2023 Volume 71 Issue 12 Pages 906-908
2023 Volume 71 Issue 12 Pages 906-908
Drug taste, which affects palatability, influences drug adherence. Sensory masking may be used to confound bitter tastes in drugs with other tastes and flavors; however, evaluation of sensory masking is difficult because of the existence of multiple tastes. In this study, a new two-bottle choice test was performed in rats to evaluate bitterness masking and determine the drug-to-sweetener ratio that significantly improves palatability. Sulfamethoxazole and trimethoprim were used as model bitter drugs, and sucralose was used as sweetener. The addition of sucralose and trimethoprim at a 0.13 : 1 ratio resulted in the greatest improvement in preference. This method is a useful new technique for evaluating the palatability of drug formulations.
The success of long-term therapies is determined by adherence, which is negatively affected by drug bitterness and poor palatability. One approach for masking drug bitterness is sensory masking, which confounds the bitter taste with other tastes and flavors. However, the evaluation of sensory masking is complicated by the existence of multiple tastes.
Although human gustatory sensation tests can comprehensively evaluate taste and palatability, these tests may lead to increased development costs. As an alternative to human gustatory sensation tests, artificial taste sensors may be used,1) which can objectively quantify single tastes. However, it may be difficult to assess the overall palatability of formulations with multiple tastes, especially those with high-sweetness sweeteners that do not react with taste sensors.
Another promising method for evaluating taste and palatability is the gustatory sensation test using rodents. This method has reduced costs and uniform subjects with smaller individual differences than humans, leading to successful tests. In this study, a two-bottle choice test in rats, whose taste preference showed good correlation with humans,2,3) was used to evaluate the sensory masking of bitterness. The drug-to-sweetener ratio was optimized using sulfamethoxazole and trimethoprim (SMX/TMP) as model bitter drugs and sucralose as sweetener.
Sulfamethoxazole (Virchow Laboratories Ltd., India) and trimethoprim (Ipca Laboratories Ltd., India) were used as model bitter drugs. Quinine hydrochloride (Q HCl) (Tokyo Chemical Industry Co., Ltd., Japan) was used as the representative bitter compound. Sucralose (San-Ei Gen F.F.I., Inc., Japan) was used as the model sweetener.
Evaluation of Bitterness IntensityThe taste sensor SA402B (Intelligent Sensor Technology, Inc., Japan) and sensor BT0, which was developed to detect basic bitter substances, was used to determine bitterness intensity of the sample solutions according to the principles and procedures described by Ishizaka et al.1) First, a reference solution was measured and the obtained electric potential (mV) was defined as Vr. Then, a sample solution (0.1 mM of each compound) was measured, and the electric potential obtained was defined as Vs. The relative sensor output (R) is represented by Vs−Vr. The electrode was dipped again into the reference solution, and the new potential of the reference solution was defined as Vr′. Vr′−Vr is defined as the change in membrane potential caused by adsorption (CPA). The measurement time was set to 30 s, and the electrodes were rinsed after each measurement. Higher R and CPA values indicate higher bitterness intensities.
Two-Bottle Choice TestExperiments were conducted using almost identical groups of six male Sprague–Dawley rats (CD/IGS) (CHARLES RIVER LABORATORIES JAPAN, Inc., Japan). Seven-week-old rats were housed in separate racks in a vivarium at an ambient temperature of 23 °C and a relative humidity of 55%. Each rat was housed in a 292 × 440 × 200 mm hanging cage. Food and at least one bottle of water were always available, and the food cups and water bottles were refilled as required. Animal bedding chips under the rats’ cages were used to collect excrement and spillage and were changed daily. The rats were acclimatized for six days before the tests began. All animal procedures were approved by the Institutional Animal Care and Use Committee of Shionogi & Co. Ltd. In compliance with ARRIVE guidelines (Approval No.: F20078D2-0000).
The rats underwent three two-bottle choice tests. First, the rats were confirmed to avoid bitter tastes (0.316 mM Q HCl solution, which has already been shown to be avoided by rats).4) These rats were then exposed to increasing concentrations of SMX/TMP and sucralose with SMX/TMP. The SMX/TMP concentrations were selected based on the results of bitterness intensity tests. Solutions were prepared within 24 h before use. Most solutions were made in 1-L batches by stirring the appropriate quantity of the taste compound into tap water.
