2023 年 71 巻 3 号 p. 229-233
2023 年 71 巻 3 号 p. 229-233
In vitro evaluation of the physical properties of biopolymer-based hydrogels can help in understanding certain phenomena, such as liquid–liquid phase separation. The formation of bovine serum albumin (BSA) hydrogels was investigated in the pH range of 1.0 to 4.0. Hydrogels were formed in the pH range of 3.0 to 4.0, whereas viscous solutions were formed in the pH range of 1.5 to 2.5. Unexpectedly, formation of BSA hydrogel was observed in extremely acidic condition (pH 1.0). The circular dichroism spectra of BSA solutions were recorded at pH values of 1.0, 2.0, 3.0, and 7.0, and α-helix contents were determined from the ellipticity data at 222 nm. The α-helix content decreased with a decrease in pH, and this decrease was associated with the partial denaturation (F-isoform) and the denaturation (E-isoform) of BSA. However, the α-helix contents at pH 1.0 and 3.0 were similar. BSA hydrogels at pH 1.0 and 3.5 showed similar dynamic viscoelastic properties, further supporting the stereo structural change of BSA from the denatured E-isoform to the partially denatured F-isoform at pH 1.0. The study also focused on measuring viscoelasticity, a fundamental physical property of hydrogels, using traditional rheometer and with minimal sample volume. A highly reproducible procedure for measuring the viscoelastic properties of hydrogels was established using sample volumes of 200 and 350 μL.
Protein-based biopolymers can significantly increase water viscosity. Liquid–liquid phase separation (LLPS) induced by biopolymers has been gaining attention lately as it helps in understanding the link between molecular-level processes and macroscopic phenomena in life.1,2) Hydrogels are water-swollen polymeric materials, and sufficient understanding of the hydrogelation process would help in further understanding of LLPS. In vitro hydrogelation of some proteins, such as collagen (gelatin), lysozyme, β-lactoglobulin, serum albumin, has been reported.3–8) The triple helix structure of collagen is unraveled by heating in water, and the random rewinding of the helix during cooling forms a three-dimensional network structure resulting in a gelatin hydrogel. pH and heat can result in the partial denaturation of globular proteins, such as β-lactoglobulin, and the exposed hydrophobic moieties form aggregates through hydrophobic interactions.4,9) Serum albumin is the most abundant protein in blood, and heat- and pH induced bovine serum albumin (BSA) hydrogel formation has been previously reported.10–12)
Gel viscoelastic properties give important information including gel strength and structure. These properties are determined by rheological measurements using a rheometer. Traditional rheometers require large sample volumes; thus, sample volume reduction is of high importance. For example, during our research on limited quantity and fragile artificial supramolecular gels, measuring viscoelastic properties was challenging.13) Consequently, a method with good reproducibility using small sample volumes (in the order of μL) was established.14–16) In this study, we examined the effect of pH on hydrogel formation of BSA, and hydrogel was formed even in strongly acidic solutions. Moreover, viscoelastic properties of BSA hydrogels were measured and further reduction of sample volume was evaluated.
The hydrogel formation of BSA at different pH values was investigated. It has reported that BSA formed hydrogels in narrow pH range (around pH 3.5) based on the partial denaturation.7) Hydrogelations of 17 wt% BSA solutions from pH 1.0 to 4.0 were investigated at room temperature or 37 °C.
At room temperature, a BSA solution with pH 4.0 remained as a solution for at least 7 d (Fig. 1a). In contrast, a semitransparent hydrogel was formed when the same solution was heated to 37 °C for 60 min (Fig. 1b). However, a BSA solution of pH 3.5 placed at room temperature resulted in a transparent hydrogel after approximately 5 d (Fig. 1c), whereas a hydrogel was obtained in 10 min upon increasing the temperature to 37 °C (Fig. 1d). Similar results were observed at pH 3.0 where the formation of a transparent hydrogel at room temperature required several days (Fig. 1e), while an immediate formation was observed at 37 °C (Fig. 1f). The more acidic BSA solutions (pH 1.5, 2.0, and 2.5) did not result in hydrogels neither with nor without heating at 37 °C (Figs. 1g–l). These results were consistent with previously reported ability of BSA to form hydrogels.11)

(a) pH 4.0 prepared at room temperature (r.t.), (b) pH 4.0 prepared at 37 °C, (c) pH 3.5 prepared at r.t., (d) pH 3.5 prepared at 37 °C, (e) pH 3.0 prepared at r.t., (f) pH 3.0 prepared at 37 °C, (g) pH 2.5 prepared at r.t., (h) pH 2.5 prepared at 37 °C, (i) pH 2.0 prepared at r.t., (j) pH 2.0 prepared at 37 °C, (k) pH 1.5 prepared at r.t., (l) pH 1.5 prepared at 37 °C, (m) pH 1.0 prepared at r.t., (n) pH 1.0 prepared at 37 °C.
