2022 年 70 巻 9 号 p. 662-668
2022 年 70 巻 9 号 p. 662-668
A number of alkaloids found in Mitragyna species belonging to the Rubiaceae family have been shown to have potent biological activity such as analgesic properties. Here, we report the asymmetric total syntheses of mitragynine, speciogynine, and 7-hydroxymitragynine, which are classified as corynantheine-type monoterpenoid indole alkaloids, isolated from Mitragyna speciosa. These syntheses were accomplished within 12 steps and in >11% total yield from commercial 3-(trimethylsilyl)propanal using an organocatalytic anti-selective Michael reaction and bioinspired transformations.
Mitragyna speciosa, which belongs to the Rubiaceae family, is endemic to the tropics of Southeast Asia, where it is called “Kratom” in Thailand and “Biak Biak” in Malaysia.1) Traditionally, the leaf of this plant has been used to alleviate the fatigue of workers laboring in the scorching heat and as a substitute for opium. Many chemists have been fascinated by its special biological activity, and isolation and structure elucidation studies of this plant have been conducted since the 1960 s.2–6) As a result, several corynantheine-type monoterpenoid indole alkaloids have been found with a methoxy group attached to the C-9 position on the indole ring (Fig. 1).

Mitragynine (1) is the major component of a base fraction of Mitragyna speciosa leaves, and speciogynine (2) has been found as the stereoisomer at the C-20 position. Recently, the in vitro and in vivo activity of 2 at serotonin receptors (5-HTRs) was reported; speciogynine (2) exhibited a high affinity for 5-HT1ARs and 5-HT2BRs.7) 7-Hydroxymitragynine (3) was found as a minor component of the leaves; this molecule had a potent analgesic activity via opioid µ receptors (mitragynine (1) also showed mild analgesic activity).6,8–16) In addition, it was discovered that this alkaloid maintains potent activity through oral administration.9) This molecule is now widely recognized as a key component in folk medicine.
Because of their interesting biological activity, several total syntheses of these alkaloids have been reported.10,17–22) Very recently, our group also achieved the total syntheses of the same class of corynantheine-type monoterpenoid indole alkaloids, corynantheidine and dihydrocorynantheine.23) However, the efficiency of these total syntheses could be improved, specifically because the goal of previous total syntheses was to reproduce the biosynthetic transformations from strictosidine in the flask.
Here, we report the practical asymmetric total syntheses of mitragynine (1), speciogynine (2), and 7-hydroxymitragynine (3). These syntheses were achieved within 12 steps from commercially available materials with total yields of >11%.
Our retrosynthetic analysis of (−)-mitragynine (1) and (+)-speciogynine (2) is outlined in Chart 1. We envisioned that our previously developed bioinspired transformations toward the syntheses of corynantheine-type alkaloids could also reveal the syntheses of (−)-1 and its C-20 epimer (+)-2. Thus, (−)-1 and (+)-2 were retrosynthesized to 9-methoxystrictosidine derivative 5, via bioinspired skeletal transformations and stereoselective reduction of the common intermediate 4. Preparation of key intermediate 5 was expected to be achieved by diastereoselective Pictet–Spengler cyclization using (R)-4-methoxy-α-cyanotryptamine (6) and secologanin derivative 7.24) Tryptamine derivative 6 was considered for the installation of a 4-methoxyindole unit to the known methyl (R)-N-benzyloxycarbonyl (Cbz)-azirizine-2-carboxylate (8).25) For the concise synthesis of secologanin derivative 7, our discovered anti-selective organocatalytic Michael reaction was selected as a key step to install all carbons of 7.26) Thus, butyraldehyde (9) and conjugated ene-yne compound 10, prepared from commercially available 3-(trimethylsilyl)propanal (11), were assumed as optimal building blocks.
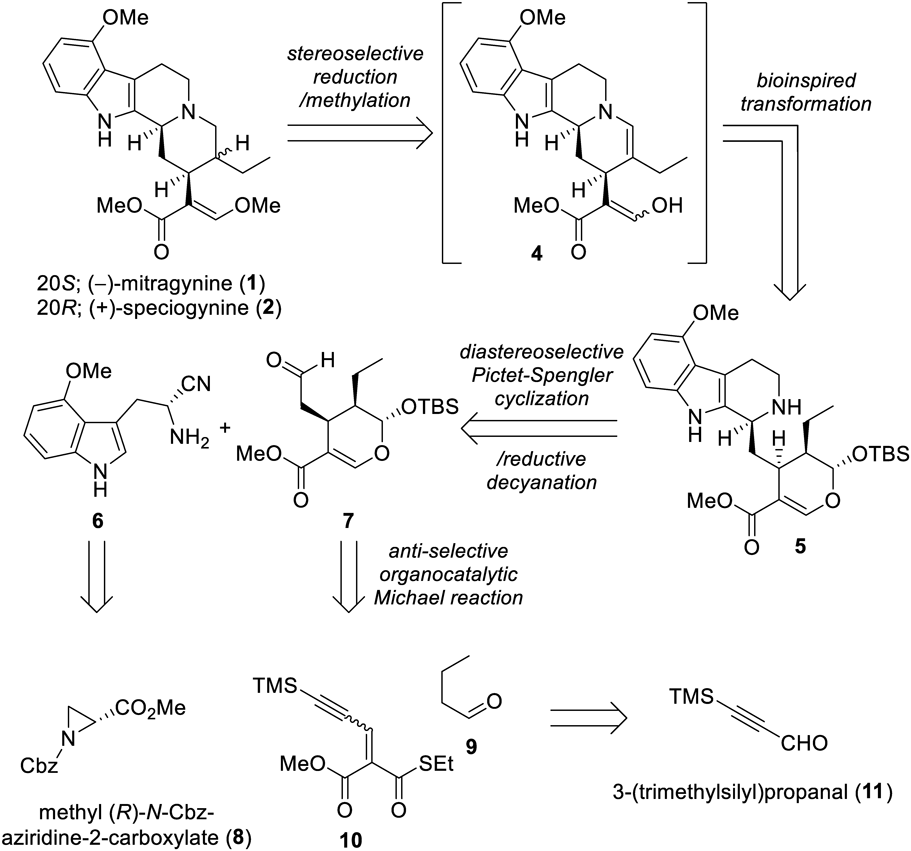
As shown in Chart 2, our synthesis commenced with the preparation of (R)-4-methoxy-α-cyanotryptamine (6). Synthesis of D-tryptophan derivative 13 was performed via a Lewis acid-mediated coupling reaction of 4-methoxyindole (12) with known chiral aziridine 8.27) After several trials, Yb(OTf)3 effectively promoted the coupling reaction and the desired 13 was obtained as a major product (1.5 equivalent (equiv.) of Yb(OTf)3; room temperature (r.t.). Hydrolysis and subsequent amidation of the crude mixture of 13 gave primary amide 14 in gram quantities (39% yield over three steps). Then, amide 14 was converted to 6 via dehydration to construct a cyano group and deprotection of the Cbz group (74% yield over two steps).
