2022 Volume 70 Issue 3 Pages 235-239
2022 Volume 70 Issue 3 Pages 235-239
Heavy atom-containing molecules cause a photoreaction by a direct S0 → Tn transition. Therefore, even in a hypervalent iodine compound with a benzene ring as the main skeleton, the photoreaction proceeds under 365–400 nm wavelength light, where UV-visible spectra are not observed by usual measurement method. Some studies, however, report hypervalent iodine compounds that strongly absorb visible light. Herein, we report the synthesis of two visible light-absorbing hypervalent iodines and their photooxidation properties under visible light irradiation. We also demonstrated that the S0 → Tn transition causes the photoreaction to proceed under wavelengths in the blue and green light region.
Photoreactions exhibit different reactivities compared with thermal reactions because light energy is much greater than heat energy. Photoreactions of hypervalent iodine compounds began to be surveyed starting around 1979, and reactions using UV light were reported in the 1980 s–1990 s1–5)(Chart 1A). UV light excites various molecules (even simple benzene), however, thereby causing undesired side reactions. Therefore, in recent years, the development of reactions using visible light has been actively investigated, and visible-light reactions with hypervalent iodine have been reported. Most of the reactions reported to date, however, are visible-light reactions using photo-catalysts6–15); some reactions directly exciting hypervalent iodine by visible light are reported16–25) (Chart 1B). The development of direct visible-light activation of hypervalent iodines has not been reported because the main skeleton of almost all hypervalent iodines is iodobenzene, whose longest absorption maximum wavelength is in the UV light region. Thus, the design and synthesis of hypervalent iodines that absorb visible light is an important research topic for hypervalent iodine photoreactions.

In 2020, we reported that photoreactions of heavy-atom-containing molecules such as hypervalent iodine proceed by a direct S0 → Tn transition26) (Chart 1C). In the same paper, we also reported a hypervalent iodine compound with an acridine skeleton that can accelerate photo-oxidation by a direct S0 → Tn transition under 450-nm blue- and 510-nm green-light irradiation (Chart 1D). We thought that this 10-phenyl-acridinile-9-dioxo-iodane (PADI) could be a breakthrough for visible-light-reactions of hypervalent iodine. Herein, we report the synthesis of visible-light–activated hypervalent iodines and their versatility for photo-oxidation.
We first prepared 4-bromo-9-phenyl-acridine (3) from commercially available 9-phenyl-acridine (2), and after regioselective mono-bromination by N-bromosuccinimide, the iodo-acridine (4) was synthesized through a halogen-lithium-exchange, followed by quenching using molecular iodine (Chart 2A). Bromo-acridine (3) could also be synthesized from commercially available 2-bromodiphenylamine (5) by microwave-promoted cyclization with benzoic acid.27,28) Finally, the desired PADI (1) was obtained by oxidation with dimethyldioxirane. Next, we attempted to synthesize the hypervalent iodine containing diphenyl anthracene moiety (DPADI (9)), which was thought to absorb visible light (Chart 2B). 9,10-Diphenyl-2-iodo-anthracene (8) was prepared using reported procedure,29) and DPADI (9) was synthesized by dimethyldioxirane oxidation. We next measured the absorption of PADI (1) and DPADI (9); the absorption maxima were observed at 362, 380, and 400 nm for PADI (1),26) and 371, 390, and 410 nm for the DPADI (9), respectively (Chart 2C).

We previously reported that the S0 → Tn transition of PADI proceeds with visible light greater than 450 nm.26) Thus, to clarify whether the S0 → Tn transition occurs with newly synthesized DPADI, we performed various spectral measurements. Using a degassed 1 : 1 mixed solution of diethyl ether and toluene (0.1 mM) as a glassing solvent system, we measured the absorption, emission, phosphorescence, and excitation. Under cooling at 77 K with liquid nitrogen, fluorescence and phosphorescence were observed between 400–500 nm and 500–650 nm, respectively. Because the phosphorescence intensity was very low, the phosphorescence bands were observed in emission spectra for which the y-axis was expanded. The phosphorescence excitation showed a weak peak between 450–510 nm, consistent with the absorption spectra. When the emission spectrum was measured by excitation with 450-nm light, we observed the same phosphorescence as under excitation with 380 nm light, leading us to conclude that the absorption between 450–510 nm was the S0 → Tn absorption band.