At the beginning of each test, the food cups and regular water bottles were removed for 6–8 h, after which two similar bottles were provided. The bottles were initially presented such that the water or reference solution was on the left and the test solution was on the right. The positions of the two bottles were switched after 8.5 h, exactly midway through the presentation duration (17 h), because the rats remembered the tastes of the solution presented in the previous test, which could lead to a positional preference. The bottles were weighed (± 0.01 g) before being placed in the cage, after being switched at 8.5 h, and after removal at 17 h.
The fluid intake in grams was converted to milliliters, assuming 1 mL = 1 g for all solutions. The total intake was obtained from the sum of the intakes from both bottles. The test solution preference was calculated as the ratio of the test solution intake to the total intake, expressed as a percentage. The experimental data were analyzed using the statistical software JMP® 17 (SAS Institute, Cary, NC, U.S.A.). The difference between the intake of the test solution and that of the reference solution was analyzed using the Student’s t-test. Statistical significance was set at p < 0.01. A test solution was considered preferred or avoided if the mean preference was significantly greater or less than 50%, respectively, according to a one-sample t-test with p < 0.01.
Two-bottle choice tests are widely used to determine the preference of rodents for two liquids by evaluating the amount of liquid consumed from each bottle.
SMX/TMP was selected as the model bitter drug. To determine the concentration of SMX/TMP avoided by the rats, the bitterness of Q HCl, SMX and TMP were compared using an artificial taste sensor. Table 1 lists the bitterness intensities of the compounds. SMX showed low R and CPA values, and those of TMP were 3–10 times lower than those of Q HCl. In a study by Kawahara et al.,5) the same result was obtained; no sensor outputs were observed in response to SMX solutions of different concentrations, and TMP solutions of 0.0625–1.0 mM showed high sensor output that increased in a concentration-dependent manner.
| Compounds | Quinine hydrochloride | Sulfamethoxazole (SMX) | Trimethoprim (TMP) |
|---|---|---|---|
| R | 73.3 | −1.7 | 21.9 |
| CPA | 30.5 | 0.3 | 3.3 |
R corresponds to the “immediate taste after ingestion,” and CPA corresponds to the aftertaste.
Then the concentration range of the SMX/TMP solutions used for the two-bottle choice tests was based on the results of bitterness intensity tests and palatability results of the 0.01 mM Q HCl solution, which showed avoidance (approximately 35% preference) and intake of 10 mL/d.4) The concentration range was calibrated because excessive avoidance of a bitter taste could affect the results of the subsequent tests and the health of the rats. A fixed ratio of 5 : 1 SMX and TMP was used for the two-bottle choice tests to reflect the compounding ratio of SMX/TMP as a combination drug. Water and SMX/TMP solutions of different concentrations (0.01, 0.04, 0.20, 0.40 mM trimethoprim) were presented in a two-bottle choice test. If the rats tasted the bitterness from the SMX/TMP solution, they preferred to drink from another bottle (water). The 0.04 mM SMX/TMP solution was avoided with 13.2% preference (Fig. 1B). The concentration of SMX/TMP did not affect the total intake of the two bottles. Higher concentrations of SMX/TMP resulted in higher avoidance, and the intakes of the 0.20 and 0.40 mM SMX/TMP solutions were only about 1 mL (Fig. 1A). Therefore, the optimal SMX/TMP concentration was determined as 0.04 mM.
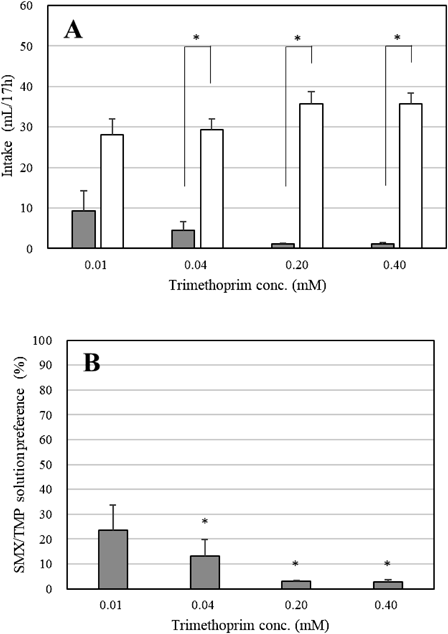
(A) Gray bars show SMX/TMP solution intakes, while white bars show water intakes. * p < 0.01 compared with water (n = 6). (B) Gray bars show the preference for SMX/TMP solution at each TMP concentration. * p < 0.01 compared with 50% (n = 6). Each bar shows the mean ± standard error (S.E.).