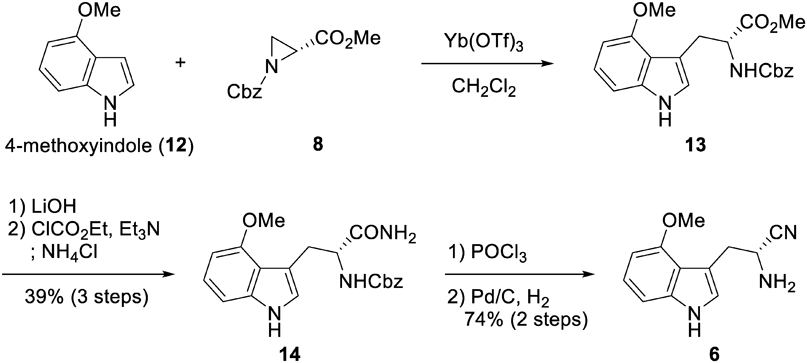
As in our previous synthesis of monoterpenoid indole alkaloids, the preparation of secologanin derivative 7 commenced with the Knoevenagel condensation with aldehyde 11 and methyl 3-ethylthio-3-oxopropanoate (15)28) in the presence of trifluoroacetic acid (TFA), affording ene-yne compound 10 (81%, E:Z = 1 : 1) (Chart 3). To install the C-15 chiral center and C-20 ethyl group in (−)-1 and (+)-2, an anti-selective organocatalytic Michael reaction to form 16 was set up. Thus, when ene-yne compound 10 and butyraldehyde (9) were treated with 3 mol% diphenylprolinol trimethylsilyl ether catalyst in Et2O at 0 °C (40 h), the anti-adduct 16 was obtained with excellent diastereoselectivity (anti:syn = 11 : 1). Subsequent Fukuyama reduction29) to convert aldehyde from a thioester of 16 and spontaneous cyclization were carried out, affording optically active dihydropyran derivative 17 in 85% yield over two steps (4.5 equiv. Et3SiH, 15 mol% of Pd/C, r.t.). Subsequent silylation of the hemiacetal hydroxy group in 17 was performed. Thus, when 17 was treated with AgNO3 and tert-butyldimethylchlorosilane (TBSCl) in dry N,N-dimethylformamide (DMF), stereoselective silylation proceeded from the sterically less hindered site to afford α-oriented silyl ether 18 as a single isomer (87%). Enantiomeric excess was determined at this stage: >99% enantiomeric excess (ee) by chiral HPLC analysis. Finally, alkyne 18 was treated with hydroboration–oxidation conditions to afford secologanin derivative 7 in excellent yield (84%, 1.2 equiv. 9-borabicyclo[3.3.1]nonane (9-BBN), tetrahydrofuran (THF), r.t. then 5.0 equiv. H2O2, 1.0 equiv. NaOH, r.t.). All protocols in the synthesis of 7 were performed in gram-scale quantities.
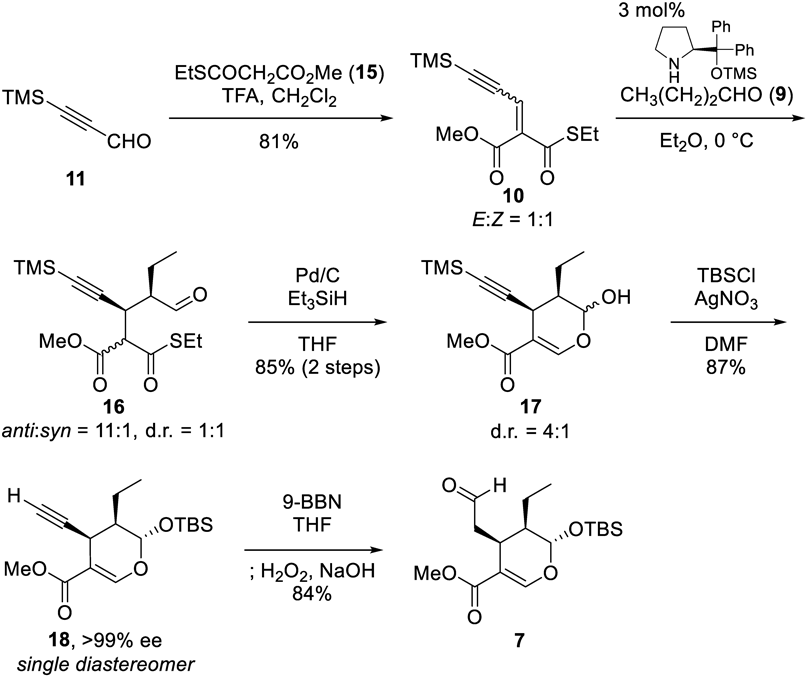
With the desired synthons 6 and 7 in hand, we next attempted bioinspired transformations for the construction of a corynantheine-type scaffold (Chart 4). To prepare 9-methoxystrictosidine derivative 5, our developed diastereoselective Pictet–Spengler cyclization/reductive decyanation sequences were examined. When a mixture of 6 and 7 was treated with 50 mol% TFA in CH2Cl2 at 0 °C (30 min), the desired 3S-isomer 19 was obtained as a single diastereomer in quantitative yield.30) Reductive removal of the cyano group in 19 was performed by treatment with NaBH3CN in the presence of AcOH in MeOH, affording 9-methoxystrictosidine derivative 5 in excellent yield (78%).
