2022 年 70 巻 2 号 p. 106-110
2022 年 70 巻 2 号 p. 106-110
Benzolactams have unique biological activity and high utility in the synthesis of valuable compounds with direct applicability to oxindole alkaloids and antibacterial agents. Despite recent advances in organic chemistry and the growing number of reported methods for synthesizing benzolactams, their preparation still requires a multistep process. C–H amination reactions can convert aromatic C(sp2)–H bonds directly to C(sp2)–N bonds, and this direct approach to C–N bond formation offers effective access to benzolactams. Hypervalent iodine reagents are promising tools for achieving oxidative C–H amination. Motivated by our ongoing research efforts toward the development of useful hypervalent-iodine-mediated oxidative transformations, we herein describe an effective intramolecular oxidative C–H amination reaction based on μ-oxo hypervalent iodine catalysis for the synthesis of benzolactams bearing various functional groups.
Benzolactams, which feature a lactam ring fused to a benzene ring as a fundamental chemical structure, are an important motif in medicinal1–4) and synthetic chemistry.5–7) Thus, a method that could give rapid access to the benzolactam skeleton is highly desirable.
Most benzolactams are synthesized via intramolecular bond-formation reactions. Among them, intramolecular condensation between aniline and carboxyl groups is one of the most conventional methods for preparing benzolactams. Although this method is generally reliable, it cannot efficiently synthesize some types of benzolactams because the preparation of starting materials bearing aniline and carboxyl groups typically requires multistep synthetic processes.8) The intramolecular Friedel–Crafts reaction9,10) may also be employed to prepare benzolactams; however, this approach requires the use of excess amounts of metal salts and high reaction temperatures. To provide more reliable and convenient access to the desired benzolactam structure, organic chemists have developed transition-metal-catalyzed C–N-bond-formation reactions; such reactions allow the effective synthesis of benzolactams via intramolecular coupling between aryl halides and side chains with an amide moiety11–14) (Fig. 1, Method A).

An alternative means to construct the C–N bond of benzolactams is oxidative C–H amination. In this reaction, the aromatic C(sp2)–H bond is directly converted to the C(sp2)–N bond15,16) (Fig. 1, Method B). This approach has received considerable attention in recent years17–19) from the viewpoints of atom and step economy because C–N bond formation is achieved without pre-functionalization of the aromatic rings of the substrates. Hence, compared with the Buchwald–Hartwig and Goldberg amination approaches, the oxidative C–H amination method offers a direct approach to benzo-fused lactams. This amination method can be realized using transition-metal catalysts and a stoichiometric amount of an oxidant under high-temperature conditions. Therefore, the development of C–H amination reactions under mild conditions is an important undertaking in the field of synthetic organic chemistry.
Hypervalent iodine reagents, which are characterized by low toxicity, high safety, and attractive reactivity20) similar to heavy-metal oxidants, hold the key to improving oxidative C–H amination reactions. These reagents are utilized in various types of oxidative transformations, including oxidative C–N-bond-formation reactions. We first reported a method to achieve aromatic C–H azidation by ligand transfer from in situ generated PhI(N3)X (X = N3 or OCOCF3) to the aromatic ring.21–23) Utilizing fluoroalcohol solvent, a series of metal-free oxidative C–H coupling reactions in which nucleophiles were introduced to aromatic rings were then realized on the basis of this strategy.24–26) In later work, Kikugawa and other scholars reported the amide route of aromatic C–H functionalization to produce benzolactams with stoichiometric amounts of hypervalent iodine reagents in fluoroalcohols.27–29)
Over the last decade, continuous efforts have been devoted toward the development of hypervalent-iodine-catalyzed reactions30–32) owing to their numerous advantages. The catalytic route of arylamide formation has also been investigated by several research groups,33–37) including our group.38) C–H amination in a typical intermolecular reaction occurs under metal-free and mild conditions in the presence of a catalytic amount of iodoarene with a suitable co-oxidant, which suggests that hypervalent iodine catalysis is an attractive alternative to other C–N-bond-formation reactions. The corresponding intramolecular reaction to produce benzolactams has been also demonstrated, and benzamide derivatives were employed in most cases.36,37) On the other hand, the cyclization of alkyl amides with benzene ring via C–H amidation was limited,39,40) and only a maximum yield of 60% is obtained by using 10 mol% iodobenzene with m-chloroperbenzoic acid (mCPBA).
