2022 年 70 巻 6 号 p. 443-447
2022 年 70 巻 6 号 p. 443-447
Urea derivatives 1 and 2, synthesized from adenosine, were designed as low-molecular-weight gelators. Hydrophobic groups have been introduced into all or part of the hydroxy groups of the hydrophilic ribose moiety of 1 and 2 to control the solvophilicity of the molecules and their aggregates. Compound 2 selectively formed supramolecular gels in halogenated solvents such as chloroform and 1,2-dichloroethane. The supramolecular gel of 2 and chloroform was thermally stable and its gel-to-sol phase transition temperature was higher than the boiling point of chloroform. The physical properties of the supramolecular gel were investigated by determining its viscoelastic properties using a rheometer. The supramolecular gel realized multiple stimuli-responsive reversible gel–sol phase transitions. The supramolecular gel showed reversible phase transition by repeated warming–cooling cycles accompanying with the gel–sol transitions. The supramolecular gel could undergo five repeated mechano-responsive gel–sol transitions. Gel-to-sol phase transition could also be achieved by adding various anions to the supramolecular gel, such as tetrabutylammonium fluoride. Regelation was realized by adding boron trifluoride etherate to the fluoride ion containing sol. Addition of methanol to the supramolecular gel also induced gel-to-sol phase transition. Regelation was realized by adding molecular sieves 4 Å to the suspension.
Supramolecular gels are formed by self-assembly of small molecules called low-molecular-weight-gelators (LMWGs).1–4) Such gels show flexible and stimuli-responsive characteristics as they contain weak non-covalent interactions. Specific stimulus-responsive supramolecular gel can be developed through a rational design of LMWG.5–8) Stimulus-responsive supramolecular gel can have a wide range of applications such as sensing, orthogonal crystallization, environmental remediation, and drug delivery system (DDS).9–14) In DDS, drugs are first adsorbed inside a supramolecular gel and are then released via degradation of the gel in response to external stimuli.
LMWGs with various structures are being developed from past several decades. Biomolecules such as sugars,15) peptides,16–18) steroids,19,20) and nucleic acids21–23) are some of the most common frameworks of LMWGs. Highly biocompatible supramolecular gels can be prepared using biomolecules such as nucleobases as the framework of LMWG. Nucleobases are nitrogenous heterocyclic compounds and form directionally controlled multiple intermolecular interactions such as in-plane multiple hydrogen-bonding interactions and cofacial π-stacking interactions perpendicular to the plane. These interactions are important in biological systems as they help stabilize the highly ordered structure of nucleic acids. In synthetic systems, these interactions are used not only to develop drugs and sensors but also to fabricate functional supramolecular architectures.21–28)
The number of LMWGs derived from nucleic acids is less than that derived from peptides and sugars. However, we believe that nucleic acid-based LMWGs can be used to obtain highly functional supramolecular gels by utilizing properties such as the formation of complementary base pairs and cofacial π-stacking. To develop LMWGs with the nucleic acid framework, we here design and synthesize urea derivatives 1 and 2 using adenosine as the candidate for LMWG (Fig. 1). Urea derivative 2 functions as a highly selective LMWG for haloalkanes. The resulting supramolecular gel responds to heat, mechanical stress, and two sets of chemical stimuli, and also undergoes reversible gel–sol phase transitions.