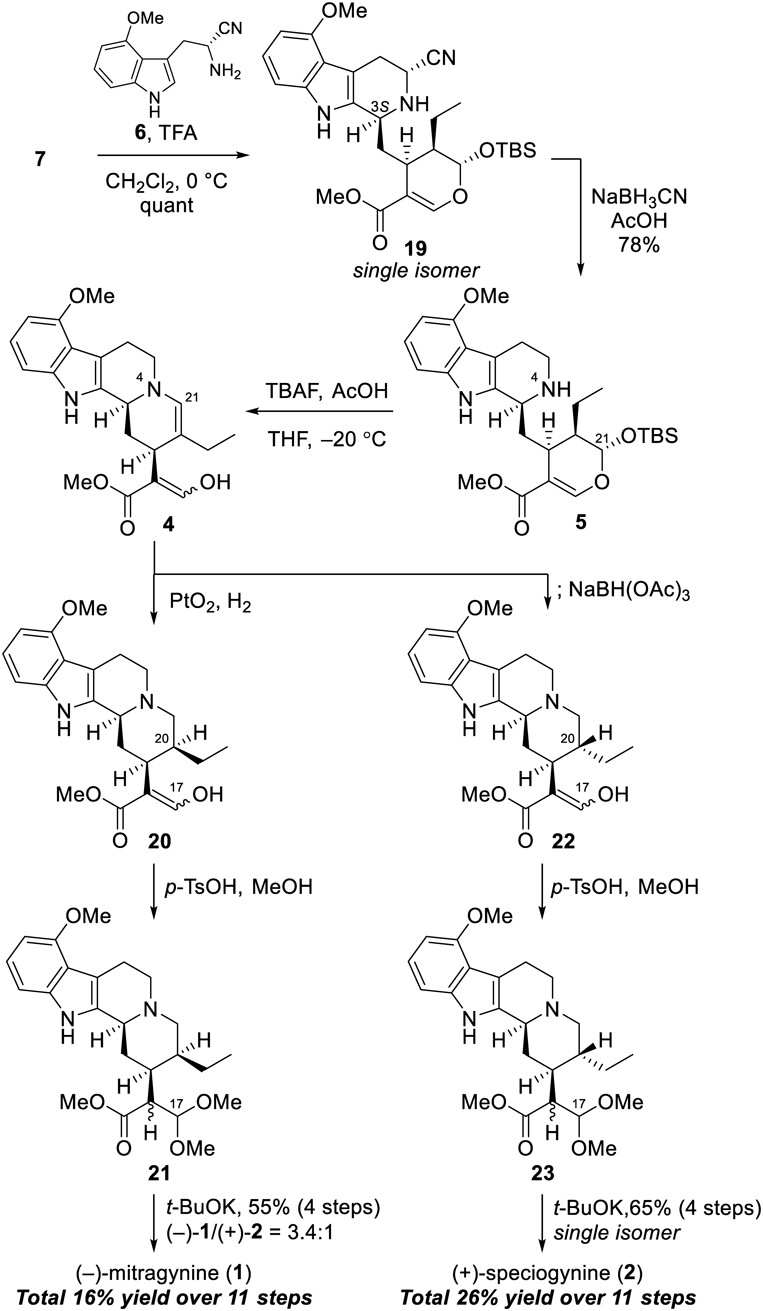
Next, key bioinspired transformations triggered by removal of the silyl group in 5 were examined. Thus, 5 was exposed to a simple desilylation condition (2.0 equiv. tetrabutylammonium fluoride (TBAF), 2.0 equiv. AcOH, THF, –20 °C) to afford enamine 4 linked by N-4 and C-21. After confirming a full conversion to 4 from 5 by NMR spectroscopic analysis, the crude mixture of 4 was directly treated with 100 wt% PtO2 under H2 atmosphere.17) As a result, kinetically reduced product 20, which has the same C-20S stereochemistry as (−)-mitragynine (1), was preferentially obtained. To complete the total synthesis of (−)-1, modification of the enol hydroxy group at the C-17 position was performed. Thus, the crude mixture of 20 was treated with p-TsOH in MeOH to afford dimethoxy acetal 21.31) Finally, stereoselective E1cB elimination of the C-17 methoxy group of 21, using t-BuOK in DMF,17) afforded a separable mixture of (−)-mitragynine (1) and (+)-speciogynine (2) [(−)-1/(+)-2 = 3.4 : 1, 55% yield over four steps].
Bioinspired transformations toward the selective synthesis of C-20R isomer (+)-speciogynine (2) were also demonstrated. After generating enamine 4 under the same conditions as for the synthesis of (−)-1, 2.0 equiv. NaBH(OAc)3 was added in a one-pot protocol. In this case, thermodynamic controlled protonation at the C-20 position followed by reduction of a generated iminium ion afforded C-20R isomer 22 as a single diastereomer. Subsequent modification of the C-17 enol hydroxy group was carried out as in the synthesis of (−)-1, and the E1cB elimination reaction, following the formation of 23, afforded (+)-speciogynine (2) in 65% overall yield within four steps from 5.
In summary, practical total syntheses of (−)-mitragynine (1, total 16% over 11 steps from 11) and (+)-speciogynine (2, total 26% over 11 steps from 11) were accomplished.
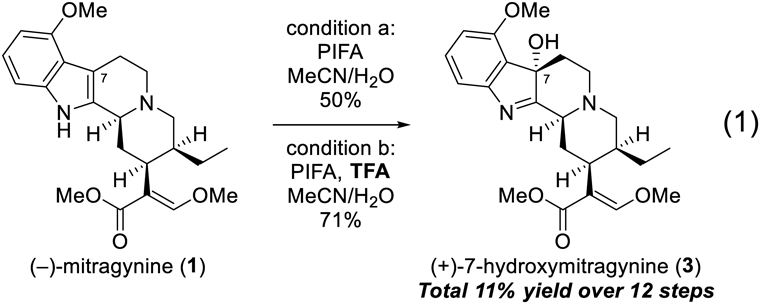
Next, to prepare (+)-7-hydroxymitragynine (3), the direct C-7 oxidation of (−)-1 was investigated (Eq. 1). Following our previously reported method, when freshly purified (−)-1 was oxidized in aqueous acetonitrile by treatment with 1.0 equiv. [bis(trifluroacetoxy)iodo]benzene (PIFA), (+)-3 was obtained in moderate yield (condition a in Eq. 1, 50%).32) After several trials toward further improvement of the chemical yield, it was determined that the addition of 2.0 equiv. TFA led to the effective production of (+)-3 (condition b in Eq. 1, 71%). We suppose that TFA masked the reactive lone pair of the N-4 nitrogen in (−)-1 as a salt, thereby suppressing undesired N-4 oxidation. As a result, the total synthesis of (+)-7-hydroxymitragynine (3) was accomplished with 11% overall yield within 12 steps from 11.
