2023 年 71 巻 2 号 p. 148-153
2023 年 71 巻 2 号 p. 148-153
This study aimed to evaluate the bitterness of famotidine (FAM) combined with each of three non-steroidal anti-inflammatory drugs (NSAIDs): ibuprofen (IBU), flurbiprofen (FLU), and naproxen (NAP), which have potential as fixed-dose combination (FDC) drugs. We evaluated the bitterness of FAM and each NSAID by taste sensor AN0 and C00, respectively. FAM showed high sensor output representing sensitivity to bitterness, whereas three NSAIDs did not show large sensor output, suggesting that the bitterness intensities of three NSAIDs were lower than that of FAM. The bitterness of FAM on sensor AN0 was suppressed in a concentration-dependent manner when mixed with IBU, FLU, or NAP. Among three NSAIDs, IBU most effectively inhibited bitterness on sensor output, and the gustatory sensation test confirmed that adding IBU to FAM reduced the bitterness of FAM in a concentration-dependent manner. MarvinSketch confirmed that the drugs were mostly present in an ionic solution when FAM was mixed with NSAIDs. The 1H-NMR spectroscopy analysis also revealed the presence of electrostatic interactions between FAM and NSAIDs, suggesting that the electrostatic interaction between FAM and NSAIDs might inhibit the adsorption of FAM on the bitter taste sensor membrane, thereby masking the bitter taste.
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used as anti-inflammatory analgesics and inhibit prostaglandin synthesis by blocking the cytochrome c oxidase (COX)-1 enzyme in the gastric mucosa. However, since prostaglandins protect against gastric acid, gastric mucosal damage has been reported as a side effect of long-term administration of NSAIDs.1,2) Therefore, fixed-dose combinations (FDC) of NSAIDs with acid secretion inhibitors have been developed with the advantage of reduced side effects, reduced number of medications taken, and improved adherence.3,4) Famotidine (FAM) is a histamine receptor antagonist that decreases gastric acid secretion, used widely for treating gastric ulcers. Even though NSAIDs do not have a severe bitter taste, FAM is known to have a strong bitter taste.5) However, in FDC, one drug could efficiently suppress the bitterness of the other drug involved in formulation to reduce the stress of medicine intake and improve adherence to FDC.
Sensory testing and chemical analysis are commonly used to evaluate the taste of pharmaceutical products. However, sensory testing is susceptible to human physical and psychological conditions and personal preferences. In addition, detecting synergistic or inhibitory effects between taste substances using HPLC could be challenging. Therefore, the “electronic tongue,” an analytical instrument with an array of non-selective chemical sensors, was developed in the mid-1990 s to measure and compare tastes.6–12)
The taste sensor developed by Toko is composed of an artificial lipid membrane that mimics the biological taste receptor mechanism and can quantify the taste of many substances. Our laboratory was the pioneer in quantitative evaluation for the bitterness of medicine or formulation using the taste sensor system (Taste Sensing System, Intelligent Sensor Technology, Inc., Atsugi, Japan) with published results.13–19) We also identified that bitter medicine acted as an agonist for human taste receptor hTAS2R14 and reported their relationship for the first time.20,21)
Different taste sensors are used for medicines with different tastes. C00 is an acidic bitterness sensor used for medicines like diclofenac sodium, a non-steroidal anti-inflammatory drug,18) AC0 and AN0 are basic bitterness sensors used for medicines like solifenacin succinate13) and amlodipine besylate.16) Due to their correlation with taste intensity, taste sensors are proven beneficial in predicting the taste of various substances and have been widely used in developing taste masking preparations.
This study aimed to evaluate the bitterness of FAM combined with each of three NSAIDs, ibuprofen (IBU), flurbiprofen (FLU), and naproxen (NAP), and their possible usage as FDC drugs. A fixed-dose combination of FAM and IBU called Duexis is marketed in the United States to reduce side effects. Therefore, we firstly adopted FAM/IBU combination. IBU belongs to propionate NSAIDs, likewise we chose FLU and NAP. We evaluated the bitterness of FAM mixed with each of three NSAIDs at different concentrations using taste sensors AN0, and C00, respectively.
