2022 年 70 巻 6 号 p. 427-434
2022 年 70 巻 6 号 p. 427-434
In the present study, four novel ginsenosides fatty acid and aromatic acid derivatives were designed and synthesized, and their cytotoxic effects on human ovarian carcinoma cells (SKOV3) were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The results demonstrated that all derivatives inhibited SKOV3 cell growth, and Compound 3 showed the most outstanding anti-proliferative effect on SKOV3 cells. The IC50 value of Compound 3 was 33.8 ± 2.21 µM, less than half of that of cis-platinum (70.1 ± 7.64 µM). Subsequent analysis revealed that Compound 3 could promote SKOV3 cell apoptosis, and the percentage of apoptotic cell population increased with increasing Compound 3 concentrations. In addition, the expression ratios of Bax/Bcl-2, cleaved-Caspase-3/Caspase-3 and cleaved-Caspase-9/Caspase-9 were gradually elevated in Compound 3-treated SKOV3 cells compared with control cells. Furthermore, translocation of Bax to mitochondria was associated with the release of Cytochrome C. Molecular docking analysis revealed three hydrogen-bonds existed in Compound 3 with poly(ADP-ribose)polymerase (PARP) receptor (PDB code: 5DSY), which may be the target of the anti-ovarian cancer effect of Compound 3. Altogether, our study indicates that Compound 3 induces SKOV3 cell apoptosis via reactive oxygen species (ROS)-dependent mitochondrial pathway, and can serve as an anti-cancer agent for treating ovarian carcinoma.
Ovarian cancer is a common malignant tumor of female reproductive organs.1,2) It is the seventh most common cancer among women, which accounts for approximately 295000 new cases and 185000 deaths annually.3) The incidence and death rate of ovarian cancer are increasing every year.4,5) At present, postoperative chemotherapy based on platinum and paclitaxel is considered to be the most effective treatment for ovarian cancer. However, patients receiving this type of chemotherapy tend to experience cumulative toxic effects and are prone to develop chemotherapeutic resistance.6) Therefore, it is urgently needed to discover a novel, effective anti-ovarian tumor drug with less toxic and side effects.
Ginseng, the roots of Panax ginseng Meyer, is one of the most well-known traditional Chinese herbs. It has been widely commonly employed as a functional agent for thousands of years in East Asian countries.7) Over the past few decades, researchers have conducted in-depth and extensive research on Ginseng. Many studies have indicated that ginsenosides, the major component of Ginseng, exhibit various pharmacological properties such as anti-tumor activity,8–10) anti-inflammation,11) cardiovascular health improvement,12,13) neuro-protection,14) immunoregulatory actions,15,16) and so on. Among them, their antitumor activity is well known, and some ginsenoside compounds have been developed into drugs and used in clinical treatment.17) For example, Shenyi capsule is the first anti-cancer agent approved by China’s State Food and Drug Administration.18) In addition, some other ginsenosides also show great potential in anti-tumor agents, 20(S)-protopanaxadiol (PPD), the principal intestinal metabolite of ginsenosides, was recognized as the pharmacophore of ginsenosides and showed increased absorption in the gastrointestinal tract.19) PPD also showed anti-ovarian cancer activity by inhibiting the growth, invasion and migration and inducing the apoptosis of human ovarian cancer cells.20) These results demonstrated that the metabolites of ginsenosides could be promising candidates for ovarian cancer treatment.