In conclusion, we accomplished the total syntheses of three bioactive Mitragyna alkaloids: (−)-mitragynine (1), (+)-speciogynine (2), and (+)-7-hydroxymitragynine (3). The synthesis of secologanin derivative 7, a key fragment in this synthesis, was carried out via an anti-selective organocatalytic Michael reaction using ene-yne compound 10 and simple aldehyde 9, with excellent enantiomeric excess. Preparation of common intermediate 4 was achieved via bioinspired transformations of strictosidine derivative 5 triggered by the removal of a silyl group, which branched into (−)-1 and (+)-2 by stereoselective reduction. In addition, (+)-3 was obtained through direct oxidation of (−)-1 via a nitrogen-masking strategy. As a result, syntheses of (−)-mitragynine (1), (+)-speciogynine (2), and (+)-7-hydroxymitragynine (3) were realized within fewer than 12 steps and in >11% overall yield. Using the synthesized natural products and their derivatives, a study of their structure–activity relationships is currently under way in our laboratory.
All reactions were monitored by TLC using Merck 60 F254 precoated silica gel plates (0.25 mm thick) and Fuji Silysia Chemical precoated amino-silica gel plates (0.25 mm thick). UV spectra were recorded in MeOH on a JASCO V-560 instrument. Specific optical rotations were measured using a JASCO P-2200 polarimeter. Circular dichroism spectra were recorded on a JASCO J-1100 spectrometer. Fourier transform (FT) IR spectra were recorded on a JASCO FT/IR-4700. 1H- and 13C-NMR spectra were recorded on a JEOL ECZ 400 (400 MHz 1H-NMR, 100 MHz for 13C-NMR), ECS 400 (400 MHz 1H-NMR, 100 MHz for 13C-NMR), JEOL ECX 500 (500 MHz 1H-NMR, 125 MHz for 13C-NMR), and ECZ 600 (600 MHz for 1H-NMR, 150 MHz for 13C-NMR) FT-NMR spectrometer instrument. Data for 1H-NMR are reported as chemical shifts (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, dd = double doublet, ddd = double double doublet, dt = double triplet, q = quartet, m = multiplet, br = broad), coupling constant (Hz), integration, and assignment. Data for 13C-NMR are reported as chemical shifts. The high-resolution mass spectra were recorded on a JEOL AccuTOF LC-plus JMS-T100LP. Flash chromatography was performed using Kanto Chemical silica gel 60N and Fuji Silysia Chemical amino-silica gel (SiO2-NH, NH-DM2035). HPLC analysis was performed on a JASCO MD-4017; UV detection was monitored at an appropriate wavelength using Daicel Chiralcel OD-H (0.46 × 25 cm).
Preparation of Compound 14To a solution of known aziridine 8 (1.0 g, 4.25 mmol) and 4-methoxyindole (12, 1.25 g, 8.50 mmol) in CH2Cl2 (17 mL) was added Yb(OTf)3 (2.64 g, 4.25 mmol) under Ar atmosphere. The reaction mixture was stirred for 24 h at r.t. under Ar atmosphere in the dark. To the resulting mixture was added additional Yb(OTf)3 (1.32 g, 2.13 mmol), followed by stirring for a further 48 h at r.t. The mixture was then quenched with saturated aqueous NaHCO3 and the resulting mixture was filtered through a Celite pad, eluted with CHCl3. The filtrate was extracted three times with CHCl3. The combined organic layer was dried over Na2SO4 and then concentrated under reduced pressure. Flash chromatography (SiO2, 22–28% AcOEt/n-hexane gradient) provided an inseparable regioisomer mixture of 13 (780 mg, r.r. = 6.7 : 1). To a solution of a mixture of 13 in aqueous THF (34 mL, THF/H2O = 10 : 1) was added 2 M aqueous LiOH (3.06 mL, 6.12 mmol) at r.t. under Ar atmosphere. The reaction mixture was stirred for 2.5 h at r.t. before quenching with 2 M aqueous HCl (pH 2). Brine was added to the resulting mixture and the aqueous layer was extracted three times with AcOEt. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. Obtained crude materials were dissolved in anhydrous THF (40.8 mL), then ethyl chloroformate (272 µL, 2.86 mmol) and triethylamine (853 µL, 6.12 mmol) were added at 0 °C under Ar atmosphere. The reaction mixture was stirred for 30 min at 0 °C. To the resulting mixture was added 1.0 M aqueous NH4Cl (3.06 mL, 3.06 mmol) at 0 °C. The reaction mixture was stirred for an additional 20 min at 0 °C. It was then quenched with saturated aqueous NaHCO3 and the aqueous layer was extracted three times with AcOEt. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. Flash chromatography (SiO2, 2%–5% MeOH/CHCl3 gradient) afforded amide 14 (607.5 mg, 39% over three steps).
Compound 14: White amorphous powder; [α]23D +2.6 (c 0.31, MeOH); IR (ATR) νmax cm−1 3448, 3338, 2945, 2480, 1674, 1620, 1589, 1525, 1502, 1423, 1356, 1313, 1257, 1153, 1095, 1039, 966, 910, 860, 775, 752, 735, 719, 694, 617; high resolution (HR)MS (electrospray ionization (ESI)) [M + Na]+ Calcd. for [C20H21N3Na1O4]+: 390.1430, Found: 390.1435; UV (MeOH) λmax 291, 281, 265, 220 nm; 1H-NMR (400 MHz, (CD3)2SO) δ: 10.8 (br s, 1H), 7.34–7.19 (m, 5H), 7.03 (br s, 1H), 6.96–6.92 (m, 3H), 6.44 (d, J = 6.4 Hz, 1H), 4.95 (s, 2H), 4.23 (dt, J = 8.8, 4.4 Hz, 1H), 3.84 (s, 3H), 3.28 (dt, J = 14.4, 3.6 Hz, 1H), 2.98 (ddd, J = 14.4, 10.0, 3.2 Hz, 1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 174.2, 155.8, 154.1, 137.8, 137.1, 128.4, 127.7, 127.4, 122.2, 121.8, 117.0, 110.6, 105.0, 98.9, 65.2, 56.2, 55.1, 39.9, 39.8, 39.7, 39.5, 39.4, 39.2, 39.1, 29.3.