Sucralose, a high-intensity sweetener, was added as a model sweetener to mask bitterness. Generally, the amount of high-intensity sweetness is difficult to quantify using taste sensors. Therefore, few taste sensors for high-intensity sweeteners have been developed.6) In this study, we confirmed that the addition of sucralose did not change the R and CPA values of SMX or TMP solutions. Therefore, we evaluated the sensory masking of bitterness using a two-bottle choice test with rats.
An SMX/TMP solution that was avoided by rats and an SMX/TMP solution containing sucralose (at concentrations of either 1.9, 3.8, or 5.7 µM) were presented in a two-bottle choice test, and the rats’ preference for the solution containing sucralose was evaluated. The addition of sucralose with a mass ratio of 0.13 (3.8 µM) to TMP resulted in an improvement in preference (Fig. 2). However, the solution with 5.7 µM sucralose showed a high variability in intake with no difference in intake of the SMX/TMP solution with or without sucralose. This result may be due to the deterioration of taste caused by large amounts of sucralose. A high concentration of high-intensity sweeteners cause bitterness and tingling.7) Saccharin, a high-intensity sweetener, was also preferred only up to a certain concentration (approximately 10 mM), and some rats had a lower preference for or avoided the 100 mM saccharin solution.4) The concentration of sucralose did not affect the total intake. From these results, the ratio of sucralose that most effectively masks bitterness from SMX/TMP and improves palatability was determined.
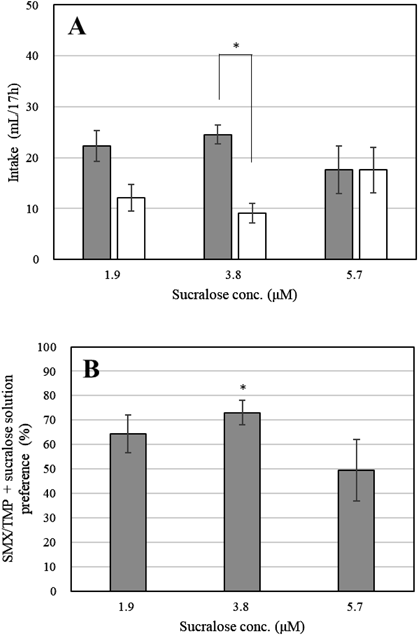
(A) Gray bars show intakes of SMX/TMP + sucralose solution, while white bars show intakes of SMX/TMP solutions. * p < 0.01 compared with SMX/TMP solutions (n = 6). (B) Gray bars show the preference for SMX/TMP + sucralose solution at each sucralose concentration. * p < 0.01 compared with 50% (n = 6). Each bar shows the mean ± S.E.
Based on this study, the newly developed SMX/TMP mini combination tablets included sucralose with a mass ratio of 0.06–0.13 : 1 to TMP. According to Saito et al.,8) most patients showed better acceptability of the mini-tablets than other SMX/TMP formulations.
In the present study, the preference for solutions containing multiple compounds with different tastes was evaluated. There are differences in the preferences and thresholds of rodents for taste compounds depending on the strain4) and life stage,9) but it is easier to control these variabilities than in humans. Therefore, it is expected that rats with palatability and thresholds closer to those of humans will be identified and that the use of these rats will improve the accuracy of taste and palatability evaluations.
In the two-bottle choice test, the addition of a 0.13 : 1 mass ratio of sucralose to TMP resulted in a significant improvement in preference. The two-bottle choice test revealed the sucralose ratio which most effectively masked the bitterness of SMX/TMP and improved its palatability. The method presented is a useful technique for evaluating the palatability of drug formulations.
The authors declare no conflict of interest.