Pseudo-sapogenin DQ [(20S, 24R)-epoxy dammarane-3β,12β,25-diol, PDQ], which is a C-24 bit R configuration ocotillol-type triterpene saponin (Fig. 1), is the main metabolite of PPD in human liver. PDQ was found to have better oral bioavailability and a lower metabolism burden in organisms compared with the parent compounds PPD and protopanoxadiol-type ginsenosides, suggesting its potential medicinal value.21) Therefore, PDQ and its derivatives have been extensively studied for drug development against a variety of conditions such as myocardial ischemic injury,22) cisplatin-induced nephrotoxicity,23) malarial infection,24) cancer,25) and inflammatory diseases.26) However, the research on PDQ or PDQ analogs with anti-ovarian cancer activity is relatively scarce. To obtain ginsenoside derivatives with better biological activity, a great deal of research has been made to structurally modify ginsenosides from the perspective of chemistry and pharmacology.27,28) Among the structural modifications of PDQ, many amino acid derivatives had been designed and synthesized. For example, the amino acid derivatives of ginsenoside AD1 have anti-liver fibrosis activity,29,30) and those of Pyxinol have P-glycoprotein (PGP)-mediated multiple drug resistance (MDR) activity.31) Furthermore, several in vivo studies have shown that fatty acid esters may be the most effective substance of ginsenosides in the body.32–34) Hence, the reaction efficiency of esterification with fatty acids may be one of the most promising design strategies.35,36)

With the efforts to discover novel ginsenoside derivatives with significant anticancer activity, four novel PDQ fatty acid and aromatic acid derivatives were designed and synthesized in this study. The cytotoxic effects of these derivatives on human ovarian carcinoma cells (SKOV3) were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The most promising compound was further evaluated to clarify the mechanisms underlying its anti-proliferative effect.
Compounds 1–4 were synthesized according to Chart 1. PDQ (Fig. 1) was dissolved in dry dichloromethane solution, and the reactions were initiated with butanedioic anhydride, phthalic anhydride, maleic anhydride and glutaric anhydride at room temperature, in the presence of trimethylamine to obtain the target compounds. The structural characterization of Compounds 1–4 was assessed by MS, 1H-NMR and 13C-NMR.
Reagents and conditions: (a) CH2Cl2, Et3N, r.t.
Compound 1: A white crystal in ethyl acetate, Yield: 86%, mp 168.8–174.6 °C. 1H-NMR (500 MHz, Pyr) δ: 4.72 (1H, dd, J = 11.7, 4.8 Hz, H-24), 3.94 (1H, dd, J = 8.7, 6.7, Hz,H-12), 3.68 (1H, dd, J = 10.5, 4.7 Hz, H-3), 1.46 (s, 3H), 1.27 (s, 3H), 1.25 (s, 3H), 0.95 (s, 3H), 0.94 (s, 3H), 0.90 (s, 3H), 0.87 (s, 3H), 0.75 (s, 3H). 13C-NMR (126 MHz, Pyr) δ: 175.00(C-34), 172.65(C-31), 86.78(C-24), 85.72(C-20), 80.84(C-3), 71.10(C-12), 70.38(C-25), 56.22(C-5), 52.22(C-14), 50.63(C-9), 49.84(C-13), 48.44(C-17), 40.03(C-8), 38.71(C-4), 38.24(C-1), 37.21(C-10), 35.03(C-7), 32.87(C-15), 32.46(C-32), 31.76(C-33), 30.41(C-11), 30.07(C-22), 28.84(C-27), 28.13(C-23), 27.78(C-28), 27.33(C-2), 27.02(C-26), 25.58(C-21), 24.06(C-16), 18.48(C-6), 18.38(C-30), 16.81(C-19), 16.52(C-29), δ 15.57(C-18). MS, m/z: 577.41 [M + H]+.
(20S, 24R)-Dammar-20, 24-Epoxy-12β, 25-Diol-3β-yl HemiphthalateCompound 2: A white crystal in ethyl acetate, Yield: 90%, mp 226.1–227.6 °C. 1H-NMR (500 MHz, Pyr) δ: 5.02 (1H, dd, J = 11.9, 4.6 Hz, H-24), δ 3.96 (1H, td, J = 10.0, 4.5 Hz, H-12), δ 3.70 (1H, td, J = 10.4, 4.6 Hz, H-3), The hydrogen signals of the benzene ring [δ 7.54–8.13 (4H, m, C6 H4)], 8.13 (dd, J = 6.9, 2.0 Hz, 1H), 7.94 (dd, J = 6.7, 2.0 Hz, 1H), 7.54 (td, J = 6.3, 1.7 Hz, 2H), 1.49 (s, 3H), 1.29 (s, 3H), 1.27 (s, 3H), 1.09 (s, 3H), 0.96 (s, 3H), 0.94 (s, 3H), 0.90 (s, 3H), 0.73 (s, 3H). 13C-NMR (126 MHz, Pyr) δ: 170.25(C-38), 168.61(C-31), 134.55(C-34), 134.38(C-35), 131.07(C-32), 131.02(C-37), 129.67 (C-36), 129.05 (C-33), 86.78 (C-24), 85.72 (C-20), 82.39 (C-3), 71.10 (C-12), 70.38 (C-25), 56.35 (C-5), 52.21 (C-14), 50.64 (C-9), 49.83 (C-13), 48.44 (C-17), 40.04 (C-8), 38.78 (C-4), 38.42 (C-1), 37.23 (C-10), 35.03 (C-7), 32.87 (C-15), 32.46 (C-11), 31.74 (C-22), 28.84 (C-27), 28.27 (C-23), 27.78 (C-28), 27.33 (C-2), 27.02(C-26), 25.57(C-21), 23.68(C-16), 18.47(C-6), 18.38(C-30), 16.89 (C-19), 16.45 (C-29), 15.55 (C-18). MS, m/z: 625.41 [M + H]+.
