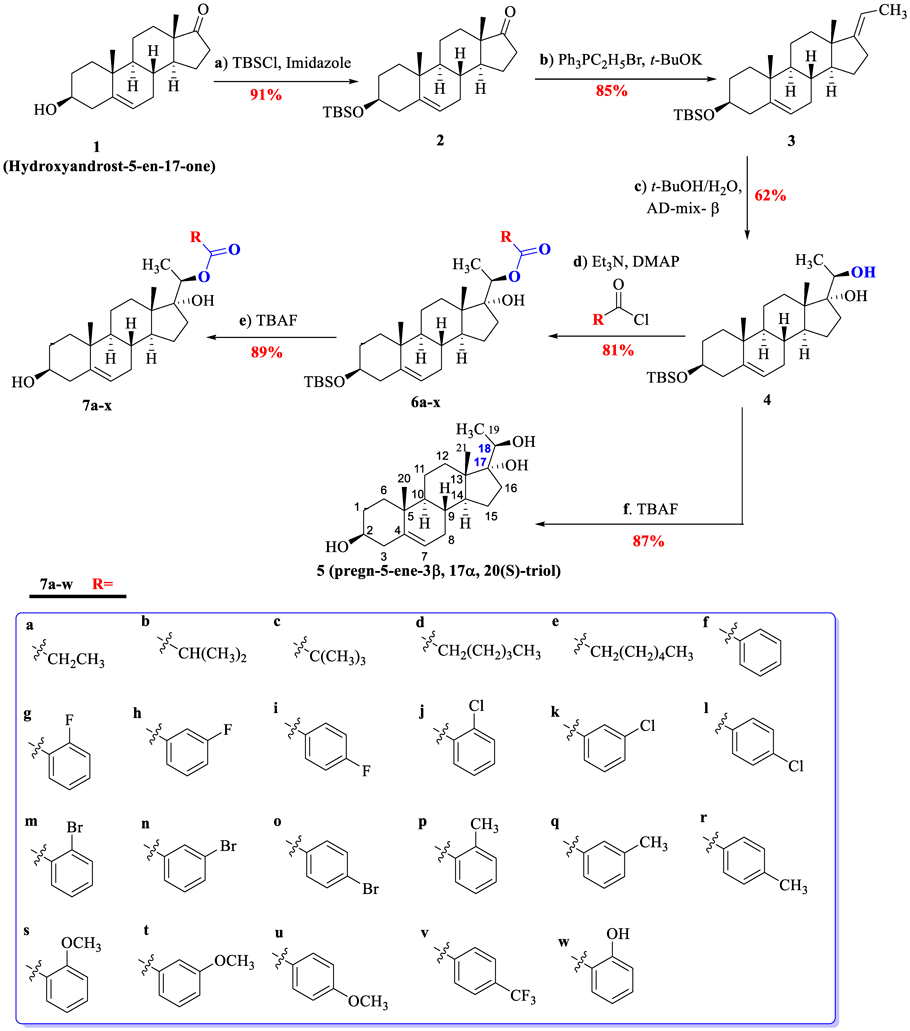
2023 年 71 巻 3 号 p. 220-228
2023 年 71 巻 3 号 p. 220-228
There is no doubt that derivation of intermediates from natural product is a very efficient way to develop new environmentally friendly pesticide. We synthesis a succession of compounds esterified with pregn-5-ene-3β,17α,20(S)-triol to evaluate its insecticidal and bacteriostatic activity. Otherwise, their structure–activity relationships (SAR) are also discussed. As a result, compounds 7g, 7h, 7j, 7l and 7o exhibit more obvious insecticidal activity against 3rd Mythimna separata Walker (LC50 = 0.60, 0.68, 0.79, 0.85 and 0.78 mg/mL, respectively) than periplocoside F (PSF). Meanwhile, compounds 7g, 7h and 7i perform well inhibitory activity against Pseudomas syringae pv. actinidiae (Psa) in vitro (minimum inhibitory concentration (MIC) values: 0.10–0.25 mg/mL, minimum bactericidal concentration (MBC) values: 0.15–0.35 mg/mL). And SAR analysis indicates that the replacement and position of fluorine atom on benzoyl are highly vital to biological activity.
One of the most crucial agricultural issues is to maximize the quality of crop through the minimization of pests and diseases harm.1) According to the statistics, the 17% grain yield has been reduced due to the pests before the harvest throughout the world, and bacterial infections are also considered to be important factors restricting crop production worldwide.2,3) On the one hand, excessive use of pesticides will cause resistance of diseases and pests, increasing the difficulty of prevention and control. On the other hand, it will cause excessive pesticide residues in agricultural products and do harm to people's lives.4–7) Therefore, there should be urgent development of new possible alternatives for efficient and selective management of pests and bacteria.8–11)
As we know, the botanical pesticides are usually produced from extracts of medicinal plants or plants used in the food industry, and they are viewed as products associated with minimum health and environmental risks.12) In our previous works, the secondary metabolites are isolated from Periploca sepium root barks (PSRB), a traditional herbal medicine in China, has been regarded as promising pesticides to control pests.13–15) For example, periplocoside F (PSF) and periplocoside (PSNW) are regarded as stomach poison against Mythimna separata Walker.16–18) Periplocoside X exhibits prominent insecticidal activities against workers of the red imported fire ants (LD50 = 0.116 mg/mL).19) However, it is properly difficult to be obtained to meet the demand for general use. Considering that our program is exploiting non-food bioactive products to obtain some ideal pesticides, the synthesis of ester derivatives form pregn-5-ene-3β,17α,20(S)-triol (Fig. 1) is necessary to carry out.20,21) Besides, we also have found that hydroxyandrost-5-en-17-one (or DHEA), owns obvious antibacterial activity.22) In addition, many compounds synthesized by esterification are prepared as pesticide such as osthole-type esters (I) andrographolide esters (II), solketal palmitate (III) and Chlorogenic acid (IV) (Fig. 1). Some of which exhibit better bioactivity than corresponding precursors.23–25) It reminds us that ester group plays a crucial part in bioactivity of compounds. And there is no report that ester derivatives of pregn-5-ene-3β,17α,20(S)-triol are assessed its biological activity against pest and bacteria.

Thus, in our preliminary study, a series of ester derivatives from pregn-5-ene-3β,17α,20(S)-triol are synthesized so as to screen compounds with conspicuous biological activity as new alternatives to control pests and bacteria in agriculture.
In general, the DHEA (hydroxyandrost-5-en-17-one) was passed five steps reactions to afford ester derivatives 7a–w (Chart 1 synthetic route). Their structures were well characterized by 1H-NMR, 13C-NMR, optical rotation, high resolution (HR)MS and m.p. (supplementary material).
The 3rd M. separata, which is pests threatening to a wide range of fruit trees and crops26–28) produced different final mortality rate (FMR) after being treated with compounds 7g, 7h, 7j, 7l and 7o. And it could be showed in Table 1, compounds 7g, 7h, 7j, 7l and 7o showed higher insecticidal activity than PSF (positive control) regarded commonly as insectifuge. Especially compound 7g exhibited the most obvious pesticidal activity with FMR of 66.67%, whereas the FMR of compound 1 and pregn-5-ene-3β,17α,20(S)-triol were only 5.26 and 19.30%. After preliminary screen, the datas showed that the fluorine atom of ortho- (7g) or meta- (7h) positions of benzoyl had more prominent insecticidal action than those with other substitutes.