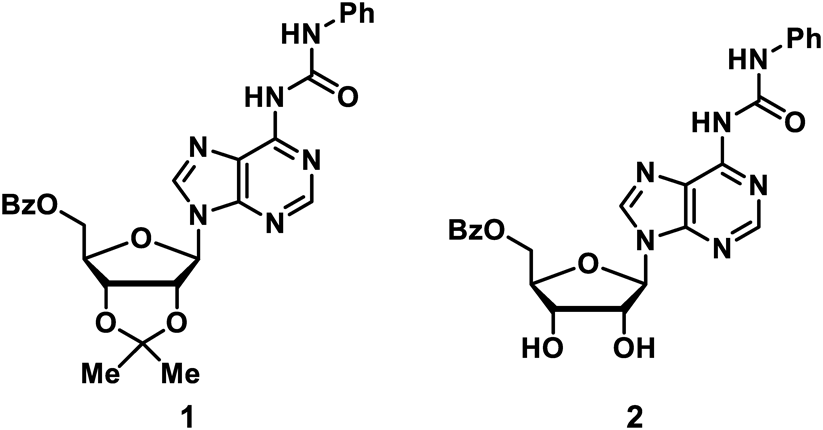
Among various nucleic acid units, we paid particular attention to using adenosine as a basic framework for LMWGs. Fuel-responsive derivatives can be designed from adenosine because phosphorylated adenosine derivatives like ATP can release energy through hydrolysis.29–31) As a result, adenosine can be used to develop functional supramolecular materials (gels) that respond to energy transfer. Urea derivatives 1 and 2 derived from adenosine were designed as LMWG candidates. The ureido group, which has a hydrogen bond acceptor and a hydrogen bond donor, is a functional group widely found in LMWGs.32,33) The primary amino group of adenosine can be converted to the ureido group by reacting with isocyanate. Controlling the solvophilicity of the molecule and their aggregate is important in the molecular design of LMWGs. Hydrophobic groups have been introduced into all or part of the hydroxy groups of the hydrophilic ribose moiety of 1 and 2. It was expected that they would exhibit appropriate solvophilicity as LMWGs.
Urea derivatives 1 and 2 were synthesized by the following procedure (Chart 1). Compound 3, in which hydroxy groups at the 2- and 3-positions of the ribose moiety were acetalized, was obtained by the reaction of adenosine with dimethoxypropane in acetone under acidic conditions.34) Compound 3 was then reacted with benzoic anhydride in the presence of pyridine to obtain compound 4, in which the hydroxy group at the 5-position of the ribose moiety was benzoylated. Urea derivative 1 was synthesized by reacting 4 with phenyl isocyanate, while 2 was obtained by the deacetalization of 1 with an aqueous solution of formic acid. The structures of 1 and 2 were confirmed by 1H- and 13C-NMR spectroscopy and mass spectrometry.

The gelation ability of 1 and 2 was evaluated using water and various organic solvents (Table 1). A mixture of 1 or 2 and solvent in a glass vial was heated until the urea derivative dissolved, whereupon the mixture was cooled slowly to room temperature. The effect of the concentration of the urea derivative was examined up to 50 mM. A sample that was stable upon inverting the test tube was defined as a gel (G). A mixture of a gel and solvent was defined as a partial gel (PG). A sample containing insoluble solids that did not form a gel was defined as a suspension (Sus). A sample that maintained the state of a solution was defined as a solution (S). Urea derivative 1 did not form gel in water or any of the examined nine organic solvents. Mixtures of 1 and water, methanol, or non-polar organic solvents formed suspensions comprising an insoluble precipitate; however, 1 was soluble in other organic solvents. Urea derivative 2 was insoluble in water and hexane and soluble in dimethyl sulfoxide (DMSO). A mixture of 2 and acetone, ethyl acetate, or liquid paraffin dissolved on heating, but became a suspension on cooling to room temperature. Urea derivative 2 selectively formed gels with halogenated solvents. A mixture of 2 and chloroform formed a cloudy supramolecular gel, with the minimum gelation concentration (MGC) of 50 mM (Fig. 2a). A partial gel was obtained from a mixture of 2 (<50 mM) and chloroform. Urea derivative 2 formed a partial gel with dichloromethane even the concentration of 2 was increased to 50 mM. When more than 50 mM of 2 was used with dichloromethane, an insoluble suspension was obtained. Gelation of 1,2-dichloroethane was also realized by 2, with an MGC of 50 mM.
| Solvents | 1 | 2 |
|---|---|---|
| H2O | Sus | Sus |
| DMSO | S | S |
| Methanol | Sus | Sus |
| Acetone | S | Sus |
| Ethyl acetate | Sus | Sus |
| n-Hexane | Sus | Sus |
| Liquid paraffin | Sus | Sus |
| CHCl3 | S | G |
| CH2Cl2 | S | PG |
| 1,2-Dichloroethane | S | G |
a) Each experiment was performed in solvents of concentration 50 mM, G = gel, PG = partial gel, Sus = suspension, S = solution.