Preparation of (R)-4-Methoxy-α-cyanotryptamine (6)To a solution of amide 14 (608 mg, 1.65 mmol) in anhydrous pyridine (11 mL) was added phosphoryl chloride (355 µL, 3.80 mmol) at 0 °C under Ar atmosphere. The reaction mixture was stirred for 50 min at 0 °C. MeOH was added at 0 °C to the resulting mixture and stirred for 10 min, followed by concentration under reduced pressure. The resulting mixture was dissolved in Et2O, and the organic layer was washed with 1 M aqueous NaOH three times, and with brine, dried over Na2SO4, and then concentrated under reduced pressure. Flash chromatography (SiO2, 1% MeOH/CHCl3) gave a dehydrated compound (510 mg, 88%). To a solution of the dehydrated compound (500 mg, 1.43 mmol) in degassed 1,4-dioxane (10.4 mL) was added 10% Pd/C (152 mg, 0.143 mmol) at r.t. under Ar atmosphere. The resulting mixture was purged with a stream of hydrogen and the reaction mixture was stirred for 13 h at r.t. under H2 atmosphere. The resulting mixture was filtered with a Celite pad with CHCl3. The filtrate was concentrated under reduced pressure and the resulting residue was purified by flash chromatography (SiO2, 60% AcOEt/n-hexane, then 5% MeOH/CHCl3) to afford (R)-4-methoxy-α-cyanotryptamine (6, 260.0 mg, 84%).
(R)-4-Methoxy-α-cyanotryptamine (6): Pale pink amorphous powder; [α]23D –15.0 (c 1.96, CHCl3); IR (ATR) νmax cm−1 3363, 3194, 2914, 1741, 1587, 1547, 1510, 1431, 1356, 1257, 1167, 1132, 1086, 1053, 955, 895, 852, 814, 683, 623; HRMS (ESI) [M–CN]+ Calcd. for [C11H13N2O1]+: 189.1028, Found: 189.1025; UV (MeOH) λmax 290, 281, 267, 220 nm; 1H-NMR (600 MHz, CDCl3) δ: 8.22 (br s, 1H), 7.11 (t, J = 7.8 Hz, 1H), 7.00 (d, J = 2.4 Hz, 1H), 6.98 (d, J = 7.8 Hz, 1H), 6.52 (d, J = 7.8 Hz, 1H), 4.18 (t, J = 7.2 Hz, 1H), 3.93 (s, 3H), 3.33 (dd, J = 13.8, 7.2 Hz, 1H), 3.27 (dd, J = 13.8, 7.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ: 154.4, 138.2, 123.3, 122.6, 122.5, 117.0, 110.0, 104.9, 99.8, 55.3, 45.4, 33.6.
Preparation of Compound 17To the solution of an E and Z mixture of half thioester malonate derivative 10 (500 mg, 1.85 mmol) and diphenylprolinol trimethyl silyl ether (18.0 mg, 0.0555 mmol) in Et2O (9.2 mL) was added n-butyraldehyde (9, 167 µL, 1.85 mmol) at 0 °C under Ar atmosphere. The reaction mixture was stirred for 40 h at 0 °C. The resulting mixture was concentrated under reduced pressure. Crude 1H-NMR revealed that anti-adduct 16 was the main product (diastereomer ratio of 16; anti:syn = 11 : 1). The crude materials of 16 were solved to degassed THF (3.7 mL), then 10% Pd/C (197 mg, 0.185 mmol) and Et3SiH (886 µL, 5.55 mmol) were added at 0 °C under Ar atmosphere. The reaction mixture was stirred for 4.5 h at r.t, whereafter an additional 10% Pd/C (98.4 mg, 0.0924 mmol) and Et3SiH (443 µL, 2.77 mmol) were added at r.t. under Ar atmosphere. This was stirred for 5 h at r.t. before the addition of 1 M aqueous HCl (3.7 mL). The resulting mixture was stirred for a further 18 h at r.t. before filtration with a Celite pad eluted with AcOEt, and partitioned between an AcOEt layer and an aqueous layer. The aqueous layer was extracted three times with AcOEt. The combined organic layer was dried over MgSO4, and concentrated under reduced pressure. Flash chromatography (SiO2, 5%–20% AcOEt/n-hexane gradient) afforded two diastereomers of dihydropyran 17 (444.5 mg, 85% over two steps, d.r. = 4 : 1).
Compound 17: Yellow waxy solid; IR (ATR) νmax cm−1 3370, 2953, 1672, 1629, 1444, 1383, 1311, 1247, 1204, 1148, 1125, 1100, 1030, 1015, 981, 945, 908, 879, 835; HRMS (ESI) [M + Na]+ Calcd. for [C14H22Na1O4Si1]+: 305.1185, Found: 305.1145; 1H-NMR (500 MHz, CDCl3) δ: 7.53 (s, 1H), 7.46 (s, 1H), 5.36 (dd, J = 12.0, 1.5 Hz, 1H), 5.22 (t, J = 7.5 Hz, 1H), 4.85 (d, J = 7.5 Hz, 1H), 3.77 (s, 3H), 3.75 (s, 3H), 3.58 (d, J = 5.0 Hz, 1H), 3.55 (d, J = 5.0 Hz, 1H), 3.34 (d, J = 7.5 Hz, 1H), 1.85–1.71 (m, 3H), 1.64–1.53 (m, 3H), 1.05 (t, J = 7.0 Hz, 3H), 1.02 (t, J = 7.0 Hz, 3H), 0.14 (s, 9H), 0.12 (s, 9H); 13C-NMR (125 MHz, CDCl3) δ: 166.9, 166.7, 153.3, 152.5, 107.2, 106.3, 104.0, 103.7, 97.1, 96.3, 90.1, 97.9, 51.7, 51.6, 42.2, 39.9, 26.8, 23.6, 21.6, 20.4, 11.4, 11.0, 0.17 (3C), 0.12 (3C).