(20S, 24R)-Dammar-20, 24-Epoxy-12β, 25-Diol-3β-yl HemimaleateCompound 3: A white crystal in ethyl acetate, Yield: 79%, mp 207.5–209.7 °C. 1H-NMR (500 MHz, Pyr) δ: 6.73 (1H, d, J = 12.1 Hz, –CH = CH–), 6.55 (1H, d, J = 12.1 Hz, –CH = CH–), 4.85 (1H, d, J = 7.3 Hz, H-24), 3.96 (1H, td, J = 10.0, 4.5 Hz, H-12), 3.69 (1H, dd, J = 10.4, 4.5 Hz, 3-H), 1.48 (s, 3H), 1.28 (s, 3H), 1.26 (s, 3H), 1.01 (s, 3H), 0.95 (s, 3H), 0.92 (s, 3H), 0.88 (s, 3H), 0.72 (s, 3H). 13C-NMR (126 MHz, Pyr) δ: 168.09(C-34), 165.79(C-31), 132.20(C-32), 128.66(C-33), 86.45(C-24), 85.40(C-20), 81.55(C-3), 70.77(C-12), 70.05(C-25), 55.96(C-5), 51.89(C-14), 50.30(C-9), 49.51(C-13), 48.12(C-17), 39.71(C-8), 38.41(C-4), 37.97(C-1), 36.87(C-10), 34.69(C-7), 32.54(C-15), 32.13(C-11), 31.44(C-22), 28.51(C-27), 27.76(C-23), 27.46(C-28), 27.01(C-2), 26.70(C-26), 25.26(C-21), 23.46(C-16), 18.14(C-6), 18.05(C-30), 16.49(C-19), 16.15(C-29), 15.23(C-18). MS, m/z: 575.39 [M + H]+.
(20S, 24R)-Dammar-20, 24-Epoxy-12β, 25-Diol-3β-yl HemiglutarateCompound 4: A white crystal in ethyl acetate, Yield: 81%, mp 176.7–182.3 °C. 1H-NMR (500 MHz, Pyr) δ: 4.70 (1H, dd, J = 11.6, 4.9 Hz, 24-H), 3.96 (1H, td, J = 10.0, 4.5 Hz, 12-H), 3.71 (1H, td, J = 10.4, 4.5 Hz, 3-H), 1.49 (s, 3H), 1.29 (s, 3H), 1.27 (s, 3H), 0.98 (s, 3H), 0.91 (s, 3H), 0.90 (s, 3H), 0.89 (s, 3H), 0.78 (s, 3H). 13C-NMR (126 MHz, Pyr) δ: 175.52(C-35), 173.01(C-31), 86.78(C-24), 85.72(C-20), 80.61(C-3), 71.11(C-12), 70.38(C-25), 56.17(C-5), 52.22(C-14), 50.65(C-9), 49.84(C-13), 48.45(C-17), 40.04(C-8), 38.72(C-4), 38.17(C-1), 37.22(C-10), 35.03(C-34), 34.24(C-32), 34.06(C-7), 32.87(C-15), 32.47(C-11), 31.76(C-22), 28.84(C-27), 28.15(C-23), 27.78(C-28), 27.32(C-2), 27.03(C-26), 25.58(C-21), 24.08(C-16), 21.33(C-33), 18.50(C-6), 18.39(C-30), 16.80(C-19), 16.54(C-29), 15.57(C-18). MS, m/z: 591.42 [M + H]+.