| Compounds | Corrected mortality rate (%)a, b) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 1 | 1.67 ± 2.36 | 5.18 ± 2.36 | 5.26 ± 0.00 |
| 5 | 16.78 ± 2.36 | 18.97 ± 2.36 | 19.30 ± 2.36 |
| 7a | 5.03 ± 2.36 | 10.35 ± 2.36 | 12.28 ± 2.36 |
| 7b | 6.71 ± 2.36 | 10.35 ± 2.36 | 14.04 ± 2.36 |
| 7c | 10.06 ± 2.36 | 12.07 ± 4.08 | 12.28 ± 2.36 |
| 7d | 8.39 ± 0.00 | 15.52 ± 2.36 | 15.79 ± 4.08 |
| 7e | 6.71 ± 2.36 | 13.80 ± 2.36 | 14.04 ± 2.36 |
| 7f | 20.13 ± 2.36 | 20.69 ± 2.36 | 21.05 ± 4.08 |
| 7g | 46.98 ± 2.36 | 58.62 ± 4.08 | 66.67 ± 2.36 |
| 7h | 46.98 ± 2.36 | 55.17 ± 4.08 | 59.65 ± 4.71 |
| 7i | 23.49 ± 4.08 | 25.38 ± 2.36 | 29.82 ± 2.36 |
| 7j | 41.94 ± 2.36 | 46.55 ± 4.71 | 52.63 ± 4.08 |
| 7k | 26.84 ± 2.36 | 27.59 ± 2.36 | 29.82 ± 2.36 |
| 7l | 38.59 ± 4.08 | 44.83 ± 2.36 | 49.12 ± 4.17 |
| 7m | 26.84 ± 4.08 | 27.59 ± 0.00 | 31.58 ± 4.08 |
| 7n | 23.49 ± 2.36 | 25.86 ± 2.36 | 31.58 ± 0.00 |
| 7o | 41.94 ± 2.36 | 46.55 ± 2.36 | 54.39 ± 4.08 |
| 7p | 20.13 ± 2.36 | 20.69 ± 2.36 | 21.05 ± 4.08 |
| 7q | 21.81 ± 2.36 | 22.42 ± 0.00 | 24.56 ± 2.36 |
| 7r | 16.78 ± 2.36 | 17.24 ± 4.08 | 19.30 ± 2.36 |
| 7s | 20.13 ± 2.36 | 27.59 ± 0.00 | 28.07 ± 2.36 |
| 7t | 15.10 ± 2.36 | 20.69 ± 2.36 | 24.56 ± 2.36 |
| 7u | 11.74 ± 2.36 | 15.52 ± 2.36 | 22.81 ± 2.36 |
| 7v | 16.78 ± 2.36 | 20.69 ± 2.36 | 21.05 ± 4.08 |
| 7w | 26.84 ± 4.08 | 44.83 ± 2.36 | 49.12 ± 4.17 |
| ckc) | 0.00 ± 2.36 | 0.00 ± 2.36 | 0.00 ± 0.00 |
| PSF | 36.91 ± 2.36 | 43.11 ± 4.08 | 49.12 ± 6.24 |
a) Corrected mortality rate = (treatment group mortality rate − CK mortality rate)/(1 − CK mortality rate). b) Values are mean ± standard error (S.E.) of three replicates. c) The blank group is dealt with acetone alone.
Some derivatives with well insecticidal activity against 3rd M. separata were further assessed (Table 2). The LC50 values of 7g, 7h, 7j, 7l and 7o were between 0.60 and 0.85 mg/mL. Notably, compounds 7g, 7h, 7j, 7l and 7o all exhibited over 2 folds insecticidal activity than pregn-5-ene-3β,17α,20(S)-triol (LC50 value: 2.30 mg/mL). Furthermore, the LC50 values of these compounds were lower than PSF (LC50 value = 0.88 mg/mL). At the same time, there were many periplocosides owning similar activity against 3rd M. separata to PSF,29,30) but periplocosides were extremely difficult to be isolated from PSRB. Hence, we synthesized derivant form pregn-5-ene-3β,17α,20(S)-triol could preferably take place of periplocosides as promising insecticide to control pests.
| Compound | Linear regression equation | Correlation coefficient | LC50 (mg/mL) | 95% confidence interval | X2 | p |
|---|---|---|---|---|---|---|
| 5 | Y = −1.05 + 2.89 X | 0.979 | 2.30 | 1.71–5.27 | 0.99 | 0.80 |
| 7g | Y = 0.52 + 2.36 X | 0.989 | 0.60 | 0.46–0.85 | 1.80 | 0.62 |
| 7h | Y = 0.39 + 2.37 X | 0.998 | 0.68 | 0.51–0.95 | 0.86 | 0.83 |
| 7j | Y = 0.27 + 2.61 X | 0.988 | 0.79 | 0.61–1.21 | 1.63 | 0.65 |
| 7l | Y = 0.14 + 2.07 X | 0.996 | 0.85 | 0.62–1.59 | 2.11 | 0.55 |
| 7o | Y = 0.23 + 2.13 X | 0.993 | 0.78 | 0.58–1.34 | 0.86 | 0.84 |
| PSF | Y = 0.11 + 1.95 X | 0.995 | 0.88 | 0.62–1.79 | 0.92 | 0.80 |
a) Regression analysis by IBM SPSS Statistics 22.0, p < 0.05.
The antibacterial activity could be seen in Table 3, compounds 7g, 7h and 7i indicated different levels of antibacterial activity against Pseudomas syringae pv. actinidiae (Psa), which was highly virulent and spread fast between plants and caused serious damage to kiwi.31) The values of inhibition zone ranged between 7 and 13 mm, but no obvious antibacterial activity was observed against Erwinia carotovora (E. carotovora) and Ralstonia solanacearum (R. solanacearum). Combined and analyzed data of bioassay, we could find a phenomenon that the inhibitory properties of the corresponding compounds were remarkable when substitutes of benzoyl were fluorine atom.
| Compounds | Diameter of inhibition zone (mm)a) | ||
|---|---|---|---|
| E. carotovora | R. solanacearum | P. syringae pv. actinidiae | |
| 1 | — | — | 2+ |
| 5 | — | — | — |
| 7a | — | — | — |
| 7b | — | — | — |
| 7c | — | — | — |
| 7d | — | — | — |
| 7e | — | — | — |
| 7f | — | — | — |
| 7g | — | + | 13++ |
| 7h | — | — | 7++ |
| 7i | + | — | 10++ |
| 7j | — | — | — |
| 7k | — | — | — |
| 7l | — | — | — |
| 7m | — | — | — |
| 7n | — | — | — |
| 7o | — | — | + |
| 7p | — | — | — |
| 7q | — | — | — |
| 7r | — | — | — |
| 7s | — | — | — |
| 7t | — | — | — |
| 7u | — | — | — |
| 7v | + | — | — |
| 7w | + | + | + |
| ck | — | — | — |
| Ampicillin | 24++ | 26+++ | 21++ |
a) The data in the Table is the average of three repetitions. “—” means no inhibitory effect, “ + ” means visible, “++” means clear, “+++” means transparent, “number” means inhibition zones values.
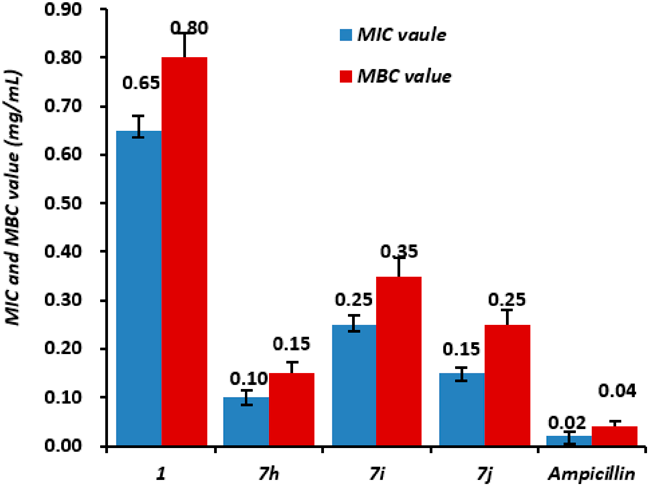
Because of obvious antibacterial activity, the 7g, 7h and 7i were further assessed minimum inhibit concentration (MIC) against Psa. Their MIC values were 0.10, 0.25 and 0.15 mg/mL, respectively. Whereas the MIC values of DHEA was 0.65 mg/mL (Fig. 2). Compounds 7g–i performed 2.6 to 6.5 times the antibacterial activity of DHEA. In addition, the minimum bactericidal concentration (MBC) values of 7g, 7h and 7i were 0.15, 0.35 and 0.25 mg/mL, respectively. Compared with MIC values, bioactive compounds could be classified into bacteriostatic (MIC < MBC) and bactericide agents (MIC = MBC). According to these parameters, compounds 7g, 7h and 7i could be considered as promising bacteriostatic agents against Psa.