We recently reported an efficient method to synthesize aromatic amides via intermolecular C–H amination catalyzed by specific biaryl-type iodoarenes at only 0.5–2 mol% loadings.38) Motivated by the potential applications of this catalytic system to the synthesis of benzolactams, in this communication, we demonstrate the feasibility of intramolecular C–N bond formation based on biaryl-based iodoarene catalysts (Chart 1) involving the in situ generation of μ-oxo hypervalent iodine species (vide infra, Fig. 2, right). We also investigate the catalytic activity of the generated catalysts in the proposed reaction system.

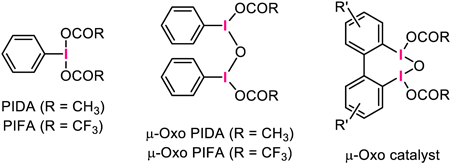
Our group has continuously developed efficient μ-oxo-hypervalent-iodine-mediated oxidation reactions. The reactivity of the μ-oxo PIFA dimer (Fig. 2, center) toward the oxidation of phenols has been thoroughly examined, and findings suggest that μ-oxo dimers are more reactive than conventional hypervalent iodine compounds, such as PIDA and PIFA41–43) (Fig. 2, left). We have also demonstrated that biaryl-type iodoarenes are smoothly transformed to the corresponding μ-oxo hypervalent iodine species (Fig. 2, right), following treatment with an appropriate oxidant.44) This μ-oxo species could serve as a reactive hypervalent iodine catalyst for oxidative C–N-bond-formation reactions. In addition to these experimental observations, theoretical calculations support the high reactivity of μ-oxo catalysts.45,46)
We, therefore, investigated the intramolecular C–H amination reaction based on μ-oxo hypervalent iodine catalysis for the efficient synthesis of benzolactam 2a (Table 1). We first screened a suitable solvent for the reaction by employing biaryl-type iodine catalyst 3a (entries 1–6). The reactions were performed at room temperature in the presence of aryl amide 1a, mCPBA, and 2 mol% catalyst 3a. When MeCN was used as a solvent, the desired benzolactam 2a was not detected at all (entry 1). Although a small amount of amide 1a was consumed in CH2Cl2 and 1,2-dichloroethane, no benzolactam formation was observed (entries 2 and 3). The use of MeNO2 resulted in the slight formation of benzolactam 2a (entry 4). The yield of 2a drastically improved when the reaction was carried out in 2,2,2-trifluoroethanol (TFE) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) which are highly polar and low nucleophilic alcoholic solvents (entries 5 and 6). In the presence of 2 equivalent (equiv.) of CF3COOH, the production of 2a was significantly boosted (entry 7). Increasing the amount of CF3COOH used in the reaction had no detectable effect on the yield of 2a (entries 8 and 9). Compound 2a was smoothly formed even when the amount of 3a was reduced to less than 1 mol% (entries 10–12). The use of 3b instead of 3a led to a higher 2a yield (entry 13); interestingly, the highest turnover number (i.e., 740) was observed in this system (entry 14). We assume that the methyl substituents in the catalyst 3b possibly affect the conformation of μ-oxo species and/or the cationic character of the iodine atoms. By contrast, the use of iodobenzene 3c and 4-iodotoluene 3d resulted in the formation of 2a at lower yields even with higher catalyst loadings (entries 15 and 16). These results indicate that the biaryl-type iodoarenes 3a and 3b that can generate μ-oxo species promote the reaction more efficiently. Furthermore, 4-iodoanisole 3e would reduce the yield presumably due to its instability under the oxidation conditions (entry 17). This result confirms that oxidative C–H amination could be catalyzed by a hypervalent iodine species because no detectable formation of benzolactam 2a was observed in the absence of iodoarenes 3 (entry 18).