At a neutral pH, BSA has a normal heart-like structure (N-isoform), while under acidic conditions it has a partially expanded cigar-like shape (F-isoform)7,17) (Fig. 2). This N–F isoform transition proceeds at pH 4.3, and the association and gelation progress by the hydrophobic interactions between the exposed hydrophobic domains of F-isoform. Below its transition point at pH 2.7, BSA denatures into its fully expanded E-isoform, consequently losing its gelation ability. However, unexpected hydrogel formation was observed in acidic BSA solutions of pH 1.0, where a transparent hydrogel was obtained at room temperature after several hours (Fig. 1m) and spontaneously at 37 °C (Fig. 1n). The reason behind hydrogels formation under acidic condition (pH 1.0) will be discussed later on. BSA hydrogels can be also obtained by thermal denaturation. In fact, spontaneous hydrogel formation was observed upon heating a BSA solution at 80 °C which was then allowed to stand at room temperature (Supplementary Fig. S1a). In contrast, a BSA solution that was not exposed to heat remained as a solution (Supplementary Fig. S1b).

Copyright 2014, American Chemical Society. https://pubs.acs.org/doi/10.1021/bm500883h.
Secondary structure of proteins can be obtained from the circular dichroism (CD) spectra. BSA with a molecular weight of 66.4 kDa is a protein rich in α-helix structure,18,19) which is observed as negative cotton effects at 208 and 222 nm in the CD spectrum. A BSA solution (3.01 × 10−4 mM or 0.02 g/L) was prepared, and the CD spectra were measured after 1 h, 1, 6 and 9 d. The CD spectrum on day 9 at various pH values is shown in Fig. 3a. The CD spectra of BSA were expressed as a molar residue ellipticity (θ) in deg.cm2.dmol−1 and the α-helix contents of BSA in each solution were calculated from θ values at 222 nm according to Experimental.19,20) From the peak at 222 nm it was observed that the θ values at pH 1.0, 2.0, 3.0, and 7.0 were −1.26 × 104, −1.00 × 104, −1.33 × 104, and −1.75 × 104, respectively. The α-helix contents at pH 1.0, 2.0, 3.0, and 7.0 were 34.0, 25.4, 36.1, and 50.1%, respectively, as calculated from the θ values at 222 nm (Fig. 3b). As the acidity of the solution increased, BSA denaturation was faster and the α-helix content tended to decrease except at pH 1.0. Similar trends were also observed in the CD spectra after 1 h, 1, and 6 d.
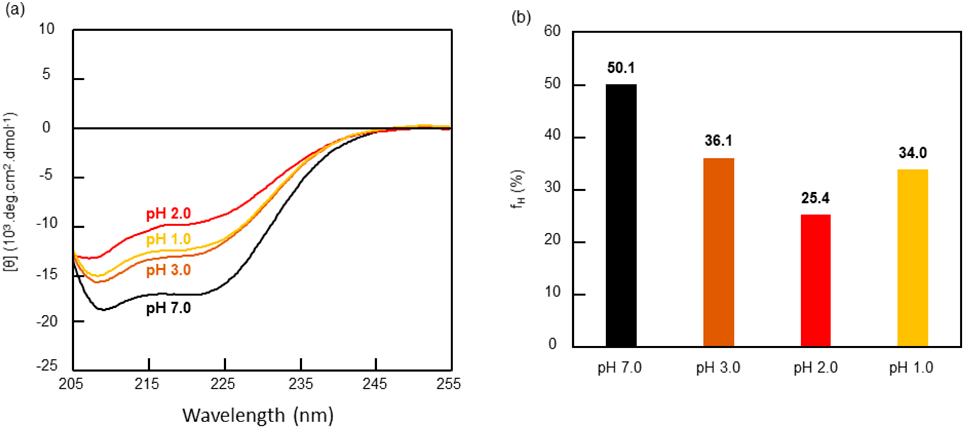
Colors: black, pH 7.0; brown, pH 3.0; red, pH 2.0; and yellow, pH 1.0.