Our results showed that FAM (1.2 mM), when mixed with different concentrations of IBU, suppressed bitterness most effectively. We also performed a gustatory sensation test to confirm the suppression of bitterness in human volunteers.
Furthermore, MarvinSketch was performed to calculate the percentage ionic or non-ionic molecular fraction of FAM combined with NSAIDs, and 1H-NMR spectral revealed the presence of electrostatic interactions between FAM and NSAIDs.
As shown in Fig. 1(a), the change in the membrane potential caused by adsorption (CPA) values of AN0 for FAM (0.3, 0.6, and 1.2 mM) increased in proportion to concentration. The AN0 CPA value for 0.03 mM quinine was considered the threshold of basic bitterness in humans (τ1). For further experiments, a FAM concentration of 1.2 mM was used.
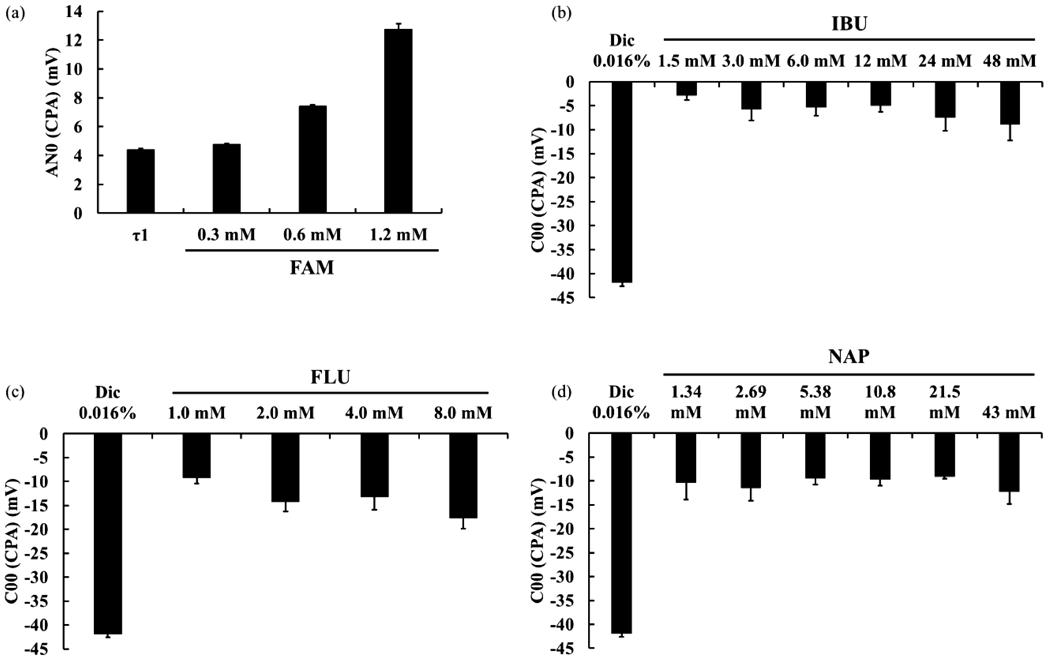
n = 3, mean ± S.D.
Taste sensor output of IBU (1.5, 3.0, 6.0, 12, 24, or 48 mM) was between −3 and −10 mV (Fig. 1(b)), that of FLU (1.0, 2.0, 4.0 or 8.0 mM) was between −10 and −18 mV (Fig. 1(c)) and that of NAP (1.34, 2.69, 5.38, 10.8, 21.5 or 43 mM) was approximately −10 mV (Fig. 1(d)). Diclofenac was used as a control for acidic bitterness control and showed a sensor output of −40 mV (Figs. 1(b)–(d)).