As we knew, DHEA was an endogenous steroidal hormone and had pharmacological activities in many ways.32,33) such as regulating diabetes, anticancer, neuroprotective properties and boosting immune function.34–37) In our experiment, the DHEA was regarded as starting materials to yield various ester derivatives, which were rarely applied in agriculture to evaluate biological activity against pest and bacteria.
Additionally, in our previous research, it could been easily found that periplocosides mainly consist of pregn-5-ene-3β,17α,20(S)-triol and various sugar units.38) Apart from it, the research of its mechanism against aimed pest has been confirmed that periplocosides destructed midgut of insect, making content flow out, and finally causing insect to death.16–19) But the specific target part remains unclear. In this study, we found that ester derivatives had more significant insecticidal activity than pregn-5-ene-3β,17α,20(S)-triol. This let us realize derivative part was particularly important for the biological activity of these compounds. So we speculate that sugar units of periplocosides might increase the penetration of periplocosides into the midgut of pests or sugar units was the target of mechanism. It still needed to be further confirmed.
In our experiment, compounds 7g and 7h were not only regarded as an insecticide but also as a bacteriostatic agent. Combined the result analysis, we could realize that fluorine atom on benzoyl played an important role in biological activity. The mechanism might be fluorine atom formed hydrogen bond with target protein to strengthen effect of compound. However, fluorine atom compound 7i was too far with target protein to form hydrogen bond, which made 7i have no potent insecticidal activity. These also needed to be further studied.
The DHEA (1) (5.0 g, 17.34 mmol) was dissolved in dry N,N-dimethylformamide (DMF) (12 mL). Then imidazole (3.54 g, 52.02 mmol) and TBSCl (3.91 g, 26.01 mmol) were added to solution at 0 °C, and the mixture was continuously stirred for 1h. When the reaction was finished, it was quenched with saturated NaCl solution. The aqueous layer was extracted with EtOAc (30 mL), The combined organic layer was washed with saturated aqueous NaHCO3 (3 × 60 mL), dried with anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc: petroleum ether = 1 : 10) to provide pure 2 (91% yield) as a white solid.36) 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.34 (dt, J = 5.1, 1.8 Hz, 1H), 3.51–3.45 (m, 1H), 2.45 (ddd, J = 19.2, 9.0, 1.0 Hz, 1H), 2.27 (ddd, J = 13.5, 10.8, 2.5 Hz, 1H), 2.21–2.17 (m, 1H), 2.12–2.04 (m, 2H), 1.97–1.91 (m, 1H), 1.86–1.80 (m, 2H), 1.74–1.62 (m, 4H), 1.60–1.43 (m, 5H), 1.30–1.24(m, 2H), 1.02 (s, 3H), 0.89–0.88 (m, 12H), 0.05 (s, 6H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 210.57, 141.85, 120.42, 72.48, 51.85, 50.37, 47.58, 42.82, 37.35, 36.76, 35.87, 32.05, 31.51, 31.50, 30.86, 25.95, 21.91, 20.39, 19.48, 18.27, 13.57, −4.56. HRMS (electrospray ionization (ESI)): Calcd for C25H42O2Si ([M + H]+), 402.3063. Found, 402.3071. m.p. 149–151 °C; [α]20D = 14.5 (c 1 mg/mL, CHCl3).
Preparation of compound 3. A stirred solution of t-BuOK (1.02 g, 9.12 mmol) and C2H5Ph3PBr (3.37 g, 9.12 mmol) in dry tetrahydrofuran (THF) (12 mL) was stirred at −10 °C for 1h and kept for 3h at room temperature (r.t.). Subsequently, 2 (0.92 g, 2.28 mmol) was added and stirred overnight. After quenching the reaction with saturated NH4Cl, the mixture was extracted with ethyl acetate (30 mL). The combined organic layer was washed with 1 N HCl (3 × 60 mL), dried with anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc: petroleum ether = 1 : 50) to provide pure 3 (85% yield) as a white solid.37) 1H-NMR (500 MHz, CDCl3): δ(ppm) 1H-NMR (500 MHz, CDCl3) δ: 5.11 (q, J = 7.0 Hz, 1H), 3.55 (tt, J = 10.2, 4.6 Hz, 1H), 2.38–2.32 (m, 1H), 2.24 (dt, J = 12.4, 3.1 Hz, 1H), 2.19–2.11 (m, 1H), 1.72–1.57 (m, 9H), 1.53–1.31 (m, 6H), 1.29–1.23 (m, 2H), 1.20–1.04 (m, 2H), 0.88 (s, 9H), 0.86 (s, 3H), 0.81 (s, 3H), 0.05 (s, 6H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 13C-NMR (125 MHz, CDCl3) δ: 150.53, 113.18, 72.21, 56.31, 54.53, 45.04, 44.42, 38.72, 37.30, 37.19, 35.60, 35.10, 32.02, 31.99, 31.46, 28.78, 25.99, 24.42, 21.45, 18.30, 16.91, 13.12, 12.37, −4.52. HRMS (ESI): Calcd for C27H46OSi ([M + H]+), 414.3327. Found, 414.3329. m.p. 150–152 °C; [α]20D = −35.5 (c 1 mg/mL, CHCl3).
Preparation of compound 4. Adding CH3SO2NH2 (3.44 g, 36.23 mmol) and a solution of 3 (5.0 g, 12.13 mmol) in CH2Cl2 (40 mL) to a stirred solution of AD-mix-β (18.6 g) in t-BuOH/H2O (80 mL/80 mL) at 0 °C, after which the solution was kept at 0 °C for 2 d, then reacted at r.t. for another 3 d. Solid Na2SO3 was added and stirred for 1 h at r.t. After extracting the solution with EtOAc and washing the organic layer with 2 N KOH and 1 N HCl respectively, the residue was dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc: petroleum ether = 1 : 10) to provide pure 4 (62% yield) as a white solid.35) 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.32–5.30 (m, 1H), 3.82 (t, J = 5 Hz, 1H), 3.45–3.51 (m, 1H), 2.50 (d, J = 15 Hz, 1H), 2.18–2.14 (m, 1H), 2.06–1.95 (m, 2H), 1.89 (d, J = 5 Hz, 1H), 1.82–1.66 (m, 6H), 1.64–1.42 (m, 7H), 1.23–1.14 (m, 4H), 1.04–0.98 (m, 4H), 0.88 (s, 9H), 0.73 (s, 3H), 0.05 (s, 6H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 141.83, 121.04, 85.79, 72.57, 72.35, 51.44, 49.74, 45.69, 42.81, 37.70, 37.39, 36.58, 33.30, 31.93, 31.90, 31.86, 31.83, 31.11, 25.96, 23.57, 20.53, 19.44, 18.61, 14.02. HRMS (ESI): Calcd for C27H48O3Si ([M + H]+), 448.3434. Found, 448.3451. m.p. 196–198 °C; [α]20D = −13.5 (c 1 mg/mL, CHCl3).