 | |||||
|---|---|---|---|---|---|
| Entry | Precatalyst 3 | CF3COOH | Solvent | Yield of 2aa) | Recovery of 1aa) |
| 01 | 3a (2 mol%) | — | MeCN | N.D. | 96% |
| 02 | // | — | CH2Cl2 | N.D. | 77% |
| 03 | // | — | ClCH2CH2Cl | N.D. | 67% |
| 04 | // | — | MeNO2 | 14% | 63% |
| 05 | // | — | TFE | 43% | 17% |
| 06 | // | — | HFIP | 72% | N.D. |
| 07 | // | 2 equiv. | // | 91% (87%)b) | N.D. |
| 08 | // | 4 equiv. | // | 93% | N.D. |
| 09 | // | 8 equiv. | // | 94% | N.D. |
| 10 | 3a (1 mol%) | 2 equiv. | // | 94% | N.D. |
| 11 | 3a (0.5 mol%) | // | // | 90% | N.D. |
| 12 | 3a (0.25 mol%) | // | // | 80% | N.D. |
| 13 | 3b (0.25 mol%) | // | // | 89% | N.D. |
| 14 | 3b (0.1 mol%) | // | // | 74% | N.D. |
| 15 | 3c (0.5 mol%) | // | // | 55% | 24% |
| 16 | 3d (0.5 mol%) | // | // | 65% | 24% |
| 17 | 3e (0.5 mol%) | // | // | 20% | 56% |
| 18 | — | // | // | N.D. | >99% |
a) NMR yield using 1,1,2,2-tetrachloroethane as an internal standard. b) Isolated yield. N.D. = Not detected.
After the optimization of catalytic conditions, we examined the substrate scope of catalytic benzolactam formation (Chart 2) using 1 mol% 3a. Excellent yields of benzolactams 2b and 2c bearing methyl groups on the six-membered lactam ring were obtained. Biphenyl amide 1d was also successfully converted to phenanthridinone 2d. Benzolactams 2e and 2f with an electron-withdrawing ester and trifluoromethyl groups were produced in moderate to good yields although the electron density of the benzene rings of substrates 1e and 1f is reduced by these substituents. The ester group of 1e appeared to be tolerant to the catalytic conditions. However, the formation of lactam 2g with a strong electron-withdrawing group was challenging because of the low nucleophilicity of the aromatic ring of 1g in the oxidative coupling, and most of the starting material was recovered after the reaction work-up. When methyl-substituted aryl amide 1h was employed as a substrate, an unexpected side reaction was observed and lactam 2h was obtained in low yield; the formation of some unidentified benzylic oxidation product was accompanied in this case. Aryl amide 1i bearing the ortho-trifluoromethyl group gave the corresponding benzolactam 2i, albeit at only acceptable yield.