NSAIDs Reduced the FAM Taste Sensor OutputThe taste sensor output of FAM (1.2 mM) was approximately 13 mV. Adding IBU (1.5–48 mM), FLU (1.0–8.0 mM), and NAP (1.34–43 mM) to FAM (1.2 mM) significantly reduced its taste sensor output (Figs. 2(a)–(c)), and all three NSAIDs significantly suppressed bitterness.

n = 3, mean ± S.D., *** p < 0.001 vs. FAM 1.2 mM (Tukey’s test).
Human gustatory sensory test results showed that the bitterness intensity of FAM increased in a concentration-dependent manner, with τ = 2.6± standard deviation (S.D.) at 1.2 mM, while IBU showed no bitterness (τ = 0.2± S.D.) even at higher concentrations (20 mM). Adding IBU to 1.2 mM FAM significantly decreased its sensor output (τ = 0.8± S.D.) in an IBU concentration-dependent manner. (p < 0.01, Tukey’s test) (Fig. 3). The sensor output corresponding to the τ 0.8 were lower than τ 1.0 which was threshold for bitterness for human, which meaning that bitterness could be acceptable for patients.
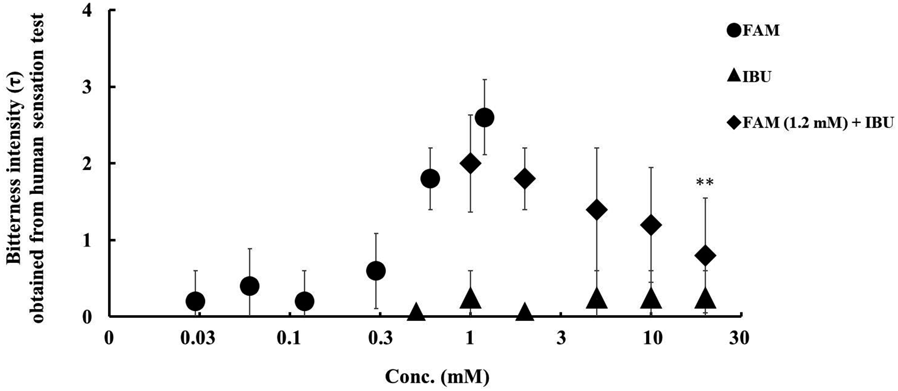
n = 5, mean ± S.D., ** p < 0.01 vs. FAM 1.2 mM (Tukey’s test).
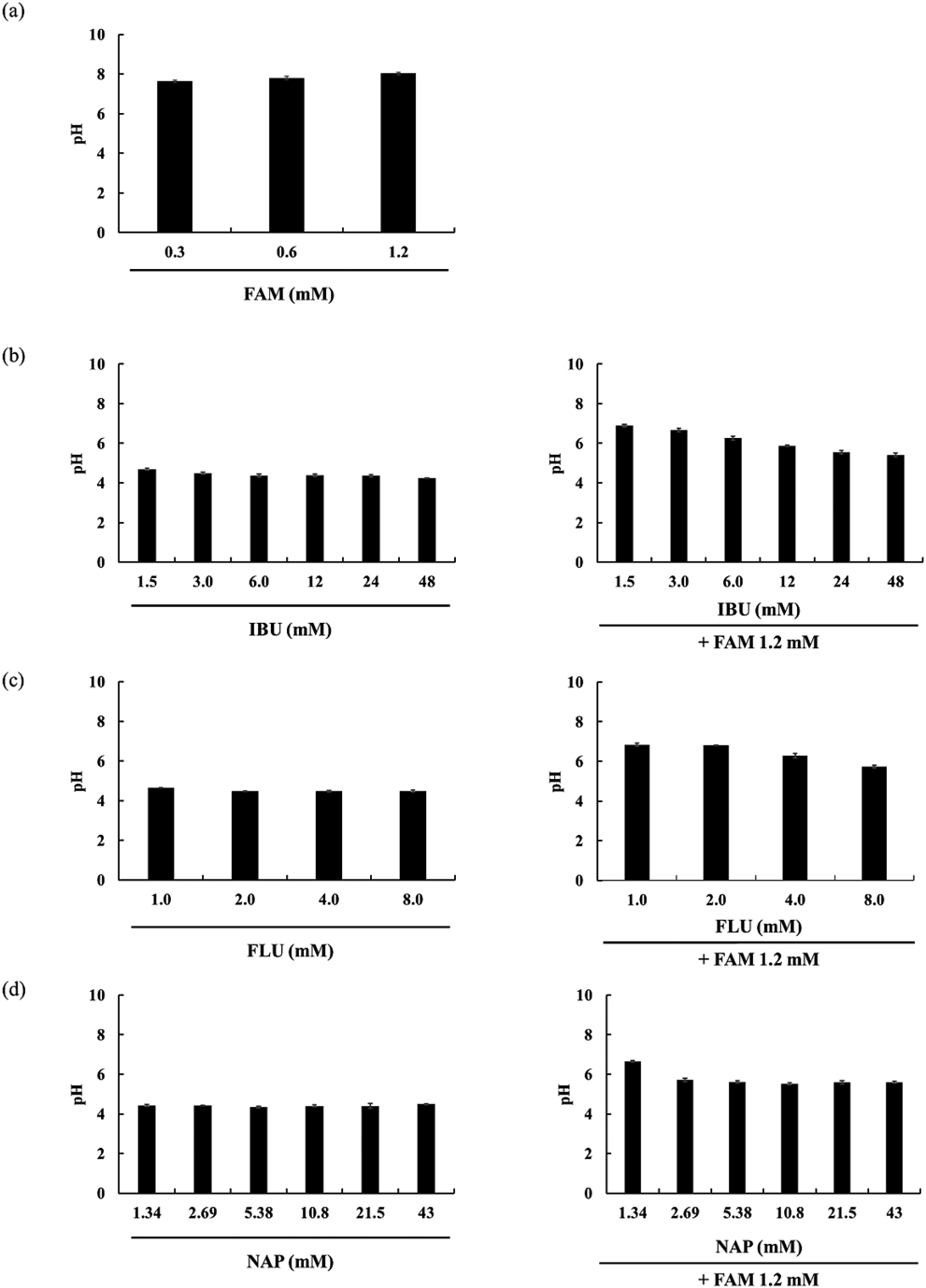
The pH values of FAM (a) alone or FAM contained IBU (b), FLU (c), and NAP (d) as mixed solutions are summarized in Fig. 4.
The pKa calculation using MarvinSketch revealed five ionized species for FAM, one for IBU, FLU, and NAP each, and a non-ionized form for each.
Non-ionized forms of FAM were predicted to account for less than 50% over the whole pH range. FAM ionized form I predominates from pH 1.8 to 8.4, ionized form II predominates from pH 9.4 to 14, and ionized form III predominates from pH 0 to 1.6 (Fig. 5(a)).

IBU ionized form predominates from pH 5.0 to 14, while the non-ionized form predominates over the pH range of 0 to 4.8. The pH of the FAM (1.2 mM) mixture with IBU (1.5–48 mM) was 6.9–5.4. In this pH range, ionized form I of FAM was predicted to predominate. At pH 6.9, when FAM (1.2 mM) was mixed with IBU (1.5 mM), it was predicted that 99.09% of IBU would be in ionized form and 0.91% in non-ionized form. At pH 5.4, when FAM (1.2 mM) was mixed with IBU (48 mM), it was predicted that 77.94% of IBU would be in ionized form and 22.06% in non-ionized form (Fig. 5(b)).