Preparation of compound 5. To a stirred solution of 4 (50 mg, 0.11 mmol) in THF (5 mL), tetrabutylammonium fluoride (TBAF)·3H2O (119 mg, 0.38 mmol) was added at r.t. and stirred overnight. The reaction mixture was diluted and extracted with CH2Cl2 (30 mL). The combined organic layer was washed with 1N HCl (3 × 10 mL), dried with anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc: petroleum ether = 1 : 20) to provide pure 5 (87% yield) as a white solid.26) 1H-NMR (500 MHz, CD3OD) δ: 5.27 (dd, J = 5.2, 2.6 Hz, 1H), 4.57 (d, J = 4.5 Hz, 1H), 4.11 (d, J = 6.5 Hz, 1H), 3.59 (p, J = 6.3 Hz, 1H), 3.45 (s, 1H), 3.25 (tq, J = 9.6, 4.8, 4.3 Hz, 1H), 2.50–2.49 (m, 2H), 2.18–2.05 (m, 2H), 1.95–1.87 (m, 2H), 1.78–1.61 (m, 4H), 1.59–1.47 (m, 4H), 1.36 (ddt, J = 28.3, 15.1, 8.4 Hz, 4H), 1.08–0.99 (m, 4H), 0.94 (s, 3H), 0.83 (td, J = 11.4, 4.3 Hz, 1H), 0.65 (s, 3H). 13C-NMR (125 MHz, CD3OD) δ: 141.75, 120.94, 84.97, 70.74, 70.47, 51.27, 50.10, 45.48, 42.73, 37.46, 37.37, 36.58, 32.13, 31.95, 31.93, 31.21, 23.69, 20.66, 19.65, 19.32, 14.50. HRMS (ESI): Calcd for C21H34O3 ([M + H]+), 335.2534. Found, 335.2595. m.p. 268–270 °C; [α]20D = −14.5 (c 1 mg/mL, CHCl3).
Preparation of compound 6a–v. Adding DMAP (2.44 mg, 0.02 mmol), Et3N (38.92 µL, 0.28 mmol) and various acid chlorides (0.11 mmol) to a stirred solution of 4 (50 mg, 0.11 mmol) at 0 °C. After the reaction was stirred for 1h, it was kept r.t. at overnight. The reaction mixture was diluted and extracted with CH2Cl2 (30 mL). The combined organic layer was washed with 1N HCl (3 × 10 mL), dried with anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc: petroleum ether = 1 : 20) to provide pure 6a–v (75–81% yield) as a white solid.27)
Preparation of compound 7a–v. To a stirred solution of 6a–v (60 mg, 0.18 mmol) in THF (5 mL), TBAF·3H2O (143 mg, 0.45 mmol) was added at r.t. and stirred overnight. The reaction process was checked by TLC analysis. Then the reaction mixture was extracted with CH2Cl2 (30 mL). The combined organic layer was washed with 1N HCl (3 × 10 mL), dried with anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc: petroleum ether = 1 : 20) to provide pure 7a–v (79–89% yield) as a white solid.39,40)
Data for 7a: Yield: 84%, white solid, m.p. 122–124 °C; [α]20D = −26.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.11 (q, J = 6.3 Hz, 1H), 3.59 (tt, J = 10.6, 4.8 Hz, 1H), 2.32 (q, J = 7.6 Hz, 2H), 1.88 (ddd, J = 14.6, 11.6, 3.0 Hz, 1H), 1.79 (dd, J = 11.8, 7.4 Hz, 2H), 1.69 (tt, J = 15.7, 3.7 Hz, 4H), 1.60–1.46 (m, 5H), 1.43–1.35 (m, 2H), 1.31–1.24 (m, 4H), 1.21 (d, J = 6.3 Hz, 3H), 1.14 (t, J = 7.6 Hz, 3H), 1.12–1.06 (m, 1H), 0.96 (tt, J = 12.2, 4.6 Hz, 2H), 0.80 (s, 3H), 0.76 (s, 3H), 0.68 (ddd, J = 12.4, 10.4, 4.0 Hz, 1H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 173.58, 140.72, 121.55, 84.80, 75.58, 71.75, 60.44, 51.16, 49.63, 45.96, 42.28, 37.87, 37.25, 36.48, 31.88, 31.65, 31.06, 27.97, 23.47, 20.57, 19.41, 15.62, 14.44, 9.34. HRMS (ESI): Calcd for C24H38O4 ([M + H]+), 391.2840. Found, 391.2842.
Data for 7b: Yield: 79%, white solid; m.p. 175–177 °C; [α]20D = −38.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.35–5.34 (m, 1H), 5.11 (q, J = 6.3 Hz, 1H), 3.53 (ddt, J = 16.0, 11.1, 4.6 Hz, 1H), 2.32–2.20 (m, 2H), 2.06 (s, 3H), 1.99 (ddt, J = 16.2, 5.4, 2.8 Hz, 1H), 1.91 (ddd, J = 14.6, 11.5, 2.9 Hz, 1H), 1.83 (td, J = 12.0, 10.9, 3.8 Hz, 2H), 1.79–1.68 (m, 3H), 1.65–1.39 (m, 11H), 1.27–1.21 (m, 4H), 1.17 (dd, J = 12.0, 6.1 Hz, 1H), 1.08 (td, J = 14.6, 13.8, 4.0 Hz, 1H), 1.01 (s, 4H), 1.00–0.94 (m, 1H), 0.78 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 170.23, 140.72, 121.53, 84.92, 75.50, 71.74, 51.14, 49.64, 45.98, 42.29, 38.94, 38.06, 37.25, 36.49, 31.91, 31.87, 31.65, 31.05, 27.21, 23.51, 20.56, 19.40, 15.43, 14.40. HRMS (ESI): Calcd for C27H43O4 ([M + H]+), 405.2535. Found, 405.2586.
Data for 7c: Yield: 85%, white solid, m.p. 145–147 °C; [α]20D = −19.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.35 (dt, J = 4.8, 2.0 Hz, 1H), 5.07 (q, J = 6.3 Hz, 1H), 3.52 (tt, J = 11.0, 4.6 Hz, 1H), 2.32–2.20 (m, 2H), 1.98 (dtd, J = 17.5, 5.2, 2.6 Hz, 1H), 1.88–1.81 (m, 3H), 1.80–1.68 (m, 3H), 1.64–1.42 (m, 11H), 1.22–1.20 (m,12H), 1.08 (td, J = 14.6, 13.8, 4.0 Hz, 1H), 1.01 (s, 3H), 0.78 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 177.32, 140.75, 121.52, 84.92, 75.50, 71.74, 51.14, 49.64, 45.98, 42.29, 38.94, 38.06, 37.25, 36.49, 31.91, 31.87, 31.65, 31.05, 27.21, 23.51, 20.56, 19.40, 15.43, 14.40. HRMS (ESI): Calcd for C26H42O4 ([M + H]+), 419.3105. Found, 419.3101.
Data for 7d: Yield: 87%, white solid; m.p. 121–123 °C; [α]20D = −37.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.36–5.33 (m, 1H), 5.11 (q, J = 6.3 Hz, 1H), 3.52 (tt, J = 11.2, 4.6 Hz, 1H), 2.32–2.27 (m, 3H), 2.23 (tq, J = 13.4, 2.8 Hz, 1H), 1.98 (dtd, J = 17.3, 5.2, 2.6 Hz, 1H), 1.90 (ddd, J = 14.6, 11.5, 3.0 Hz, 1H), 1.86–1.80 (m, 2H), 1.78 (d, J = 4.0 Hz, 1H), 1.72 (ddd, J = 9.7, 7.8, 2.8 Hz, 1H), 1.69–1.44 (m, 11H), 1.30 (tq, J = 6.5, 4.2, 2.5 Hz, 4H), 1.22 (d, J = 6.3 Hz, 3H), 1.16 (dd, J = 12.0, 6.1 Hz, 1H), 1.12–1.05 (m, 1H), 1.00 (s, 3H), 0.89 (t, J = 6.8 Hz, 3H), 0.78 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 172.93, 140.74, 121.52, 84.77, 75.51, 71.73, 51.16, 49.64, 45.97, 42.28, 37.90, 37.26, 36.48, 34.67, 31.88, 31.64, 31.32, 31.07, 24.83, 23.48, 22.33, 20.57, 19.40, 15.64, 14.42, 13.93. HRMS (ESI): Calcd for C27H44O4 ([M + H]+), 433.3173. Found, 433.3161.