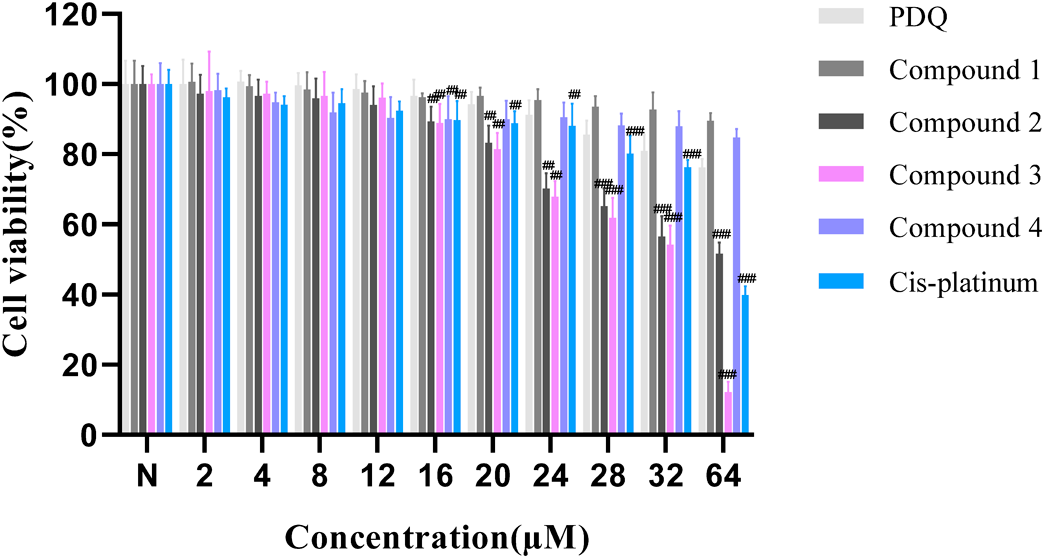
Anti-proliferative Activities of Compounds 1–4The effects of Compounds 1–4 on SKOV3 cell proliferation were analyzed by MTT assay. It was found that PDQ, Compounds 1 and 4 had no significant inhibitory activity against the proliferation of SKOV3 cells at 0–64 µM, while Compound 2, Compound 3 and cis-platinum exhibited significant anti-proliferative effects on SKOV-3 cells in concentration-dependent fashion (Fig. 2). The IC50 values of Compound 2, Compound 3 and cis-platinum against SKOV3 cells were 53.6 ± 4.35, 33.8 ± 2.21 and 70.1 ± 7.64 µM, respectively, at 48 h. These findings suggest that Compound 3 can suppress the proliferation of SKOV3 cells in vitro.
After treatment with Compound 3 for 48 h, the growth rate of SKOV3 cells was reduced, intracellular vacuoles appeared, and a large number of cell fragments were floating on the surface of the culture medium (Fig. 3A). Compared with the normal group, the cell density was dose-dependently attenuated in Compound 3 treatment group, along with cell membrane rupture, nucleus dissociation and aggregation (Fig. 3B).

Apoptosis is considered as one of the major modes of cancer cell death. To examine whether Compound 3 can induce apoptosis, fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI) assays and flow cytometric analysis were performed. The results demonstrated that Compound 3 could induce SKOV3 cell apoptosis (Fig. 4), and the apoptotic cell populations were found to be 0.06, 6.63, 8.41, 34.27 and 43.74% after treatment with Compound 3 (0, 16, 20, 24 µM) and cis-platinum (32 µM) for 24 h, respectively. The findings indicated that treatment with Compound 3 decreased the apoptotic rate of SKOV3 cells in a concentration-dependent fashion. More importantly, the apoptotic rate of Compound 3 was relatively similar to that of cis-platinum.