FLU ionized form predominates from pH 4.6 to 14, while the non-ionized form predominates over the pH range of 0–4.4. The pH of the FAM (1.2 mM) mixture with FLU (1.0–8.0 mM) was 5.7–6.8, and in this pH range, the ionized form of FAM was predicted to dominate. When FAM (1.2 mM) was mixed with FLU (1.0 mM), the pH of the solution was 6.8, and it was predicted that 99.58% of FLU would be in ionized form and 0.42% in non-ionized form (Fig. 5(c)).
NAP ionized form predominates from pH 4.2 to 14, and the non-ionized form predominates over the pH range of 0–4.0. The pH of the FAM (1.2 mM) mixture with NAP (1.34–43 mM) was 5.5–6.6, where the ionized form of FAM was predicted to dominate. When FAM (1.2 mM) was mixed with NAP (1.34 mM), the solution pH was 6.6, and it was predicted that 99.61% of NAP would be in ionized form and 0.39% in non-ionized form (Fig. 5(d)).
Overall, with FDC pH values, the FAM and the NSAIDs were primarily present in the ionic form. Owing to interaction between ionic form of FAM and ionic form of each NSAID used in the present study was expected to contribute in bitterness inhibition of FAM.
1H-NMR Spectroscopic AnalysisTo understand the mechanism underlying the bitterness-suppressing effect of NSAIDs on FAM, the interactions between FAM and each NSAID were examined by 1H-NMR spectroscopic analysis. Chemical shifts of each proton of FAM with/without IBU (1 : 1, 4, 33), FLU (1 : 1, 2, 8), or NAP (1 : 1, 4, 33) are shown in Table 1 and Supplementary Figs. S1–3. 1H-NMR spectrum of FAM was assigned as previously described.22) In the 1H-NMR spectrum of FAM with IBU, the proton signal in the guanidino group of FAM was at 6.789 ppm (FAM alone; Table 1(a)), 6.796 ppm (mixing ratio of IBU to FAM; 1; Δ0.007), 6.812 ppm (mixing ratio 4; Δ0.023) and 6.817 ppm (mixing ratio 33; Δ0.028).
| (a) | ||||
|---|---|---|---|---|
| Chemical shift (ppm) | ||||
| FAM | FAM : IBU = 1 : 1 | FAM : IBU = 1 : 4 | FAM : IBU = 1 : 33 | |
| Proton | 6.789 | 6.796 | 6.812 | 6.817 |
| (b) | ||||
| Chemical shift (ppm) | ||||
| FAM | FAM : FLU = 1 : 1 | FAM : FLU = 1 : 2 | FAM : FLU = 1 : 8 | |
| Proton | 6.789 | 6.801 | 6.809 | 6.824 |
| (c) | ||||
| Chemical shift (ppm) | ||||
| FAM | FAM : NAP = 1 : 1 | FAM : NAP = 1 : 4 | FAM : NAP = 1 : 33 | |
| Proton | 6.789 | 6.797 | 6.814 | 6.820 |
Similarly, in the 1H-NMR spectrum of FAM with FLU, the proton signal in the guanidino group of FAM was at 6.789 ppm (FAM alone; Table 1(b)), 6.801 ppm (mixing ratio of FLU to FAM; 1; Δ0.012), 6.809 ppm (mixing ratio 2; Δ0.020) and 6.824 ppm (mixing ratio 8; Δ0.035).
In the 1H-NMR spectrum of FAM with NAP, the proton signal in the guanidino group of FAM was at 6.789 ppm (FAM alone; Table 1(c)), 6.797 ppm (mixing ratio of NAP to FAM; 1; Δ0.008), 6.814 ppm (mixing ratio 4; Δ0.025) and 6.820 ppm (mixing ratio 33; Δ0.031).
We observed that the NSAIDs caused the proton signal in the guanidino group of FAM to shift downfield in a dose-depending manner, suggesting that proton donation by NSAIDs resulted in a decrease in the electron density at their particular location, causing a downfield shift occurring due to a deshielding effect.