Data for 7e: Yield: 83%, white solid; m.p. 121–123 °C; [α]20D = −33.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 5.34 (dd, J = 5.1, 2.4 Hz, 1H), 5.11 (q, J = 6.3 Hz, 1H), 3.52 (tt, J = 11.1, 4.5 Hz, 1H), 2.31–2.27 (m, 3H), 2.22 (ddd, J = 13.4, 10.9, 2.7 Hz, 1H), 1.98 (dtd, J = 17.4, 5.2, 2.5 Hz, 1H), 1.89 (ddd, J = 14.6, 11.5, 3.0 Hz, 1H), 1.82 (ddt, J = 11.8, 8.6, 3.6 Hz, 1H), 1.72 (dddd, J = 24.1, 14.5, 7.3, 3.9 Hz, 5H), 1.65–1.41 (m, 9H), 1.29 (dt, J = 7.3, 4.9 Hz, 5H), 1.22 (d, J = 6.3 Hz, 3H), 1.16 (dq, J = 12.3, 6.1 Hz, 1H), 1.07 (td, J = 14.6, 13.5, 3.6 Hz, 1H), 1.02–0.95 (m, 4H), 0.92–0.83 (m, 3H), 0.78 (s, 3H).13C-NMR (125 MHz, CDCl3): δ(ppm) 172.95, 141.73, 121.52, 84.77, 75.51, 7172, 51.16, 49.64, 45.95, 42.27, 37.90, 37.25, 36.43, 34.72, 31.88, 31.63, 31.45, 31.07, 28.84, 25.12, 23.47 22.50, 20.56, 19.43, 15.64, 14.24, 14.42, 14.04. HRMS (ESI): Calcd for C28H45O4 ([M + H]+), 447.3444. Found, 447.3323.
Data for 7f: Yield: 83%, white solid; m.p. 196–198 °C; [α]20D = −22.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 1H-NMR (500 MHz, CDCl3) δ: 8.02 (dd, J = 8.3, 1.4 Hz, 2H), 7.59–7.54 (m, 1H), 7.45 (t, J = 7.8 Hz, 2H), 5.39–5.34 (m, 1H), 3.54 (tt, J = 11.2, 4.7 Hz, 1H), 2.33–2.20 (m, 2H), 2.07–1.94 (m, 2H), 1.84 (dtd, J = 16.7, 9.4, 8.7, 4.0 Hz, 4H), 1.72–1.50 (m, 10H), 1.37 (d, J = 6.4 Hz, 3H), 1.20 (td, J = 11.9, 5.3 Hz, 1H), 1.13–1.05 (m, 1H), 1.03 (s, 3H), 0.93–0.88 (m, 1H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 165.74, 140.74, 133.00, 130.55, 129.55, 128.60, 128.44, 123.73, 121.54, 85.06, 76.50, 71.76, 51.18, 49.67, 46.17, 42.30, 37.95, 37.27, 36.50, 31.91, 31.84, 31.67, 31.11, 23.48, 20.61, 19.42, 15.70, 14.57. HRMS (ESI): Calcd for C28H38O4 ([M + H]+), 439.2880. Found, 439.2843.
Data for 7g: Yield: 85%, white solid; m.p. 187–189 °C; [α]20D = −5.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.84 (dd, J = 8.1, 1.5 Hz, 1H), 7.40 (td, J = 7.5, 1.4 Hz, 1H), 7.26–7.23 (m, 2H), 5.40–5.34 (m, 2H), 3.53 (tt, J = 11.2, 4.7 Hz, 1H), 2.33–2.19 (m, 2H), 2.09–1.96 (m, 2H), 1.88–1.79 (m, 3H), 1.82–1.79 (m, 1H), 1.73 (ddd, J = 12.1, 7.3, 2.7 Hz, 1H), 1.67–1.58 (m, 7H), 1.57–1.46 (m, 1H), 1.37 (d, J = 6.3 Hz, 3H), 1.20 (dd, J = 12.1, 5.9 Hz, 1H), 1.09 (td, J = 14.5, 13.8, 4.0 Hz, 1H), 1.02 (s, 3H), 0.86 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.69, 163.56, 161.59, 132.75, 130.06, 125.28, 120.04, 116.46, 84.91, 77.08, 71.29, 53.84, 50.87, 46.51, 44.86, 38.18, 37.80, 37.03, 35.45, 32.08, 31.51, 31.33, 28.68, 23.32, 20.77, 15.61, 14.85, 12.32. HRMS (ESI): Calcd for C28H37FO4 ([M + H]+), 457.2784. Found, 457.2748.
Data for 7h: Yield: 87%, white solid, m.p. 188–190 °C; [α]20D = −7.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.82 (dd, J = 7.7, 1.7 Hz, 1H), 7.50–7.40 (m, 2H), 7.36–7.30 (m, 1H), 5.40 (q, J = 6.3 Hz, 1H), 5.35 (dd, J = 5.1, 2.3 Hz, 1H), 3.54 (tt, J = 11.2, 4.6 Hz, 1H), 2.34–2.19 (m, 2H), 2.10–1.94 (m, 2H), 1.88–1.80 (m, 5H), 1.79–1.68 (m, 3H), 1.67–1.57 (m, 4H), 1.56–1.45 (m, 3H), 1.40 (d, J = 6.3 Hz, 3H), 1.21 (dq, J = 11.9, 6.5 Hz, 1H), 1.09 (td, J = 14.5, 13.8, 4.0 Hz, 1H), 1.02 (s, 3H), 0.85 (s, 3H).13C-NMR (125 MHz, CDCl3): δ(ppm) 164.83, 140.74, 132.12, 132.05, 125.78, 121.51, 115.68, 85.05, 76.64, 71.75, 51.16, 49.66, 46.22, 42.29, 37.85, 37.27, 36.50, 31.90, 31.84, 31.66, 31.12, 29.73, 20.60, 19.41, 15.69, 14.60.
Data for 7i: Yield: 89%, white solid; m.p. 193–195 °C; [α]20D = −10.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) δ: 8.07–7.96 (m, 2H), 7.11 (t, J = 8.6 Hz, 2H), 5.35 (t, J = 6.2 Hz, 2H), 2.27 (dddd, J = 26.9, 13.1, 7.8, 2.5 Hz, 2H), 2.01 (ddd, J = 16.8, 12.6, 3.9 Hz, 2H), 1.91–1.71 (m, 5H), 1.66–1.58 (m, 7H), 1.56–1.46 (m, 3H), 1.36 (d, J = 6.3 Hz, 3H), 1.19 (dd, J = 12.1, 6.0 Hz, 1H), 1.10 (td, J = 14.5, 13.8, 4.0 Hz, 1H), 1.02 (s, 3H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.83, 140.74, 132.12, 132.05, 126.78, 121.51, 115.68, 115.60, 85.05, 76.64, 71.75, 51.16, 49.66, 46.22, 42.29, 37.85, 37.27, 36.50, 31.90, 31.66, 31.12, 29.73, 23.46, 20.60, 19.41, 15.68, 14.60.
Data for 7j: Yield: 84%, white solid; m.p. 181–183 °C; [α]20D = −29.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.98 (t, J = 1.9 Hz, 1H), 7.91 (dd, J = 7.8, 1.3 Hz, 1H), 7.53 (ddd, J = 8.0, 2.2, 1.1 Hz, 1H), 7.39 (t, J = 7.9 Hz, 1H), 5.37 (q, J = 6.1 Hz, 2H), 3.54 (tt, J = 11.0, 4.6 Hz, 1H), 2.33–2.21 (m, 2H), 2.04–1.96 (m, 3H), 1.89–1.82 (m, 3H), 1.81–1.69 (m, 2H), 1.66–1.57 (m, 4H), 1.51 (ddt, J = 21.2, 13.0, 5.0 Hz, 3H), 1.37 (d, J = 6.3 Hz, 3H), 1.19 (qd, J = 12.1, 6.0 Hz, 1H), 1.10 (td, J = 14.5, 13.7, 4.0 Hz, 1H), 1.02 (s, 3H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.61, 140.73, 134.61, 133.03, 132.30, 129.77, 129.59, 127.71, 121.52, 85.00, 76.64, 71.77, 51.15, 49.65, 46.25, 42.27, 37.83, 37.27, 36.49, 31.90, 31.84, 31.64, 31.11, 23.45, 20.60, 19.41, 15.66, 14.61. HRMS (ESI): Calcd for C28H36ClO4 ([M + H]+), 473.2400. Found, 473.2453.