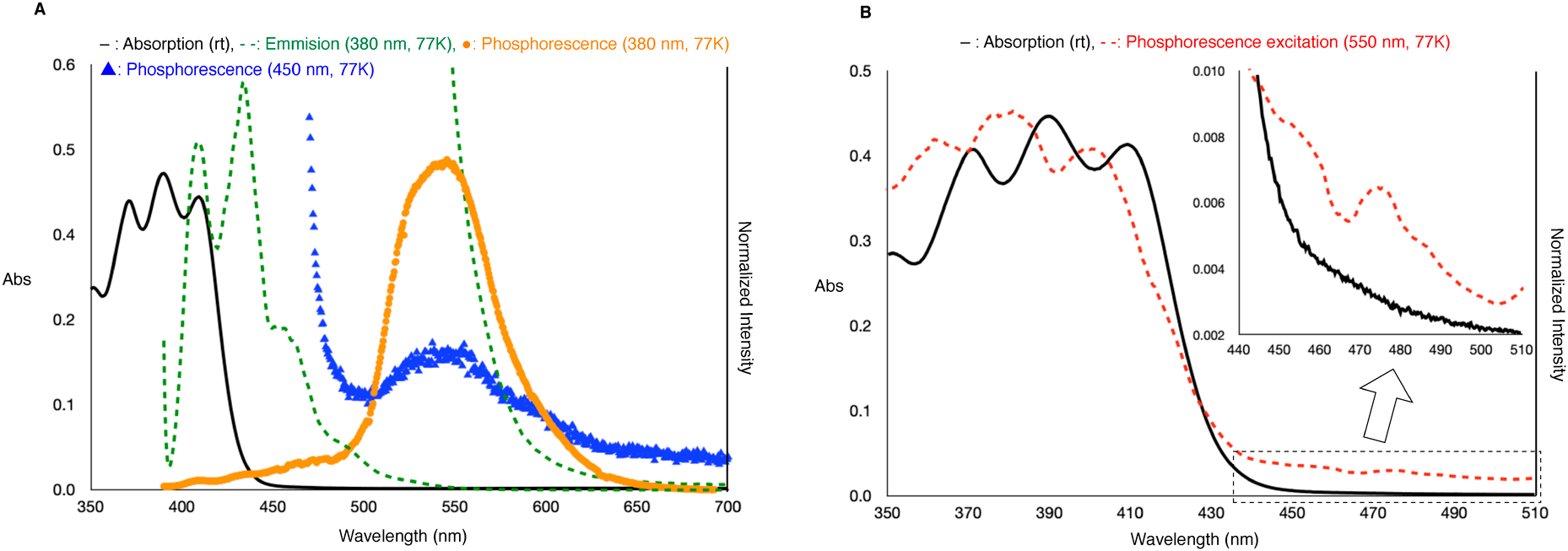
Photo-oxidation of phenyl ethyl alcohol using the visible-light–absorbing hypervalent iodine was investigated next.26) Under light-shielding dark conditions with PADI (1), only trace amounts of the ketone were observed even when heated to 40 °C (entries 1 and 2), and the oxidation proceeded in 84% yield at 80 °C (entry 3). Next, we performed the photo-oxidation under irradiating 400-, 450-, and 510-nm light for 5 h at room temperature (entries 4–6). The reaction successfully proceeded under these conditions, and 450-nm blue light produced the best yield (entry 5). A longer reaction time improved the yield to 95% (entry 7), and the photo-oxidation even proceeded at −40 °C to give the product in 98% yield (entry 8). Since the 450 nm blue LED contains light in the 420–470 nm range (see supplementary materials for details), the S0 → S1 and S0 → Tn transitions are considered to be mixed. On the other hand, it is interesting to note that the photo-oxidation reaction proceeded in 41% yield using 510 nm green LED (490–530 nm), indicating that the oxidation reaction was completely based on the S0 → Tn transition with this light (entry 6). The absorption spectra of PADI (1) and iodoacridine (4) which was produced in situ were measured in single solutions and 1 : 1 mixed solution at various concentrations, but no complexes such as self-aggregation or exciplex were observed. (See supplementary materials for details) When DPADI (9) was applied to this reaction, the reaction hardly proceeded under light-shielding conditions (entry 9), and the photo-oxidation proceeded under irradiation by 450- and 510-nm light to give the ketone in 59% and 40% yields (entries 10, 11), respectively. Although both hyper valent iodines showed the photo-oxidation property, PADI (1) gave higher yield, indicating that DPADI (9) was less active for the oxidation or a number of the molecular of triplet state is smaller. Thus, we decided to used PADI (1) to further examine the visible light oxidation reaction.