Changes in the chemical shifts of the 1H-NMR data clearly indicated that the guanidino group of FAM was involved in the electrostatic interaction, and the MarvinSketch results suggested that the interaction was due to the ionization of N (part of (γ) in Fig. 6(a)) in the guanidino group and that of carboxy group of NSAIDs (Figs. 6(b)–(d)).
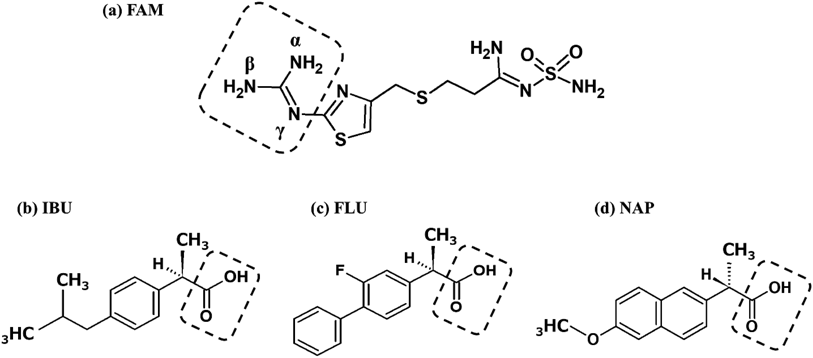
Most molecular species existed with ionic form as a result of MarvinSketch. And chemical shift perturbation was confirmed by 1H-NMR spectra. From these results, electrostatic interaction between FAM and each NSAID was suggested.
The study aimed to quantitatively evaluate the bitterness of FAM mixed with each of three kinds NSAIDs, IBU, FLU, or NAP, and to estimate the mechanism of bitterness suppression of FAM by NSAIDs mixture.
The bitterness of FAM on sensor AN0 was suppressed in a concentration-dependent manner when FAM was mixed with IBU, FLU, or NAP. IBU was most effective in inhibiting bitterness on sensor output among the three NSAIDs. Moreover, the human gustatory sensation test confirmed that a drastic reduction in the bitterness of FAM was observed with an increase in IBU concentration.
MarvinSketch results confirmed that both drugs are primarily present in ionic form in the mixed solution of FAM and NSAIDs.
1H-NMR analysis revealed potential interactions of FAM and the three NSAIDs based on chemical shift perturbations, suggesting a possible occurrence of electrostatic interaction between FAM and each NSAID.
Further studies will be planned on other commercial fixed dose combination drugs on the standpoint of bitterness inhibition.
The NSAIDs, flurbiprofen (FLU) purchased from TCI Co., Ltd., Japan), ibuprofen (IBU), and (S)-(+)-naproxen (NAP) purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) was used in the study. FAM was purchased from FUJIFILM Wako Pure Chemical Corporation. Quinine hydrochloride, used as a standard for basic bitterness, was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Diclofenac Sodium, used as a standard of acid bitterness, was purchased from FUJIFILM Wako Pure Chemical Corporation.
Taste Sensor MeasurementUsing the taste sensor SA402 (Intelligent Sensor Technology, Inc.), we measured the electric potential of sample solutions and evaluated their bitterness. The detecting sensor consists of a reference electrode and a taste sensor which acts as the working electrode and is composed of various lipid/polymer membranes. The lipid/polymer membranes, C00 (tetradodecylammonium bromide/2-nitrophenyl octyl ether) and AN0 (phosphoric acid di-n-decyl ester/dioctyl phenylphosphonate), developed specifically to detect the bitterness of basic substances,15,20) was used in the taste sensor to determine the bitterness intensities of the sample solutions. The electrode set is attached to a mechanically controlled robot arm. The electrodes consist of an internal cavity filled with 3.3 M KCl solution. The difference between the electric potential of the working electrode and the reference electrode was measured using a high-input impedance amplifier connected to a computer. Fresh 30 mM KCl solution containing 0.3 mM tartaric acid (corresponding to saliva) was used as the reference solution and to rinse the electrode after every measurement.