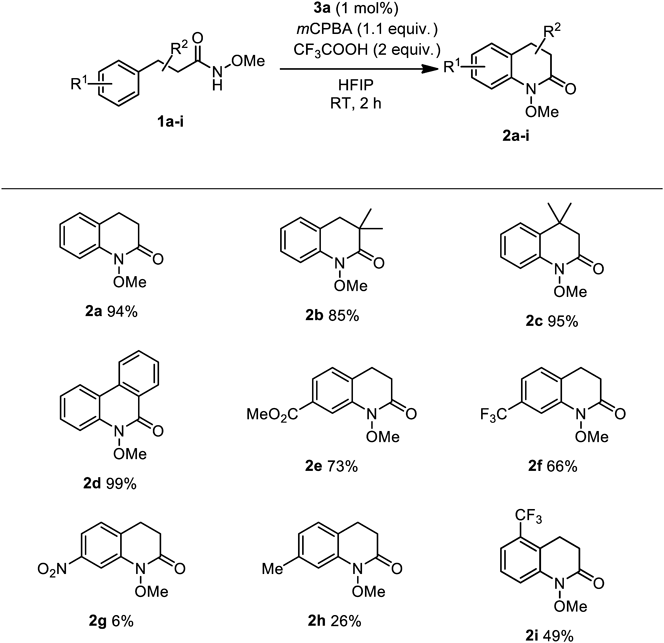
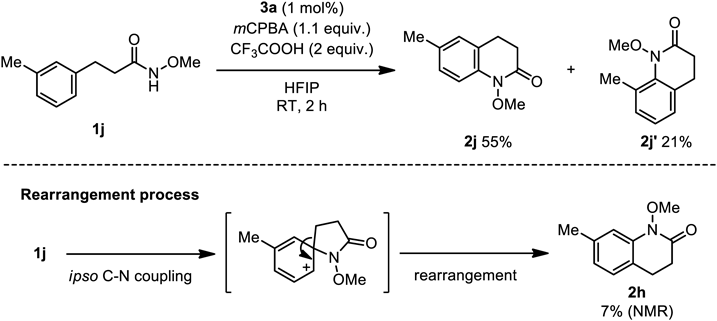
In contrast to para-substituted 1h, aryl amide 1j bearing a methyl group at the meta position smoothly reacted (Chart 3) to give lactams 2j (55%) and 2j′ (21%) as an inseparable regioisomeric mixture. 1H-NMR analysis of this reaction mixture also suggested the formation of a small amount of benzolactam 2h (7%) because of the occurrence of methylene group-rearrangement during lactam formation. This result would indicate that the C–H amination is the major pathway for the observed benzolactam formations, rather than the spirocyclization reactions.
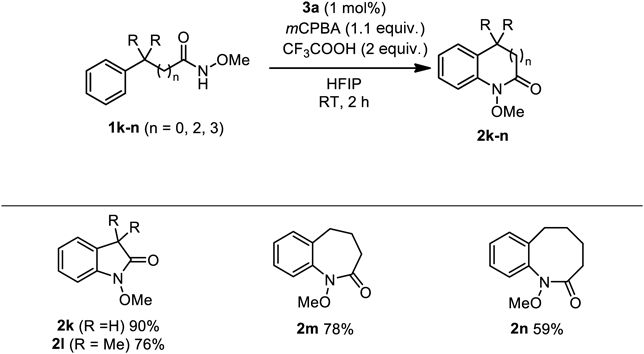
The proposed intramolecular oxidative C–H amination reaction system was compatible with the formation of five-, seven-, and even eight-membered benzolactams (Chart 4). Indolinone 2k and its derivative 2l bearing two methyl groups were obtained in excellent to good yields. The seven- and eight-membered lactams 2m and 2n were also formed in good yields47); no intermolecular C–H amination product was detected in these cases without high dilution. Thus, our reaction system based on μ-oxo hypervalent iodine catalysis can effectively provide various benzolactams 2 at surprisingly low catalyst loading (approx. 1 mol%) with good functional group tolerance under mild conditions.48)
We developed an effective method to achieve the intramolecular oxidative C–H amination of aromatic rings mediated by a μ-oxo hypervalent iodine catalyst. The use of the μ-oxo catalyst allowed intramolecular C–H amination at a remarkably low catalyst loading. The catalysts also showed a very high turnover number, confirming its applicability as an oxidation organocatalyst. Hypervalent iodine catalysis was proven to be highly effective for the synthesis of benzolactams bearing various functional groups that could be applied to the formation of five- to eight-membered compounds.
This work was supported in part by JSPS KAKENHI Grant Number 19K05466 (T. D.) and 18H02014 (K. K.), and JST CREST Grant Number JPMJCR20R1. T. D. also acknowledges support from the Ritsumeikan Global Innovation Research Organization (R-GIRO) project, and thanks Central Glass Co., Ltd. for generous gift of fluoroalcohol. H.S. thanks the Pharmaceutical Society of Japan (PSJ) for support of the Nagai Memorial Research Encouragement.
The authors declare no conflict of interest.
This article contains supplementary materials.