Data for 7k: Yield: 86%, white solid, m.p. 175–177 °C; [α]20D = −23.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.98 (t, J = 1.9 Hz, 1H), 7.90 (dt, J = 7.8, 1.4 Hz, 1H), 7.54 (ddd, J = 8.0, 2.2, 1.1 Hz, 1H), 7.39 (t, J = 7.9 Hz, 1H), 5.39–5.33 (m, 2H), 3.53 (tt, J = 11.0, 4.7 Hz, 1H), 2.36–2.16 (m, 2H), 2.00 (dddd, J = 16.7, 7.9, 5.8, 2.8 Hz, 2H), 1.88–1.82 (m, 2H), 1.81–1.70 (m, 2H), 1.66–1.59 (m, 7H), 1.55–1.45 (m, 4H), 1.37 (d, J = 6.4 Hz, 3H), 1.20 (tt, J = 12.1, 6.0 Hz, 1H), 1.15–1.06 (m, 1H), 1.02 (s, 3H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.59, 140.74, 134.61, 133.02, 132.31, 129.76, 127.70, 121.50, 84.97, 77.06, 71.75, 51.16, 49.66, 46.25, 42.29, 37.86, 37.27, 36.50, 31.90, 31.66, 31.11, 29.72, 23.46, 20.60, 19.41, 15.66, 14.61.
Data for 7l: Yield: 87%, white solid; m.p. 229–231 °C; [α]20D = −18.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.95 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 8.5 Hz, 2H), 5.34 (q, J = 6.3 Hz, 1H), 3.59 (tt, J = 10.7, 4.8 Hz, 1H), 1.97 (ddd, J = 14.6, 11.5, 2.9 Hz, 1H), 1.81 (td, J = 11.6, 7.2 Hz, 2H), 1.73–1.56 (m, 8H), 1.42 (td, J = 11.5, 11.1, 3.6 Hz, 2H), 1.35 (d, J = 6.3 Hz, 3H), 1.31–1.22 (m, 5H), 1.13 (ddd, J = 15.6, 8.9, 4.4 Hz, 2H), 0.97 (tdd, J = 12.4, 9.0, 4.7 Hz, 2H), 0.82 (s, 6H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.95, 139.46, 130.94, 129.00, 128.78, 84.96, 76.86, 71.31, 53.85, 50.89, 46.50, 44.85, 38.19, 37.80, 37.03, 35.50, 35.42, 32.08, 31.52, 31.34, 28.68, 23.32, 20.77, 15.63, 14.85, 12.33.
Data for 7m: Yield: 88%, white solid; m.p. 161–163 °C; [α]20D = −30.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.78 (dd, J = 7.6, 2.0 Hz, 1H), 7.71–7.61 (m, 1H), 7.35 (dtd, J = 21.8, 7.5, 1.8 Hz, 2H), 5.43–5.33 (m, 2H), 3.54 (tt, J = 10.8, 4.7 Hz, 1H), 2.35–2.20 (m, 3H), 2.11–1.95 (m, 2H), 1.83 (ddd, J = 18.7, 10.2, 4.2 Hz, 3H), 1.78–1.68 (m, 2H), 1.66–1.57 (m, 3H), 1.50 (dtd, J = 20.3, 12.4, 11.6, 4.8 Hz, 3H), 1.40 (d, J = 6.4 Hz, 3H), 1.25–1.17 (m, 1H), 1.12–1.06 (m, 1H), 1.02 (s, 3H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 165.47, 140.68, 134.35, 132.64, 131.50, 127.32, 121.59, 121.25, 84.77, 77.98, 71.80, 51.19, 49.64, 46.14, 42.23, 38.27, 37.24, 36.49, 31.89, 31.61, 31.07, 23.61, 20.58, 19.42, 15.70, 14.43. HRMS (ESI): Calcd for C28H37BrO4 ([M + H]+), 517.1940. Found, 517.1948.
Data for 7n: Yield: 85%, white solid, m.p. 151–153 °C; [α]20D = − 41.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 8.13 (t, J = 1.9 Hz, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.69 (dd, J = 8.1, 2.0 Hz, 1H), 7.33 (t, J = 7.9 Hz, 1H), 5.39–5.34 (m, 2H), 3.54 (tt, J = 10.7, 4.6 Hz, 1H), 2.35–2.20 (m, 2H), 2.00 (ddd, J = 14.8, 10.0, 3.7 Hz, 2H), 1.88–1.70 (m, 7H), 1.62 (ddd, J = 16.5, 7.8, 3.8 Hz, 5H), 1.56–1.42 (m, 3H), 1.37 (d, J = 6.3 Hz, 3H), 1.19 (dd, J = 12.2, 6.0 Hz, 1H), 1.09 (td, J = 14.6, 13.8, 3.7 Hz, 1H), 1.02 (s, 3H), 0.84 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.48, 140.72, 135.97, 132.50, 132.47, 130.03, 128.17, 122.54, 121.53, 84.98, 77.10, 71.76, 51.15, 49.63, 46.24, 42.27, 37.85, 37.26, 36.49, 31.90, 31.88, 31.64, 31.10, 23.46, 20.59, 19.43, 15.68, 14.61.
Data for 7o: Yield: 86%, white solid, m.p. 227–229 °C; [α]20D = − 40.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3):δ(ppm) 7.88 (d, J = 8.5 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H), 5.36 (dd, J = 7.7, 5.3 Hz, 2H), 3.53 (tt, J = 11.2, 4.6 Hz, 1H), 2.33–2.21 (m, 2H), 2.04–1.95 (m, 2H), 1.85 (dtd, J = 12.7, 7.0, 2.4 Hz, 3H), 1.79–1.69 (m, 4H), 1.61 (ddd, J = 13.0, 9.7, 6.2 Hz, 4H), 1.55–1.45 (m, 3H), 1.36 (d, J = 6.4 Hz, 3H), 1.19 (qd, J = 11.9, 5.8 Hz, 1H), 1.09 (td, J = 14.5, 13.7, 3.9 Hz, 1H), 1.02 (s, 3H), 0.84 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm)165.07, 140.74, 131.78, 131.08, 129.44, 128.12, 121.50, 85.02, 76.82, 71.75, 51.16, 49.67, 46.24, 42.28, 37.82, 37.27, 36.50, 31.90, 31.65, 31.12, 23.45, 20.60, 19.41, 15.67, 14.60.
Data for 7p: Yield: 83%, white solid; m.p. 184–186 °C; [α]20D = −33.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.81 (d, J = 7.6 Hz, 2H), 7.36 (d, J = 7.5 Hz, 1H), 7.31 (t, J = 7.6 Hz, 1H), 5.34 (q, J = 6.3 Hz, 1H), 3.59 (tt, J = 10.7, 4.8 Hz, 1H), 2.39 (s, 3H), 1.98 (ddd, J = 14.5, 11.5, 2.7 Hz, 1H), 1.81 (qd, J = 11.4, 10.9, 5.7 Hz, 2H), 1.75–1.64 (m, 6H), 1.62–1.52 (m, 3H), 1.41 (td, J = 11.1, 2.7 Hz, 2H), 1.34 (d, J = 6.4 Hz, 3H), 1.30–1.24 (m, 3H), 1.20–1.08 (m, 2H), 0.97 (tdd, J = 12.4, 9.0, 4.7 Hz, 2H), 0.81 (d, J = 2.5 Hz, 6H), 0.70 (ddd, J = 12.5, 10.5, 4.1 Hz, 1H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 165.93, 138.23, 133.74, 130.51, 130.06, 128.32, 126.70, 85.01, 76.44, 71.29, 53.87, 50.91, 46.43, 44.87, 38.19, 37.87, 37.04, 35.50, 35.43, 32.10, 31.52, 31.34, 28.70, 23.35, 21.32, 20.78, 15.67, 14.81, 12.33. HRMS (ESI): Calcd for C29H40O4 ([M + H]+), 453.2912. Found, 453.2953.