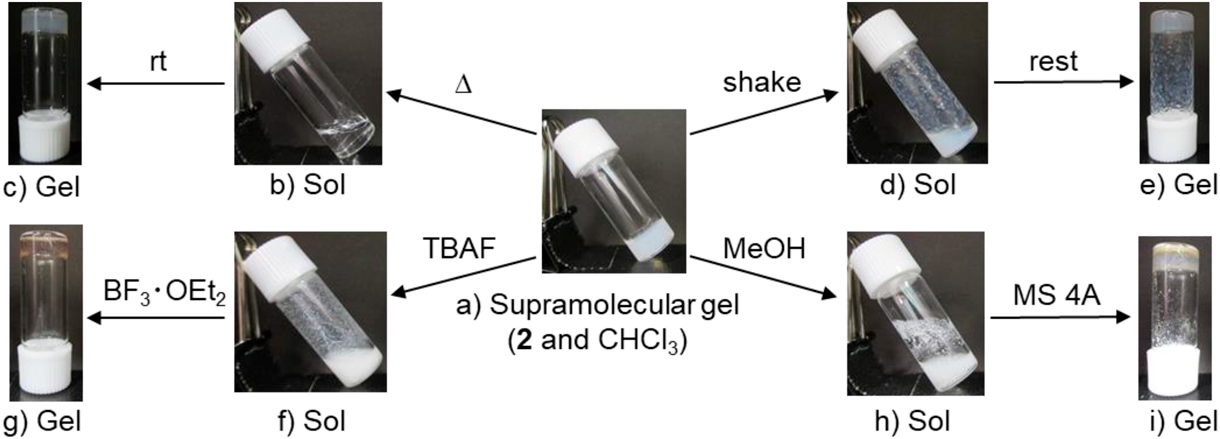
The thermal stability of the supramolecular gel was evaluated by measuring the gel-to-sol phase transition temperature (Tgel), which was determined by the inverse flow method.35) The initial temperature of the oil bath was 25 °C, which was increased at a rate of 0.2 °C/min. The Tgel value of the 50 mM supramolecular gel comprising 2 and chloroform was 64–70 °C. This Tgel was higher than the boiling point of chloroform (61 °C), thereby implying that the supramolecular gel has high thermal stability. The physical properties of the supramolecular gel were investigated by determining its viscoelastic properties using a rheometer.36,37) Both the storage moduli (G′) and loss moduli (G″) were almost independent of the frequency between 0.01 and 15.0 Hz, which is typical of a supramolecular gel (Fig. 3a). The G′ and G″ values of the supramolecular gel comprising 2 and chloroform are 20.3 and 2.7 kPa, respectively, at 0.1 Hz. A strain sweep measurement of this supramolecular gel demonstrated the elastic response typically found in supramolecular gels (Fig. 3b). The linear region was moderate and the crossover point (G′ = G″) showed 12% strain.

A gel-to-sol phase transition in response to external stimuli is characteristic of supramolecular gels containing highly reversible intermolecular interactions. The thermoreversible gel–sol transition is a notable feature of supramolecular gels. The supramolecular gel of 2 showed reversible phase transitions by several repeated warming–cooling cycles accompanying the gel-to-sol and sol-to-gel transition events (Figs. 2b, c). The supramolecular gel of 2 also showed mechano-responsive behavior, i.e., thixotropy.38,39) A low-viscosity liquid was obtained after briefly shaking the supramolecular gel of 2 using a vortex vibrator for several seconds (Fig. 2d). The supramolecular gel was recovered from the mechano-induced liquid state within 5 min (Fig. 2e). The mechano-responsive gel–sol phase transition was repeatable, although it required longer periods to recover the gel state: 30 min for the second time, several hours for the third and fourth times, and a few days for the fifth time. However, regeneration of the supramolecular gel could not be realized in the sixth attempt.
The supramolecular gel changed into insoluble suspensions when various anions were added owing to anion recognition by the ureido moiety of 2.40) Halide ions led to gel-to-sol phase transitions (Fig. 2f). The addition of 0.1, 0.2, 0.5, and 8.0 equivalent (equiv.) of fluoride, chloride, bromide, and iodide ions as tetrabutylammonium (TBA) salts, respectively, was needed for gel-to-sol phase transition of the supramolecular gel of 2. Addition of tetrafluoroborate ions did not allow complete phase transition of the gel, whereas 20 equiv. of tetrafluoroborate ions caused a slight melting of the supramolecular gel. Interaction between the anions and ureido group would prevent the self-assembly of 2. The removal of an anion may lead to the reformation of intermolecular hydrogen-bonded aggregation of 2 for gelation. Boron trifluoride etherate (BF3·OEt2) acted as a fluoride ion-selective regelation reagent (Fig. 2g). In the mixture, BF3·OEt2 and fluoride ions formed tetrafluoroborate ions,41) which have little capability for gel-to-sol phase transition of the supramolecular gel of 2.