We next investigated the substrate generality of the photo-oxidation of secondary alcohol under optimum conditions. The reaction proceeded in a high yield with substrates having a fluoro-, chloro-, and methoxy-substituted benzene (12–14). Benzyl alcohol with an alkyl chain and propargyl benzyl alcohol also afforded the corresponding ketone (15, 16). Biaryl and naphthyl alcohol were also applicable for this oxidation (17, 18). The oxidation proceeded in high yield even using cyclic alcohol (19, 20), adamantane (21), and a steroid skeleton (22). Notably, none of these reactions proceeded at all under dark conditions. With primary benzyl alcohol (23) as a substrate, carboxylic acid (24) was obtained under light irradiation conditions. The C–C bond of diol (25) was cleaved and oxidized to form carboxylic acid (24) under light irradiation conditions; whereas the aldehyde (26) was obtained in 48% yield under dark conditions. Sulfoxide (27) was oxidized to sulfone (28) under light irradiation conditions. These results clearly demonstrated that the visible light irradiation remarkably increased the oxidizing ability of the hypervalent iodine.

We next clarified the reaction mechanism in detail by quantum calculations. Density functional theory (DFT) calculations were performed using the Gaussian 16 package, and based on our benchmark study,30,31) the MN1532) functional with SDD33) (for I) and cc-pVTZ34) (for the other atoms) basis sets were used in gas phase. The oxidation reaction was calculated to proceed through the coordination of alcohol to PADI (TS1), proton transfer (TS2), and oxidative deprotonation (TS3). The energy of all triplet intermediates (purple diagram) is higher than the activation energy of all transition states in the S0 state (black diagram), meaning that the radiation-less transition from T1 → S0 easily traverses the transition states of grand states. The activation energy of oxidation step (TS3) was 27.3 kcal/mol, supporting the experimental results that the oxidation did not proceed below 80 °C in the dark (Table 1, entries 1–3).
 | |||||
|---|---|---|---|---|---|
| Entry | HVI | hn | Temp. | Time | Yield |
| 1 | PADI | None | r.t. | 13 h | Trace |
| 2 | PADI | None | 40 °C | 13 h | Trace |
| 3 | PADI | None | 80 °C | 13 h | 84% |
| 4 | PADI | 400 nm | r.t. | 5 h | 22% |
| 5 | PADI | 450 nm | r.t. | 5 h | 80% |
| 6 | PADI | 510 nm | r.t. | 5 h | 41% |
| 7 | PADI | 450 nm | r.t. | 13 h | 95% |
| 8 | PADI | 450 nm | −40 °C | 24 h | 98% |
| 9 | DPADI | None | r.t. | 13 h | Trace |
| 10 | DPADI | 450 nm | r.t. | 13 h | 59% |
| 11 | DPADI | 510 nm | r.t. | 13 h | 40% |

SM: starting material. CP: complex. TS: transition state. INT: intermediate. PD: product. 03: triplet state. FC: Franck-Condon state.
We successfully designed and synthesized visible-light–activated hypervalent iodine, and demonstrated that the mechanism of the photo-oxidation proceeds via a forbidden direct S0 → Tn transition. We anticipate that this molecular design and reaction via the S0 → Tn transition will find wide application.
The authors declare no conflict of interest.
This article contains supplementary materials.