Data for 7q: Yield: 85%, white solid, m.p. 188–190 °C; [α]20D = −6.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.91 (d, J = 8.2 Hz, 2H), 7.24 (d, J = 8.0 Hz, 2H), 5.35 (q, J = 6.4 Hz, 2H), 3.53 (tt, J = 11.1, 4.6 Hz, 1H), 2.41 (s, 3H), 2.33–2.20 (m, 2H), 2.05–1.96 (m, 2H), 1.85 (ddd, J = 11.4, 6.8, 3.3 Hz, 3H), 1.81–1.76 (m, 1H), 1.72 (dddd, J = 12.2, 10.0, 7.4, 2.9 Hz, 1H), 1.62 (dddt, J = 15.1, 9.1, 6.8, 2.7 Hz, 6H), 1.55–1.46 (m, 3H), 1.36 (d, J = 6.4 Hz, 3H), 1.19 (tt, J = 12.1, 6.1 Hz, 1H), 1.10 (td, J = 14.5, 13.8, 4.0 Hz, 1H), 1.02 (s, 3H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm)165.75, 157.17, 140.72, 139.84, 131.93, 131.72, 130.24, 128.56, 121.56, 84.87, 74.49, 71.74, 51.21, 49.67, 45.96, 42.30, 37.94, 37.26, 36.49, 31.91, 38.84, 31.66, 31.10, 27.45, 23.48, 22.53, 20.97, 20.59, 19.40, 15.74, 14.42.
Data for 7r: Yield: 83%, white solid; m.p. 222–224 °C; [α]20D = −15.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) δ: 7.99–7.93 (m, 2H), 7.29 (d, J = 8.0 Hz, 2H), 5.40 (q, J = 6.4 Hz, 2H), 3.58 (tt, J = 11.1, 4.6 Hz, 1H), 2.46 (s, 3H), 2.39–2.22 (m, 2H), 2.14–1.98 (m, 2H), 1.90 (ddd, J = 11.4, 6.8, 3.3 Hz, 3H), 1.85–1.73 (m, 2H), 1.66 (dtt, J = 12.2, 6.8, 3.5 Hz, 5H), 1.59–1.50 (m, 3H), 1.41 (d, J = 6.3 Hz, 3H), 1.23 (dd, J = 12.1, 5.9 Hz, 1H), 1.14 (td, J = 14.5, 13.8, 4.0 Hz, 1H), 1.07 (s, 3H), 0.89 (s, 3H).13C-NMR (125 MHz, CDCl3): δ(ppm) 165.79, 143.70, 140.74, 129.58, 129.14, 127.80, 121.54, 85.09, 76.23, 71.76, 51.19, 49.68, 46.14, 42.30, 37.94, 37.27, 36.50, 31.91, 31.67, 31.11, 29.72, 23.47, 21.67, 20.60, 19.41, 15.71, 14.55.
Data for 7s: Yield: 86%, white solid, m.p. 161–163 °C; [α]20D = −47.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.60 (dt, J = 7.7, 1.2 Hz, 1H), 7.53 (dd, J = 2.6, 1.5 Hz, 1H), 7.33 (dd, J = 8.8, 7.1 Hz, 1H), 7.08 (dd, J = 8.3, 2.7 Hz, 1H), 5.37–5.32 (m, 2H), 3.83 (d, J = 1.8 Hz, 3H), 3.52 (ddt, J = 15.9, 11.0, 4.6 Hz, 1H), 2.31–2.19 (m, 2H), 2.07–1.94 (m, 3H), 1.82 (ddt, J = 15.2, 10.7, 4.1 Hz, 4H), 1.71 (dddd, J = 11.7, 9.5, 7.4, 2.5 Hz, 1H), 1.66–1.56 (m, 4H), 1.49 (tdd, J = 18.8, 8.5, 6.2 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.17 (qd, J = 11.8, 11.4, 5.5 Hz, 1H), 1.11–1.04 (m, 1H), 1.00 (s, 3H), 0.83 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 165.66, 159.59, 140.77, 131.86, 129.46, 121.88, 121.48, 119.18, 114.39, 85.03, 76.63, 71.70, 68.53, 55.48, 51.15, 49.67, 46.17, 42.26, 37.86, 37.27, 36.49, 31.90, 31.61, 31.11, 23.47, 20.60, 19.41, 15.68, 14.58. HRMS (ESI): Calcd for C29H40O5 ([M + H]+), 469.2930. Found, 469.2950.
Data for 7t: Yield: 82%, white solid, m.p. 132–134 °C; [α]20D = −17.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 1H-NMR (500 MHz, Chloroform-d) δ: 7.60 (d, J = 7.7 Hz, 1H), 7.54 (t, J = 2.2 Hz, 1H), 7.35 (t, J = 7.9 Hz, 1H), 7.11 (dd, J = 8.2, 2.7 Hz, 1H), 5.36 (dd, J = 10.0, 5.2 Hz, 1H), 3.85 (s, 3H), 3.54 (tt, J = 11.0, 4.7 Hz, 1H), 2.34–2.21 (m, 2H), 2.00 (tdd, J = 11.0, 8.6, 3.1 Hz, 2H), 1.88–1.82 (m, 3H), 1.78–1.67 (m, 3H), 1.66–1.58 (m, 5H), 1.51 (tdd, J = 17.1, 11.9, 4.8 Hz, 4H), 1.37 (d, J = 6.3 Hz, 3H), 1.24–1.16 (m, 1H), 1.10 (td, J = 14.4, 13.6, 3.7 Hz, 1H), 1.02 (s, 3H), 0.85 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 165.62, 159.61, 140.73, 131.87, 129.47, 124.86, 121.55, 119.20, 114.36, 85.04, 76.64, 71.76, 55.49, 51.17, 49.66, 46.17, 42.29, 37.97, 37.26, 36.50, 31.90, 31.66, 31.10, 23.48, 20.60, 19.42, 15.69, 14.57.
Data for 7u: Yield: 85%, white solid, m.p. 164–166 °C; [α]20D = −9.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 7.97 (d, J = 8.9 Hz, 2H), 6.92 (d, J = 8.8 Hz, 2H), 5.36–5.32 (m, 3H), 3.86 (s, 2H), 3.53 (tt, J = 11.1, 4.7 Hz, 1H), 2.33–2.19 (m, 2H), 2.05–1.92 (m, 4H), 1.88–1.78 (m, 4H), 1.77–1.66 (m, 2H), 1.66–1.57 (m, 4H), 1.50 (dddd, J = 20.4, 14.4, 11.8, 4.6 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.18 (qd, J = 12.0, 5.7 Hz, 1H), 1.09 (td, J = 14.4, 13.7, 3.9 Hz, 1H), 1.02 (s, 3H), 0.84 (s, 3H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 165.49, 163.43, 140.73, 131.58, 122.91, 121.54, 113.68, 85.15, 76.64, 71.76, 55.48, 51.19, 49.68, 46.14, 42.28, 37.90, 37.27, 36.50, 31.91, 31.65, 31.12, 23.47, 20.61, 19.41, 15.75, 14.56.