A polar organic solvent can also inhibit the intermolecular hydrogen bond of 2 and thus induce a gel-to-sol phase transition of the supramolecular gel. The supramolecular gel of 2 turned into a suspension on adding methanol (Fig. 2h). The phase transition of the supramolecular gel prepared using 300 µL of chloroform required ≥5 µL of methanol. It seems that not only the polarity of the solvent but also the affinity with chloroform is important for inducing phase transition, and the phase transition did not occur even when water was added instead of methanol. The suspension would gel again on removing the methanol. Regelation of the suspension was realized by adsorbing methanol on molecular sieves 4 Å (MS4A). The supramolecular gel was regenerated by adding 50 mg of MS4A to 300 µL of the suspension, allowing it to stand at room temperature for 2 h, and then heating and cooling the mixture (Fig. 2i).
We developed urea derivative 2, which acts as an LMWG for haloalkanes, synthesized from adenosine. The resulting supramolecular gel responded to multiple stimuli and achieved a reversible gel–sol phase transition. The supramolecular gel of 2 exhibited thixotropic property in addition to the thermal responsiveness found in typical supramolecular gels. Since the intermolecular hydrogen bonds of the ureido group are used as the driving force for self-assembly, the gel-to-sol phase transitions occurred when anions and a polar solvent were added. The mixture undergoing a phase transition due to fluoride ions converted to the supramolecular gel when BF3·OEt2 is added, which transforms fluoride ions into tetrafluoroborate ions. The suspension generated by the addition of methanol could be re-gelled by adding MS4A. This study shows that nucleosides such as adenosine are excellent frameworks for developing functional supramolecular gels. Further research is currently underway in our laboratory.
Chemicals and solvents required were obtained from commercial suppliers. 1H- and 13C-NMR spectra were recorded on JEOL JNM-AL 400 and JNM-ECS 400 spectrometers. Mass spectra were measured on JMS-T100LC spectrometer. Rheology measurements were performed by a TA Instruments DHR 2.
Synthesis of Compound 4To a solution of 3 (922 mg, 3.00 mmol) in pyridine (4.0 mL) was added benzoic anhydride (4.07 g, 18.0 mmol, 6.0 equiv.). The reaction mixture was stirred at 60 °C for 16 h. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography (SiO2, hexane/ethyl acetate 11/9–ethyl acetate). The product (1.45 g) was diluted with methanol (40 mL), and heated at 110 °C in a sealed tube and stirred for 22 h. The solvent was removed under reduced pressure. The crude product was purified by column chromatography (SiO2, chloroform/methanol 93/7–23/2) to afford compound 4 (1.06 g, 86%) as a colorless solid, mp 72.9–81.3 °C; 1H-NMR (CDCl3, 400 MHz) δ: 1.43 (3H, s), 1.65 (3H, s), 4.48 (1H, dd, J = 13.1, 6.6 Hz), 4.61–4.66 (2H, overlapped), 5.18 (1H, dd, J = 6.3, 3.1 Hz), 5.59 (1H, dd, J = 6.3, 2.1 Hz), 5.83 (1H, br s), 5.89 (1H, br s), 6.12 (1H, d, J = 2.1 Hz), 7.37 (2H, t, J = 7.5 Hz), 7.54 (1H, m), 7.90–7.93 (3H, overlapped), 8.32 (1H, s); 13C-NMR (CDCl3, 100 MHz) δ: 25.4, 27.2, 64.3, 81.6, 84.2, 85.0, 91.4, 114.7, 120.4, 128.3, 129.3, 129.6, 133.3, 139.6, 149.2, 153.2, 155.5, 166.1; [α]25D −28.0 (c = 0.001 g/mL, chloroform); high resolution (HR) MS-electrospray ionization (ESI) (m/z) [M + Na]+ Calcd for C20H21N5NaO5 434.1440. Found 434.1440.