Preparation of Compound 18To a solution of two diastereomers of dihydropyran 17 (1.0 g, 3.54 mmol) in dry DMF (11.8 mL) were added AgNO3 (782 mg, 4.60 mmol) and TBSCl (800.5 mg, 5.31 mmol) at 0 °C under Ar atmosphere. The reaction mixture was stirred for 1 h at r.t. under Ar atmosphere. Additional AgNO3 (391 mg, 2.30 mmol) and TBSCl (400 mg, 2.66 mmol) were then added under Ar atmosphere. The reaction mixture was stirred for 2.5 h at r.t. Additional AgNO3 (391 mg, 2.30 mmol) was then added under Ar atmosphere and the mixture was stirred for 15 min at r.t. The resulting mixture was directly filtered through a short plug of silica gel eluted with 20% AcOEt/n-hexane and the filtrate was added to saturated NaHCO3. The aqueous layer was extracted three times with AcOEt. The combined organic layer was washed four times each with water and brine, dried over MgSO4, and concentrated under reduced pressure. Flash chromatography (SiO2, 1% AcOEt/n-hexane) afforded 18 (1.0 g, 87%). Enantiomeric excess was determined to be over 99% by HPLC (Chiralcel OD-H column) (see Supporting Information). Results for compound 18: 1.5% i-PrOH/n-hexane, 0.500 mL/min; major enantiomer tR = 7.27 min, minor enantiomer tR = 8.30 min.
Compound 18: Colorless oil; [α]22D –235.6 (c 0.71, CHCl3); IR (ATR) νmax cm−1 3315, 3269, 2954, 2931, 2883, 2858, 1714, 1639, 1462, 1437, 1392, 1298, 1254, 1228, 1167, 1130, 1088, 1001, 949, 924, 879, 837, 781, 764, 679, 663, 638, 617; HRMS (ESI) [M + Na]+ Calcd. for [C17H28Na1O4Si1]+: 347.1655, Found: 347.1669; 1H-NMR (400 MHz, CDCl3) δ: 7.48 (s, 1H), 5.18 (d, J = 8.4 Hz, 1H), 3.75 (s, 3H), 3.56 (dd, J = 5.2, 2.4 Hz, 1H), 2.15 (d, J = 2.4 Hz, 1H), 1.78 (m, 1H), 1.59 (m, 1H), 1.47 (m, 1H), 1.00 (t, J = 7.2 Hz, 3H), 0.93 (s, 9H), 0.15 (s, 3H), 0.15 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 167.1, 153.9, 106.6, 97.6, 82.4, 71.4, 51.6, 42.5, 25.8, 20.3, 18.1, 11.1, –4.1, –5.1.
Preparation of Secologanin Derivative 7To a solution of alkyne 18 (1.0 g, 3.1 mmol) in anhydrous THF (10.3 mL) was added 9-BBN (1.0 M in THF solution, 7.4 mL, 3.70 mmol) under Ar atmosphere. The reaction mixture was stirred for 2.5 h at r.t. The resulting mixture was quenched with H2O (1.0 mL) and then stirred for an additional 10 min. To the resulting mixture were added 30% aqueous H2O2 (1.57 mL, 15.4 mmol) and 1.0 M aqueous NaOH (3.08 mL, 3.08 mmol) at 0 °C. This reaction mixture was stirred for 80 min at r.t. under Ar atmosphere. After the addition of saturated aqueous NH4Cl, the aqueous layer was extracted three times with AcOEt. The combined organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure. Flash chromatography, repeated three times (SiO2, 5%–50% AcOEt/n-hexane gradient then 10% MeOH/CHCl3) afforded secologanin derivative 7 (887 mg, 84%).
Secologanin Derivative 7: Colorless oil; [α]23D –141.1 (c 1.89, CHCl3); IR (ATR) νmax cm−1 2954, 2931, 2887, 2858, 1703, 1633, 1466, 1439, 1385, 1362, 1309, 1281, 1254, 1167, 1138, 1101, 1080, 1007, 918, 874, 837, 781, 675, 640; HRMS (ESI) [M + Na]+ Calcd. for [C17H30Na1O5Si1]+: 365.1760, Found: 365.1769; 1H-NMR (400 MHz, CDCl3) δ: 9.76 (dd, J = 3.2, 2.0 Hz, 1H), 7.49 (s, 1H), 5.06 (d, J = 7.6 Hz, 1H), 3.69 (s, 3H), 3.30 (dt, J = 7.6, 5.2 Hz, 1H), 2.48 (ddd, J = 15.2, 5.2, 2.0 Hz, 1H), 2.35 (ddd, J = 15.2, 7.6, 3.2 Hz, 1H), 1.74–1.59 (m, 2H), 1.15 (m, 1H), 0.98 (t, J = 7.2 Hz, 3H), 0.91 (s, 9H), 0.14 (s, 3H), 0.13 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ: 201.6, 167.7, 154.0, 108.5, 96.4, 51.4, 45.1, 43.6, 26.8, 25.8, 19.3, 18.1, 11.5, –4.2, –5.1.
Preparation of Compound 19To a solution of secologanin derivative 7 (320 mg, 0.934 mmol), (R)-4-methoxy-α-cyanotryptamine (6, 201.1 mg, 0.934 mmol), and powdered MS 4 Å (1 g) in CH2Cl2 (18.7 mL) was added TFA (1.0 M in CH2Cl2, 467 µL, 0.467 mmol) at 0 °C under Ar atmosphere. The reaction mixture was stirred for 30 min at 0 °C. The resulting mixture was filtered through a cotton plug with CHCl3 and the filtrate was added to an excess amount of saturated aqueous NaHCO3 solution at 0 °C. The aqueous layer was extracted three times with CHCl3. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. Flash chromatography (SiO2, 15% AcOEt/n-hexane) afforded 19 (504.3 mg, quantitative yield) as a single diastereomer.