Data for 7v: Yield: 80%, white solid; m.p. 193–195 °C; [α]20D = −14.5 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, CDCl3): δ(ppm) 8.13 (d, J = 8.0 Hz, 2H), 7.70 (d, J = 8.2 Hz, 2H), 5.38 (q, J = 6.4 Hz, 1H), 3.59 (tt, J = 10.7, 4.8 Hz, 1H), 1.98 (ddd, J = 14.6, 11.5, 2.9 Hz, 1H), 1.81 (td, J = 11.6, 7.2 Hz,2H), 1.73–1.68 (m, 2H), 1.66–1.54 (m, 6H), 1.42 (td, J = 11.2, 3.9 Hz, 2H), 1.36 (d, J = 6.4 Hz, 3H), 1.28 (td, J = 12.3, 10.6, 5.1 Hz, 4H), 1.20–1.09 (m, 2H), 0.97 (tdd, J = 12.7, 10.5, 4.8 Hz, 2H), 0.82 (d, J = 3.9 Hz, 6H), 0.71 (ddd, J = 12.5, 10.5, 4.1 Hz, 1H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 164.62, 140.71, 130.56, 129.97, 125.51, 121.51, 85.03, 77.22, 71.79, 51.15, 49.65, 46.30, 42.25, 37.80, 37.26, 36.49, 31.89, 31.62, 31.12, 23.45, 20.60, 19.41, 15.66, 14.63. HRMS (ESI): Calcd for C29H37F3O4 ([M + H]+), 507.2744. Found, 507.2717.
Data for 7w: Yield: 53%, white solid; m.p. 184–186 °C; [α]20D = −27.0 (c 1 mg/mL, CHCl3); 1H-NMR (500 MHz, Chloroform-d) δ: 10.79 (s, 1H), 7.81 (dd, J = 8.0, 1.8 Hz, 1H), 7.46 (ddd, J = 8.7, 7.2, 1.8 Hz, 1H), 6.99 (dd, J = 8.4, 1.1 Hz, 1H), 6.93–6.83 (m, 1H), 5.39 (q, J = 6.3 Hz, 1H), 3.60 (tt, J = 10.7, 4.7 Hz, 1H), 1.98 (ddd, J = 14.7, 11.6, 2.9 Hz, 1H), 1.81 (dt, J = 11.5, 5.6 Hz, 2H), 1.71 (ddt, J = 16.3, 10.3, 3.2 Hz, 3H), 1.62–1.55 (m, 5H), 1.42 (td, J = 11.2, 4.0 Hz, 2H), 1.37 (d, J = 6.4 Hz, 3H), 1.27 (dd, J = 16.4, 4.2 Hz, 4H), 1.15 (ddd, J = 18.2, 11.1, 5.3 Hz, 2H), 0.98 (dtd, J = 18.0, 9.3, 8.6, 5.0 Hz, 2H), 0.82 (d, J = 1.9 Hz, 6H), 0.71 (ddd, J = 12.6, 10.6, 4.1 Hz, 1H). 13C-NMR (125 MHz, CDCl3): δ(ppm) 169.26, 161.76, 135.77, 129.72, 119.20, 117.74, 112.70, 84.85, 77.21, 71.32, 53.82, 50.88, 46.56, 44.83, 38.18, 37.84, 37.02, 35.50, 35.41, 32.08, 31.52, 31.31, 28.66, 23.32, 20.76, 15.62, 14.85, 12.34. HRMS (ESI): Calcd for C28H39O5 ([M + H]+), 454.2719. Found, 454.2726.
InsectsThe 3rd M. separata were provided by the Pesticide Research Institute (NWAFU). In the laboratory, tested insects were maintained without exposure to any insecticides under controlled conditions (22–26 °C, 70–80% relative humidity and photoperiod of light/dark = 14/10 h cycle). The larvae of M. separata was fed with wheat leaf without any application of insecticides. After selecting the larvae with the same stage, they were raised separately to obtain consistent 3rd instar larvae.
Stomach Poison Bioassays with 3rd M. separataThe leaf disc method was used to evaluate activity of compounds 7a–v against 3rd M. separata. Newly molted 3rd instar larvae were starved for 12 h and then fed with fresh wheat leaf discs (0.5 × 0.5 cm) soaked in 1 mg/mL compound solution (diluted with acetone) for 3 s. PSF was used as a positive control. Blank control group was fed with leaf discs treated with acetone alone. The bioassay was replicated three times for every treatment.19)
Filter Paper MethodAccording to the method described by Moncef’s group,41) the antibacterial activity of compounds 7a–v were evaluated against three kinds of bacteria. The Miller Hinton agar was spread into 200 µL culture suspensions containing microorganisms (1.0 × 106 cfu/mL). Then the filter paper (5 mm in diameter) loaded 20 µL compound solution at 1 mg/mL (diluted with acetone) was put on the agar layer. After 24 h of incubation at 37 °C, the antibacterial activity was evaluated by calculating the region inhibiting growth around the wells. The positive standard and negative control were Ampicillin and acetone, respectively. Each treatment was carried out in triplicate.
MIC and MBC DeterminationThe value of MIC was determined as the lowest concentration of sample inhibiting visible growth.31) To estimate the MIC value, each well of sterile 96-well Microplates had a final volume of 100 µL. The 7g, 7h and 7i were dissolved in acetone, water and Tween 80 (1% (w/v)), followed by two-fold serial dilution in medium LB. Each well of the Microplates included 10 µL of diluted compounds, 80 µL of LB medium and 10 µL of organism suspension (1.0 × 106 cfu/mL), and was incubated for 12 h at 37 °C. The MBC value was defined as the maximum dilution which organisms did not occur. Twenty microliter suspending liquid was removed from each well t and inoculated on the LB medium to confirm the MBC. Surviving organisms number was calculated after incubation at 37 °C for 12 h. Bacteria only treated with Ampicillin sodium in LB medium and 1% Tween 80 respectively, which was used as positive and negative controls. The determination of MIC and MBC values was assessed in triplicate.42)
Statistical AnalysisAll bioassays average results obtained from triplicates with standard errors of the mean (S.E.). The least significant difference test (LSD, p < 0.05) was used to analyze the significant differences on the final mortality rates of tested pest. And the data of insecticidal activities were analyzed by slope of the regression lines, LC50, 95% confidence limits and chi-square values which were calculated by probit analysis. All analysis were calculated with SPSS (version 19.0) software.
Twenty-three compounds derived from pregn-5-ene-3β,17α,20(S)-triol (all of which were novel) were designed and synthesized. All of them were evaluated their insecticidal and bacteriostatic activity. Compounds with highest activity against 3rd M. separata exceeded 3 fold more than PSF. Similarly, the inhibition rate against Pas of the best compound was 6 times better than DHEA.
In the Fig. 3, based on the results of the bioassay against M. separata, SAR analysis showed that benzoyl group with fluorine atomic at ortho- or meta-position had much higher insecticidal activity than with fluorine atomic at para-position. And the substituents of o-chlorobenzoyl and p-chlorobenzoyl could also increase the activity against M. separata than m-chlorobenzoyl. Combining analysis result of antibacterial activity, it could be observed that the fluorine atom belonging to ortho- or pata-positions of benzoyl performed better inhibitory activity than m-fluorobenzoyl. To sum up, compounds 7g and 7h were both salient insectifuges and bacteriostatic agents, which was worthy of being noted and further studied.

All in all, this study established basics for further design and structural modifications of pregn-5-ene-3β,17α,20(S)-triol as potentially insecticidal and bacteriostatic agents in crop protection.
The authors are grateful to Dr. Ahmed A. A. Aioub (Plant Protection Department, Faculty of Agriculture, Zigzagged University, 44511 Zagazig, Egypt) for editing English of the manuscripts. This study was supported financially by the Grant of the National Key Research and Development Program of China (2016YFD0201005) and the Fundamental Research Funds for the Central Universities of China (TGZX2016-37).
The Dr. Tian Li and Yuxiao Hu are mainly responsible for doing experiments. And Pro. Baojun Shi is responsible for writing manuscripts, revising manuscripts and submitting manuscripts. In addition, Pro. Wenjun Wu provided many suggestion and materials for experiments.
The authors declare no conflict of interest.
This article contains supplementary materials.