These results are in good agreement with the results that showed hydrogel formation at pH 1.0, 3.0, and 3.5, and solution formation in the pH range 1.5–2.5. BSA is known to reversibly change conformations upon pH variation.21) Under acidic conditions, dramatic conformational changes were observed at pH 4.3 and 2.7.11,22) In near-neutral pH, BSA has a globular heart-shaped structure (N-isoform).17) At pH values lower than 4.3, BSA has a partially elongated cigar-shaped structure (F-isoform), and under extremely acidic conditions (pH lower than 2.7) BSA denatures into a completely expanded structure (E-isoform). The decreases in the α-helix content observed in the CD spectral analysis upon decreasing the pH were in good agreement with these structural transformations. BSA gelation in an aqueous solution, which was not observed at pH 2.0, indicated the high gelation ability of the partially denatured N-isoform of BSA, versus a low ability of the completely denatured E-isoform. To the best of our knowledge, the stereo structure of BSA at pH below 1.0 does not exist. Since the α-helix content of BSA at pH 1.0 was close to that at pH 3.0, the conformation conversion from a fully denatured E-isoform at pH 2.0 to a partially denatured isoform with an α-helix rewind must be assessed. In fact, it was difficult to determine whether a partially denatured isoform at pH 1.0 was an N-isoform or a different partially denatured isoform. However, the E-isoform of BSA at pH 2.0 might be a rewind to the N-isoform at pH 1.0 because of the similar physical characteristics observed in the hydrogels obtained at pH 3.5 and pH 1.0 (vide infra).
The viscoelastic features of the BSA hydrogels formed at different pH were assessed. Unfortunately, measuring these properties for gels with a fragile structure is not easy, especially with the need for large sample volumes in the rheometer. A method for measuring viscoelasticity, even for fragile samples, on a sub-milliliter scale with good reproducibility using a traditional rheometer has been developed.14) The hydrogel (350 μL) was placed in an aluminum cup with a diameter of 14 mm, which was then placed in the rheometer. Viscoelastic properties were measured using a jig with a diameter of 8 mm. This method was applied to measure the viscoelastic features of the BSA hydrogels.
The storage moduli (G′) and loss moduli (G″) values at 1.0 Hz of the BSA hydrogel prepared at pH 4.0 were 60 and 6 Pa, respectively. The G′ and G″ values increased as the frequency increased from 0.1 to 10.0 Hz (Supplementary Fig. S2a), which is a trend typically observed in weak gels.23) The results obtained from the strain sweep measurement, conducted with the physical hydrogel, revealed an elastic response that was typical for physical hydrogels, and the crossover points (G′ = G″) of the hydrogel was observed at a strain of 35% (Supplementary Fig. S2b).
A frequency sweep measurement of the BSA hydrogel prepared at pH 3.5 revealed that both G′ and G″ were almost independent of the frequency from 0.01 to 10 Hz (Fig. 4a) with values of 2630 and 325 Pa, respectively, at 1.0 Hz. A strain sweep measurement of this hydrogel displayed a wide linear region, and the crossover point (G′ = G″) was observed at a strain of approximately 90% (Fig. 4b). A BSA solution at pH 3.0 resulted in a highly viscous gel-like material. The G′ and G″ values of the mixture increased with an increase in frequency from 0.1 to 10.0 Hz, with values of 262 and 173 Pa, respectively, at 1.0 Hz (Fig. 4c). In the strain sweep measurement of the mixture, no change was observed in the values of G′ and G″ until a strain beyond 100% (Fig. 4d). A frequency sweep measurement of the BSA hydrogel with pH 1.0 revealed that both G′ and G″ were nearly independent of the frequency in the range of 0.01 to 10 Hz (Fig. 4e), and the values at 1.0 Hz were 1780 and 398 Pa, respectively. A strain sweep measurement of the hydrogel demonstrated a wide linear region, and the crossover points (G′ = G″) was observed at strain of about 60% (Fig. 4f). The viscoelastic properties of this unexpectedly formed hydrogel were similar to those of the BSA hydrogel obtained at pH 3.5. Comparing these two hydrogels, the BSA hydrogel obtained at pH 1.0 seemed to be slightly fragile with smaller G′ value (1780 vs. 2630 Pa) and crossover point (60 vs. 90%).