ROS plays an essential role in regulating mitochondria-dependent cell death. Therefore, we assessed whether ROS level is increased in SKOV3 cells after treatment with Compound 3. 2,7-Dichlorodi-hydro fluorescein diacetate (DCFH-DA) and flow cytometric analysis were performed to detect the cellular levels of ROS. After exposure to 16, 20 and 24 µM Compound 3 for 24 h, the cellular levels of ROS in SKOV3 cells were 2.85 ± 0.21, 6.25 ± 0.33 and 17.63 ± 0.56%, respectively, when compared to the control group (0 µM). Meanwhile, the cellular level of ROS was 20.03 ± 0.71% after being exposed to 32 µM cis-platinum. Altogether, the results demonstrated that Compound 3 could induce SKOV3 cell apoptosis in a ROS-dependent fashion (Fig. 5).

Given that Bcl-2 and Caspase family proteins are involved in the regulatory network of cell apoptosis, we further measured the expression levels of anti- and pro-apoptotic proteins in SKOV3 cells through Western blotting. From the results in Fig. 6, we found that Compound 3 increased the expression levels of cleaved-Caspase-3 (CC-3)/Caspase-3 (C-3), cleaved-Caspase-9 (CC-9)/Caspase-9 (C-9) and Cytochrome C (Cytc), while increased the ratio of Bax/Bcl-2 in a concentration-dependent fashion.

The molecular docking results showed that Compound 3 could form hydrogen bond with poly(ADP-ribose)polymerase (PARP) receptor (PDB code: 5DSY). The 24-position OH of Compound 3 forms hydrogen bonds with the hydroxyl group of TYR-460, and the maleic acid structure of Compound 3 has two hydrogen bonds with the guanidine and amino sites of ARG-431 in the receptor. Thus, it is speculated that Compound 3 may induce SKOV3 cell apoptosis by targeting PARP.
Although conventional chemotherapy is widely used in the treatment of ovarian cancer, anti-ovarian cancer agents are often toxic not only to tumor cells but also to healthy cells.37) Therefore, natural compounds with anti-ovarian cancer properties have attracted considerable interest among researchers owing to their safety and efficacy.38,39) Studies have shown that various ginsenosides, such as 20(S)-protopanaxadiol, Rg3 and Rb1, can play an important role in anti-ovarian cancer by inducing apoptosis, inhibiting proliferation and regulating angiogenesis.6) Our previous findings proved that PDQ could have great prospects towards ovarian cancer treatment. Therefore, in this study, four different carboxylic acid derivatives were synthesized by using PDQ as raw materials. The results of MTT assay showed that the anti-SKOV3 activities of Compound 2 and Compound 3 were significantly greater than those of the other two compounds. Further molecular docking analysis of Compound 3 and PARP (Fig. 7) and the differences in their structural properties (Chart 1) indicated both Compound 2 and Compound 3 had π bonds at the C-3 position and could form π–π with AgG-431, which may be the key factor that allows these two compounds to bind more closely to the target. If the C-3 position of PDQ is linked to butyric acid or pentanoic acid (Compounds 1 and 4), these two compounds are not able to form hydrogen bonds with the receptor, which results in a weak binding and thus being less active. In addition, the steric hindrance of Compound 2 may be the main reason why its activity is weaker than that of Compound 3. Therefore, Compound 3 was selected to further evaluate its possible anti-ovarian cancer mechanism.

Apoptosis is an important mechanism underlying the effect of many anticancer drugs,40) and mitochondrial pathway is one of the main pathways to induce tumor cell apoptosis.41–43) As mentioned above, Compound 3 has a significant inhibitory effect on SKOV3 cell growth (Fig. 2). Annexin V-FITC-PI apoptosis detection confirmed that Compound 3 could induce the apoptosis of SKOV3 cells (Fig. 4). In addition, based on the results of Western blot analysis (Fig. 6), Compound 3 could upregulate the expression levels of cleaved-Caspase-9/Caspase-9, cleaved-Caspase-3/Caspase-3 and Cytochrome C. The level of Cytochrome C was increased after treatment with Compound 3, indicating that Compound 3 can induce mitochondrial membrane permeability and lead to the release of Cytochrome C. Altogether, these findings demonstrate that Compound 3 can promote cell apoptosis.
