 169.1 (3′-OCOMe), 169.1 (2′-OCOMe), 167.2 (C-22), 152.1 (C-17), 136.8 (C-8), 134.1 (C-2), 127.2 (C-13), 122.1 (C-11), 120.0 (C-10), 118.4 (C-9), 112.0 (C-12), 111.4 (C-16), 106.9 (C-7), 99.3 (C-21), 98.9 (C-1′), 73.1 (C-2′), 72.6 (C-5′), 71.8 (C-3′), 69.0 (C-19), 68.7 (C-4′), 63.9 (C-5), 61.8 (C-6′), 58.2 (C-3), 56.6 (C-18), 51.6 (C-24), 51.0 (C-25), 45.2 (C-20), 39.2 (C-14), 33.2 (C-15), 25.2 (C-6), 20.4 (6′-OCOMe), 20.3 (4′-OCOMe), 20.2 (3′-OCOMe), 20.2 (2′-OCOMe).
To a solution of 8 (27.0 mg, 0.0349 mmol) in MeOH (700 µL), 1M aqueous NaOH solution (700 µL) was added at 0 °C under Ar atmosphere. The reaction mixture was stirred for 2 h at 0 °C. The resulting mixture was quenched with 1M aqueous HCl solution (700 µL) and concentrated by lyophilization under reduced pressure. PTLC (SiO2, 30% MeOH/CHCl3) followed by HPLC (CAPCELLPAK C18, 1.0 mL/min, 20–70% MeCN/H2O gradient) afforded (−)-secorubenine (1, 15.2 mg, 74%) as a pale yellow amorphous powder; [α]D24 –91 (c 0.049, MeOH); 1H- and 13C-NMR spectra were compared as HCl salt of 1; see details in supporting information.
The authors declare no conflict of interest.
This article contains supplementary materials.