Compound 19: White amorphous powder; [α]23D –230.1 (c 0.85, CHCl3); IR (ATR) νmax cm−1 3328, 2928, 1688, 1630, 1572, 1509, 1463, 1437, 1384, 1357, 1312, 1279, 1254, 1207, 1167, 1137, 1102, 1029, 1018, 974, 938, 892, 871, 837; HRMS (ESI) [M + H]+ Calcd. for [C29H42N3O5Si1]+: 540.2894, Found: 540.2859; UV (MeOH) λmax 292, 225 nm; 1H-NMR (600 MHz, CDCl3) δ: 7.82 (br s, 1H), 7.59 (s, 1H), 7.04 (t, J = 7.8 Hz, 1H), 6.91 (d, J = 7.8 Hz, 1H), 6.47 (d, J = 7.8 Hz, 1H), 5.26 (d, J = 9.0 Hz, 1H), 4.37 (dd, J = 4.2, 3.6 Hz, 1H), 4.07 (ddd, J = 10.8, 3.6, 1.8 Hz, 1H), 3.88 (s, 3H), 3.79 (s, 3H), 3.35–3.29 (m, 2H), 2.97 (dt, J = 12.0, 4.2 Hz, 1H), 1.79 (ddd, J = 12.6, 11.4, 3.0 Hz, 1H), 1.73–1.63 (m, 2H), 1.55 (td, J = 12.6, 2.4 Hz, 1H), 1.18 (m, 1H), 0.98 (t, J = 7.2 Hz, 3H), 0.93 (s, 9H), 0.19 (s, 3H), 0.18 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ: 169.3, 154.6, 154.4, 137.4, 132.4, 122.8, 120.0, 117.5, 109.0, 105.6, 104.3, 100.0, 97.2, 55.3, 52.2, 46.8, 44.4, 43.8, 36.9, 27.8, 26.9, 25.8, 20.2, 18.1, 11.1, –3.9, –4.9.
Preparation of 9-Methoxystrictosidine Derivative 5Acetic acid (265 µL, 4.63 mmol) and sodium cyanoborohydride (582 mg, 9.26 mmol) were added to a solution of 19 (500 mg, 0.936 mmol) in MeOH (4.6 mL) at r.t. under Ar atmosphere. The reaction mixture was stirred for 24 h at r.t. To the resulting mixture were added additional acetic acid (265 µL, 4.63 mmol) and sodium cyanoborohydride (582 mg, 9.26 mmol) under Ar atmosphere. The reaction mixture was stirred for a further 36 h at r.t. The resulting mixture was quenched with saturated aqueous NaHCO3 solution at 0 °C. The aqueous layer was extracted three times with AcOEt. The combined organic layer was washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The crude materials were filtered through a short plug of amino-silica gel (SiO2-NH), eluted with 5% MeOH/CHCl3, to remove an excess amount of sodium cyanoborohydride. Flash chromatography (SiO2, 25% AcOEt/n-hexane then 20% MeOH/CHCl3) afforded the 9-methoxystrictosidine derivative 5 (371.6 mg, 78%) with 80.5 mg of starting material 19.
9-Methoxystrictosidine Derivative 5; White amorphous powder; [α]23D –189.9 (c 0.93, CHCl3); IR (ATR) νmax cm−1 2933, 2850, 1698, 1630, 1510, 1460, 1436, 1383, 1357, 1308, 1276, 1252, 1166, 1137, 1096, 1031, 1007, 975, 950, 927, 908, 872, 861, 836, 825; HRMS (ESI) [M + H]+ Calcd. for [C28H43N2O5Si1]+: 515.2941, Found: 515.2955; UV (MeOH) λmax 292, 223 nm; 1H-NMR (600 MHz, CDCl3) δ: 8.33 (br s, 1H), 7.55 (s, 1H), 7.02 (t, J = 7.8 Hz, 1H), 6.94 (d, J = 7.8 Hz, 1H), 6.46 (d, J = 7.8 Hz, 1H), 5.23 (d, J = 8.4 Hz, 1H), 4.01 (dd, J = 9.0, 3.0 Hz, 1H), 3.88 (s, 3H), 3.74 (s, 3H), 3.29 (m, 1H), 3.05–2.95 (m, 4H), 1.92 (ddd, J = 13.8, 10.8, 3.6 Hz, 1H), 1,71–1.61 (m, 2H), 1.52 (ddd, J = 13.8, 10.2, 3.6 Hz, 1H), 1.27 (m, 1H), 0.99 (t, J = 7.2 Hz, 3H), 0.92 (s, 9H), 0.17 (s, 3H), 0.16 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ: 169.3, 154.8, 154.6, 137.3, 133.6, 122.3, 117.5, 109.8, 108.6, 104.5, 99.9, 97.3, 55.4, 51.8, 50.5, 44.4, 42.1, 36.7, 28.0, 25.8, 24.2, 20.1, 18.1, 11.6, –4.0, –5.0.
Synthesis of (−)-Mitragynine (1)To a solution of 9-methoxystrictosidine derivative 5 (150 mg, 0.291 mmol) in degassed anhydrous THF (2.9 mL) were added acetic acid (83.3 µL, 1.46 mmol) and tetrabutylammonium fluoride (1.0 M in THF solution, 583 µL, 0.583 mmol) at –20 °C under Ar atmosphere. The reaction mixture was stirred for 5 h at –20 °C. The resulting mixture was directly filtered through a short plug of silica gel eluted with 40% AcOEt/n-hexane. The residue of 4 was dissolved in degassed MeOH (14.6 mL) to which was added PtO2 (150 mg, 100 wt%) at r.t. under Ar atmosphere. The resulting mixture was purged with a stream of hydrogen and the reaction mixture was stirred for 1 h at r.t. under H2 atmosphere. The resulting mixture was filtered with a Celite pad with AcOEt, and the filtrate was concentrated under reduced pressure. The resulting residue of 20 was dissolved in degassed MeOH (14.6 mL) to which was added CH(OMe)3 (52.7 µL, 0.481 mmol) and p-TsOH·H2O (211 mg, 1.11 mmol), and the mixture was stirred at 70 °C for 16 h. The resulting mixture was quenched with saturated aqueous NaHCO3 solution, and was concentrated under reduced pressure. The aqueous layer was extracted three times with CHCl3. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. The crude materials of 21 were dissolved in dry degassed DMF (5.8 mL) to which was added t-BuOK (98.1 mg, 0.874 mmol) at 0 °C under Ar atmosphere. The reaction mixture was stirred for 45 min at r.t. under Ar atmosphere. The resulting mixture was quenched with water and the aqueous layer was extracted three times with CHCl3. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. Flash chromatography (SiO2, 25–45% AcOEt/n-hexane gradient then 5% MeOH/CHCl3) afforded (−)-mitragynine (1, 48.7 mg, 42% over four steps) and (+)-speciogynine (2, 15.2 mg, 13% over four steps). All spectral data of the obtained (−)-mitragynine (1) and (+)-speciogynine (2) were identical to those of natural products.33,34)
(−)-Mitragynine (1): Yellow amorphous powder; [α]24D –103.1 (c 0.57, CHCl3) [lit. [α]24D –126 (c 1.2, CHCl3)]; IR (ATR) νmax cm−1 3359, 2926, 1697, 1622, 1567, 1508, 1461, 1434, 1350, 1273, 1253, 1188, 1170, 1146, 1104, 1078, 1020, 977, 903, 885, 862; HRMS (ESI) [M + H]+ calculated for [C23H31N2O4]+: 399.2284, found: 399.2291; CD (0.3 mM, MeOH, 23 °C) λ nm (Δε): 320 (0), 291 (2.27), 283 (2.15), 273 (2.45), 261 (0), 239 (–8.18), 229 (–7.33), 219 (–11.32), 206 (0); UV (MeOH) λmax 292, 224 nm; 1H- and 13C-NMR see supporting information.