Mitochondria are the main ROS creators in mammalian cells, and their dysfunction can lead to an increase in ROS levels, which in turn induces cell apoptosis. Therefore, the intracellular levels of ROS were evaluated by DCFH-DA dye and flow cytometry. Notably, the relative fluorescence intensities of ROS in 16, 20, and 24 µM Compound 3-treated cells were 2.85 ± 0.21, 6.25 ± 0.33 and 17.63 ± 0.56%, respectively (Fig. 5), suggesting that Compound 3 can promote the generation of ROS levels in a concentration-dependent fashion. These findings clearly showed that Compound 3 induced ROS production in SKOV3 cells, which transmitted the apoptosis process through mitochondria.
In this study, four novel PDQ fatty acid and aromatic acids derivatives were designed and synthesized. Among them, Compound 3 exhibited the greatest anti-proliferative effect on SKOV3 cells, with an IC50 value of 33.7 ± 2.21 µM. Further morphological analysis revealed that after treatment with Compound 3, the number of SKOV3 cells was reduced, intracellular vacuoles appeared, and a large number of cell fragments were floating on the surface of the culture medium. In addition, FITC-annexin V/PI staining demonstrated that the apoptotic percentages of 16, 20 and 24 µM Compound 3-treated SKOV3 cells were 6.63, 8.41 and 34.27%, respectively. Moreover, Compound 3 could promote SKOV3 cell death by regulating ROS production. Furthermore, Compound 3 upregulated the expression levels of CC-3/C-3, CC-9/C-9 and Cytc, while downregulated the ratio of Bax/Bcl-2. Our findings suggest that Compound 3 induces ovarian carcinoma cell apoptosis via activation of ROS-dependent mitochondrial pathway. Thus, Compound 3 can serve as a lead to develop novel therapeutic agents for treating human ovarian carcinoma.
PDQ was obtained by using (20S)-protopanaxadiol as a raw material through a chiral oxidation ring in our laboratory, and its purity was >95% by HPLC analysis. Other chemical reagents were procured from Macklin Biochemical Technology (Shanghai, China), and were used as supplied. To monitor the reactions, TLC analysis was conducted with pre-coated silica gel HSGF254 plates, and 10% sulfuric acid solution was used as a visualizing reagent. Column chromatography was conducted using a silica gel (200–300 mesh, Qingdao, China). The melting point was determined by YRT-3 melting point meter (Tianjin, China). 1H- and 13C-NMR spectral data were obtained using a Bruker Avance 500-MHz spectrometer (Bruker, Germany), with TMS in pyridine-d5 as an internal standard. High-resolution mass spectrometry detection was conducted on a Triple TOF 5600+ system coupled with an electrospray ionization (ESI) source (AB SCIEX, U.S.A.).
Preparation of Compounds 1–4The carboxylic acid (Compounds 1–4) was synthesized according to the route depicted in Chart 1. PDQ (0.5 g, 1.05 mmol, 1 equivalent (equiv.)) was dissolved in 10 mL dry 1,2-dichloromethane. Subsequently, 1 mL triethylamine and different anhydrides (Compound 1: 6 equiv. butanedioic anhydride, 0.6 g, 6.096 mmol; Compound 2: 6 equiv. phthalic anhydride, 0.9 g, 6.096 mmol; Compound 3: 6 equiv. maleic anhydride, 0.6 g, 6.096 mmol; and Compound 4: 6 equiv. glutaric anhydride, 0.72 g, 6.3 mmol) were mixed and stirred at room temperature for 50 h. After rinsing 3 times with distilled water, the organic phase was dried over Na2SO4 and concentrated in vacuo. The obtained crude products were analyzed by silica gel column chromatography (petroleum ether ethyl acetate solutions in different proportions). Finally, the pure Compounds 1–4 were obtained.
Cell CultureThe human ovarian cancer cell line SKOV3 was supplied by the American Type Culture Collection (VA, U.S.A.). After culturing in RPMI 1640 medium containing 10% fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 mg/mL), the cells were maintained at 37 °C and 5% CO2.
Cytotoxicity AssayThe log-phase SKOV3 cells (5 × 103 cells/mL) were subcultured in 96-well plates for 24 h, and then treated with 10 µL of Compound 3 (0–64 µM) for another 24 h. Cell viability was assessed using the MTT assay.44) The percentage of viable cells was calculated as follows: survival (%) = experimental absorbance/control absorbance × 100%.