Viscoelasticity measurements were performed on smaller sample volumes, and an aluminum cup with a diameter of 10 mm was used. Hydrogels (200 μL) were drawn up in the aluminum cups, and a jig with a diameter of 5 mm was used. A frequency sweep measurement of the BSA hydrogel formed at pH 3.5 revealed that both G′ and G″ were nearly independent of the frequency in the range of 0.01 to 10 Hz (Fig. 5a), with values of 2680 and 453 Pa, respectively, at 1.0 Hz. A strain sweep measurement of the hydrogel demonstrated a wide linear region, and the crossover points (G′ = G″) was observed at a strain of about 150% (Fig. 5b). Changing the size of the aluminum cup and jig allowed a reduction in sample volume by about 40% (from 350 to 200 μL). In order to realize reproducible measurements, both the size of the aluminum cup and that of the jig were changed. Measurements with an aluminum cup with a diameter of 10 mm and a jig with a diameter of 8 mm did not yield reliable results. Reproducible measurements of viscoelastic properties using 200 μL of hydrogels in an aluminum cup with a diameter of 10 mm and a jig with a diameter of 3 mm were possible. A frequency sweep measurement of the BSA hydrogel formed at pH 3.5 showed that both G′ and G″ were nearly independent of the frequency in the range of 0.01 to 10 Hz (Fig. 5a), with values of 3010 and 500 Pa, respectively, at 1.0 Hz. A strain sweep measurement had a wide linear region, and the crossover points (G′ = G″) were observed at a strain of about 85% (Fig. 5b). These results revealed that a jig having a diameter smaller than 3 mm can be used. However, reliable measurements could not be obtained with aluminum cups having a diameter smaller than 10 mm probably because of the surface tension at the edge of the cup. Therefore, to further reduce the sample volume, some artifices in sample preparation are necessary.

Hydrogel formation of BSA at different pH values was studied. Hydrogels of BSA were formed at pH 3.0–4.0, whereas viscous solutions were formed at pH 1.5–2.5. Unexpectedly, BSA hydrogel formation was observed at pH 1.0. From the CD spectra, the stereo structure of BSA at each pH was determined. The α-helix content corresponded to a partially denatured F-isoform at pH 3.0 and to a fully denatured E-isoform at pH 2.0. Rewinding of the α-helix was expected at pH 1.0 since the α-helix content was higher than that at pH 2.0. The results of hydrogelation, CD spectra, and rheological studies revealed that BSA might transform to a partially denatured F-isoform at pH 1.0. Highly reproducible rheological measurements with reduced sample volume were achieved with a traditional rotary rheometer, where an 8 mm jig allowed using a sample volume of 350 μL. Furthermore, using 3 or 5 mm jigs allowed using a sample volume of 200 μL.
BSA (fatty acid free) and water (ultrapure water) were purchased from FUJFILM Wako Pure Chemical Corporation (Osaka, Japan). Hydrochloric acid (35%) was purchased from NACALAI TESQUE, INC. (Kyoto, Japan). CD spectra were recorded on a model J-820 instrument (Jasco, Tokyo, Japan). Rheology measurements were performed by a TA Instruments DHR 2.
Gelation ExperimentsA BSA solution (about 20 wt%) was prepared by mixing BSA and ultrapure water in a glass vial. The pH and concentration (17 wt%) of the solution was adjusted by adding HCl (0.5 N) and ultrapure water, respectively. The obtained solution was kept either at room temperature or at 37 °C until hydrogels were formed (up to 60 min.). Hydrogelation was examined at different pH values: 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0. When the glass vial was inverted, the mixture remaining in the vial was defined as the hydrogel.
Circular Dichroism SpectroscopyThe CD spectra of BSA (0.02 g/L) in ultrapure water (pH 7, 3, 2, 1; pH was adjusted by HCl aq) were recorded using a 1 cm pathlength cell at 20 °C. For each spectrum, the spectrum of the corresponding solutions was subtracted, and these data were not further processed (e.g., by smoothing). The CD spectra of BSA was expressed as a molar residue ellipticity (θ) in deg.cm2.dmol−1 according to the following equation:
 |
where Cp is the molar concentration of the protein, n is the number of amino acid residues and l is the path length in cm. The α-helical contents of BSA (fH) in each pH solution were calculated from θ values at 222 nm using the following equation:
 |
A 17 wt% of BSA solution (350 μL) was placed in an aluminum cup with a diameter of 14 mm. The solution was warmed at 37 °C for 1 h, and the obtained hydrogel was then placed in the rheometer. Viscoelastic properties were measured using a jig (plate-plate geometry) with a diameter of 8 mm. The gap height was approx. 2200 μm. Each measurement was performed three times to confirm reproducibility. Strain- and frequency-sweeps were performed.
This work was supported by Grant-in-aid for the Scientific Research (No. 20K06977 for M.Yo.; 20K05704 for T.O.; 17H06374 and 21K06485 for M.Ya.) the Japan Society for the Promotion of Science (JSPS) or the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the NOVARTIS Foundation (Japan) for the Promotion of Science (for S.K.), and Scholarship Fund for Young/Women Researchers from The Promotion and Mutual Aid Corporation for Private Schools of Japan (for S.K.). We thank Prof. G. A. Ameer and Prof. I. Szleifer (Northwestern University, IL, United States) for providing ribbon diagrams of albumin.
The authors declare no conflict of interest.
This article contains supplementary materials.