(+)-Speciogynine (2): Yellow amorphous powder; [α]24D +17.0 (c 1.02, CHCl3) [lit. [α]24D +26.8 (c 0.85, CHCl3)]; IR (ATR) νmax cm−1 3363, 2931, 2846, 2796, 2736, 1699, 1633, 1568, 1508, 1460, 1434, 1355, 1335, 1317, 1276, 1252, 1222, 1187, 1144, 1102, 1013, 989, 915, 894, 862, 834, 810; HRMS (ESI) [M + H]+ Calcd. for [C23H31N2O4]+: 399.2284, Found: 399.2273; CD (0.3 mM, MeOH, 23 °C) λ nm (Δε): 309 (0), 291 (2.93), 281 (3.56), 269 (4.47), 253 (3.36), 237 (9.55), 230 (0), 219 (–20.57), 203 (0); UV (MeOH) λmax 291, 225 nm; 1H- and 13C-NMR see supporting information.
Synthesis of (+)-Speciogynine (2)To a solution of 9-methoxystrictosidine derivative 5 (30 mg, 0.058 mmol) in degassed anhydrous THF (580 µL) were added acetic acid (16.7 µL, 0.291 mmol) and tetrabutylammonium fluoride (1.0 M in THF solution, 117 µL, 0.117 mmol) at –20 °C under Ar atmosphere. The reaction mixture was stirred for 5 h at –20 °C. To the resulting mixture was added sodium triacetoxyborohydride (24.7 mg, 0.117 mmol) at –20 °C. The reaction mixture was stirred for 2.5 h at –20 °C. The resulting mixture was quenched with saturated aqueous NaHCO3 solution and the aqueous layer was extracted three times with AcOEt. The combined organic layer was washed with brine then dried over Na2SO4. The resulting residue of 22 was dissolved in degassed MeOH (2.9 mL), to which was added CH(OMe)3 (10.5 µL, 0.0962 mmol) and p-TsOH·H2O (42.1 mg, 0.221 mmol), and then stirred at 70 °C for 12 h. The resulting mixture was quenched with saturated aqueous NaHCO3 solution and then concentrated under reduced pressure. The aqueous layer was extracted three times with CHCl3. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. The crude materials of 23 were dissolved in dry degassed DMF (1.2 mL) to which was added t-BuOK (19.6 mg, 0.175 mmol) at 0 °C under Ar atmosphere. The reaction mixture was stirred for 40 min at r.t. under Ar atmosphere. The resulting mixture was quenched with water and the aqueous layer was extracted five times with CHCl3. The combined organic layer was dried over Na2SO4, and concentrated under reduced pressure. Flash chromatography (SiO2, 45% AcOEt/n-hexane) afforded (+)-speciogynine (2, 15.2 mg, 65% over four steps).
Synthesis of (+)-7-Hydroxymitragynine (3)To a solution of freshly purified (−)-mitragynine (1, 30 mg, 0.075 mmol) in MeCN (900 µL) and water (100 µL) was added TFA (11.5 µL, 0.151 mmol) at 0 °C, and the reaction mixture was stirred for 5 min at 0 °C. PIFA (32.4 mg, 0.0753 mmol) was added to the stirred mixture at 0 °C under Ar atmosphere. The reaction mixture was stirred for 20 min at 0 °C, followed by quenching with saturated aqueous NaHCO3 solution. The aqueous layer was extracted three times with CH2Cl2. The combined organic layer was washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Flash chromatography (SiO2-NH, 40% AcOEt/n-hexane) afforded (+)-7-hydroxymitragynine (3, 22.0 mg, 71%). All spectral data of the obtained (+)-7-hydroxymitragynine (3) were identical to those of natural product.6,8)
(+)-7-Hydroxymitragynine (3): Pale yellow amorphous powder; [α]23D +62.5 (c 0.50, CHCl3) [lit. [α]23D +47.9 (c 0.55, CHCl3)]; IR (ATR) νmax cm−1 3420, 2947, 2872, 2835, 2811, 2745, 1698, 1644, 1597, 1486, 1460, 1435, 1376, 1266, 1237, 1187, 1143, 1104, 1074, 1018, 989, 959, 935, 920, 870, 841; HRMS (ESI) [M + H]+ Calcd. for [C23H31N2O5]+: 415.2233, Found: 415.2256; CD (0.3 mM, MeOH, 23 °C) λ nm (Δε): 344 (0), 305 (0.53), 282 (0), 258 (0.82), 243 (0), 229 (−1.13), 214 (0); UV (MeOH) λmax 307, 245, 220 nm; 1H- and 13C-NMR see supplementary materials.
We gratefully acknowledge financial support through a Grant-in-Aid for Scientific Research (B) (21H02608 to H. I. and 20H03395 to M. K.) from JSPS, and a JSPS Research Fellowships for Young Scientists (21J20696) to J. S.
The authors declare no conflict of interest.
This article contains supplementary materials.