The measurement procedure was as follows: The electrodes are dipped first into the reference solution, and the electric potential obtained (mV) is defined as Vr0. Then a sample solution is measured, and the electric potential obtained is defined as Vs. The electrodes are subsequently rinsed with a fresh reference solution for 6 s. When the electrodes are dipped into the reference solution again, the new potential of the reference solution is defined as Vr1. The difference between the potentials of the reference solution before and after sample measurement (Vr1 − Vr0) is defined as the ‘Change in the membrane potential caused by Adsorption’ (CPA), which is a specific expression of bitterness. In this study, the CPA of AN0 was used to evaluate bitterness.
First, aqueous solutions of the four drugs, FAM (0.3, 0.6, and 1.2 mM), IBU (1.5, 3.0, 6.0, 12, 24, and 48 mM), FLU (1.0, 2.0, 4.0, and 8.0 mM) and NAP (1.34, 2.69, 5.38, 10.8, 21.5, and 43 mM), were measured individually. Later, FAM (1.2 mM) was combined with each of the other three drugs IBU (1.5, 3.0, 6.0, 12, 24, and 48 mM), FLU (1.0, 2.0, 4.0, and 8.0 mM), and NAP (1.34, 2.69, 5.38, 10.8, 21.5, and 43 mM), and the change in bitterness intensities was evaluated using the taste sensor.
Evaluation of the Effect of IBU on the Bitterness of FAM by Human Gustatory Sensory TestingHuman gustatory sensory test to determine the intensity of bitterness were performed as previously described.16,23) Quinine hydrochloride was used as the bitter taste standard in the human gustatory sensory test. Subjects were given 0.01, 0.03, 0.1, 0.3, and 1.0 mM quinine hydrochloride solutions in their mouths, and subjects memorized the bitterness of each concentration on a scale of 0 to 4 as the intensity of bitterness (τ). Then, FAM solution (0.03, 0.06, 0.12, 0.3, 0.6, 1.0, and 1.2 mM), IBU solution (0.5, 1.0, 2.0, 5.0, 10, and 20 mM), and FAM (1.2 mM) mixed with IBU (1.0, 2.0, 5.0, 10, and 20 mM) were held in the mouth, and the taste was evaluated after 5 s. The respondents then rated the bitterness of the solutions on a scale of τ 0 to τ 4 and compared it with the bitterness of quinine, a bitter taste standard. This protocol (No. 16-42) was approved on 23 July, 2016 by the ethical committee of Mukogawa Women’s University.
1H-NMR Spectroscopic Analysis1H-NMR spectra were measured on a JEOL 500 MHz spectrometer using DMSO-d6 as a solvent. Sample solutions FAM, IBU, FLU, NAP, and the mixtures of FAM and each NSAID were prepared. The mixing ratios of IBU and NAP to FAM in the sample solution were 1, 4, and 33 by molar ratio. The mixing ratios of FLU to FAM in the sample solution were 1, 2, and 8 by molar ratio. We determined drug ratios as described above to confirm the signal shifts of proton in an NSAIDs dose-dependent manner.
Estimation of Ionization for DrugsThe pKa values of FAM, IBU, FLU, and NAP over the pH range 0–14 at 298 K were calculated using MarvinSketch (ChemAxon).
Statistical AnalysisBellCurve for Excel® was used for statistical analysis. Results of taste sensor measurements and human gustatory sensory testing were expressed as mean ± S.D. and evaluated with the Tukey’s test. Results from taste sensors and human gustatory sensory testing were considered significant at 0.1 and 1% probability, respectively.
This work was supported by a Grant-in-aid for Scientific Research (S) from the Japan Society for Promotion of Science 21H05006.
The authors declare no conflict of interest.
This article contains supplementary materials.