Histological StainingThe SKOV3 cells (2 × 105 cells/well) were grown in 6-well plates for 24 h, and then exposed to 16, 20 and 24 µM Compound 3 or 32 µM cis-platinum for another 24 h. Dimethyl sulfoxide (DMSO) (0.1%) was employed as a control group. The cells were fixed in paraformaldehyde (4%) for 4 h, dehydrated through a gradient concentration of alcohol, and then embedded in paraffin. After dewaxing in xylene and rehydrating through graded ethanol series, the sections were rinsed with phosphate-buffered saline (PBS) and subjected to hematoxylin and eosin (H&E) staining. Finally, cell morphology was examined using a phase-contrast microscope (Olympus, Tokyo, Japan).
Assessment of ROS LevelsThe levels of ROS we detected by DCFH-DA assay. Briefly, SKOV3 cells (1 × 106) were exposed to either 16, 20 and 24 µM Compound 3 and cis-platinum (32 µM) or 1 mM H2O2 for 24 h. After harvesting and rinsing with ice-cold PBS, the cells were stained with DCFH-DA (100 µM) at 37 °C for 15 min in the dark. The stained cells were washed and resuspended in 1 mL PBS, and then examined using a FACS Caliber flow cytometer (BD Bioscience, NJ, U.S.A.) at 488/530 nm (excitation/emission). The fluorescent signals were analyzed by Cell Quest software. ROS levels are presented as mean fluorescence intensity.
Apoptosis AnalysisApoptotic cells were quantified by flow cytometry after staining with FITC-Annexin V/PI according to a previous method.45) Briefly, SKOV3 cells (6 × 105) were grown in 25-cm2 flasks for 24 h, and then treated with Compound 3 for another 24 h. Afterwards, the floating and non-floating cells were harvested, rinsed twice with ice-cold PBS, resuspended in 200 mL of 1× binding buffer containing 40 ng/sample of PI and FITC-Annexin V (1 : 50), and incubated at 37 °C for 15 min in the dark. The FACS Caliber flow cytometer was used to quantify the total number of apoptotic cells at 610 nm for PI and 488/525 nm (excitation/emission) for FITC. Data analysis was performed with Cell Quest software (BD Bioscience).
Western Blot DetectionSKOV3 cells (1 × 105 cells/well) were grown in 10-cm culture dishes for 24 h, followed by treatment with 0 (control), 16, 20 and 24 µM Compound 3 for 48 h. Cells lysed in radio immunoprecipitation assay (RIPA) buffer containing phenylmethylsulfonyl fluoride (PMSF) for Western blotting. Protein was separated through 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skimmed milk, the membranes were exposed to the following primary antibodies: Bax (1 : 1000), Bcl-2 (1 : 1000), C-3 (1 : 1000), CC-3 (1 : 1000), C-9 (1 : 1000), CC-9 (1 : 1000), Cytc (1 : 1000) and β-actin (1 : 1000) overnight, and then with secondary antibodies. The protein concentrations of Bax, Bcl-2, C-3, CC-3 C-9, CC-9, and Cytc in SKOV3 cells were determined.
Molecule Docking between Compound 3 and PARPThe binding of Compound 3 to PARP receptor was studied with Schrodinger molecular docking software. The three dimensional (3D) structures of PARP (PDB code: 5DSY46)) and Compound 3 were imported, and the interaction of the butt pose was analyzed by Sybyl 6.91 software package.
Statistical AnalysisThe GraphPad Prism v8.0.4 software was employed to perform statistical tests. Data are representative of 3 independent assays, and values are shown as mean ± standard deviation (S.D.). Statistically significant differences are represented by #, in which # p < 0.05, ## p < 0.01, ### p < 0.001.
This work was supported by the “Thirteenth Five-Year” Science and Technology Development Plan Technology Project of Jilin Provincial Education Department (JJKH20200465KJ), Jilin Science and Technology Innovation Development Plan Project (20190104148), Doctoral Research Start-up Fund of Jilin Medical University (JYBS2018009) and National University Student Innovation and Entrepreneurship Training Program (202013706005).
The authors declare no conflict of interest.
This article contains supplementary materials.