Synthesis of Compound 1To a solution of 4 (493.7 mg, 1.20 mmol) in CH2Cl2 (30.0 mL) was added phenyl isocyanate (342 µL, 3.00 mmol, 2.5 equiv.). The reaction mixture was stirred at room temperature for 71 h. The mixture was diluted with H2O (40 mL) and then extracted with chloroform (3 × 40 mL). The combined extract was washed with saturated aqueous NaHCO3 solution (60 mL), dried. The solvent was removed under reduced pressure, and the crude product (747 mg) was purified by column chromatography (SiO2, hexane/ethyl acetate 23/27–ethyl acetate) to afford compound 1 (635 mg, quant.) as a colorless solid, mp 95.4–100.9 °C; 1H-NMR (CDCl3, 400 MHz) δ: 1.45 (3H, s), 1.66 (3H, s), 4.48 (1H, dd, J = 10.6, 4.8 Hz), 4.64–4.71 (2H, overlapped), 5.18 (1H, dd, J = 6.2, 3.0 Hz), 5.60 (1H, dd, J = 6.2, 2.6 Hz), 6.19 (1H, d, J = 2.6 Hz), 7.13 (1H, m), 7.31–7.40 (4H, overlapped), 7.49 (1H, m), 7.65 (2H, m), 7.84 (2H, m), 8.27 (1H, br s), 8.56 (1H, s), 8.63 (1H, br s), 11.73 (1H, br s); 13C-NMR (CDCl3, 100 MHz) δ: 25.4, 27.2, 64.3, 81.6, 84.3, 85.2, 91.8, 114.8, 120.4, 121.2, 124.0, 128.3, 129.1, 129.2, 129.5, 133.3, 137.9, 142.3, 149.8, 150.1, 151.0, 151.3, 165.9; [α]25D −7.7 (c = 0.001 g/mL, chloroform); HRMS-ESI (m/z) [M + Na]+ Calcd for C27H26N6NaO6 553.1812. Found 553.1804.
Synthesis of Compound 2A mixture of 1 (265 mg, 0.50 mmol) in 70% aqueous formic acid solution (70%, 10.0 mL) was stirred at room temperature for 23 h. The reaction mixture was concentrated under reduced pressure, and the crude product (249 mg) was purified by column chromatography (SiO2, chloroform/methanol 19/1) to afford compound 2 (211 mg, 86%) as a colorless solid, mp 165.5–175.1 °C (decomp); 1H-NMR (DMSO-d6, 400 MHz) δ: 4.27 (1H, dd, J = 9.5, 4.8 Hz), 4.45–4.51 (2H, overlapped), 4.61 (1H, dd, J = 12.0, 3.6 Hz), 4.80 (1H, br d, J = 4.7 Hz), 5.50 (1H, br s), 5.70 (1H, br s), 6.04 (1H, d, J = 4.7 Hz), 7.08 (1H, m), 7.35 (2H, t, J = 7.4 Hz), 7.51 (2H, t, J = 6.9 Hz), 7.60–7.66 (3H, overlapped), 7.92 (2H, m), 8.62 (1H, s), 8.63 (1H, s), 10.14 (1H, br s), 11.72 (1H, br s); 13C-NMR (DMSO-d6, 100 MHz) δ: 64.2, 70.2, 72.9, 81.7, 88.3, 119.4, 120.7, 123.3, 128.8, 129.0, 129.2, 129.4, 133.5, 138.5, 142.8, 150.0, 150.5, 150.9, 165.6; [α]25D −13.1 (c = 0.001 g/mL, DMSO); HRMS-ESI (m/z) [M + Na]+ Calcd for C24H22N6NaO6 513.1499. Found 513.1482.
Gelation ExperimentA mixture of 1 and buffer in a glass vial bottle was heated on a hot plate (200 °C) until dissolved. Obtained solution was gradually cooled to ambient temperature. Gel formation was evaluated by the inverted tube test. A mixture remaining at the top of an inverted glass vial bottle was defined as a gel.
This work was supported by Grant-in-Aid for the Scientific Research (No. 20K06977 for M. Yo; 17H06374 and 21K064852 for M. Ya) the Japan Society for the Promotion of Science (JSPS) or the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the NOVARTIS Foundation (Japan) for the Promotion of Science (for S.K.)
The authors declare no conflict of interest.