2023 Volume 71 Issue 4 Pages 277-281
2023 Volume 71 Issue 4 Pages 277-281
This study aimed to develop a new and effective application form for the liver surface. We designed a two-layered sheet for the controlled release and local disposition of the anticancer drug, 5-fluorouracil (5-FU), without leakage into the peritoneal cavity. We employed poly(lactic-co-glycolic acid) (PLGA) and hydroxypropyl cellulose (HPC) to form two-layered sheets by attaching a cover sheet and a drug-containing sheet. The prepared two-layered sheets released 5-FU constantly for up to 14 d without any significant leakage from the cover side in vitro. Furthermore, we have applied sheets containing 5-FU to the rat liver surface in vivo. Notably, 5-FU could be detected in the liver attachment region even 28 d after application. The distribution ratio of 5-FU in the attachment region compared to the other liver lobes varied among the sheet formulations with different additive HPC compositions. The area under the liver concentration–time curve (AUC) of 5-FU in the attachment region from 0 to 28 d was the highest in the case of HPC 2% (w/w). This is probably due to the enhanced 5-FU released amount and controlled absorption rate from the liver surface by released HPC. No critical toxic effects were evident by the application of the two-layered sheets from the body weight change and alanine aminotransferase/aspartate aminotransferase (ALT/AST) activities. Consequently, the possible advantage of the two-layered sheets for prolonged retention of a drug in a specific region in the liver was clarified.
The recommended treatment option for hepatocellular carcinoma (HCC) is selected based on five factors: namely, hepatic reserve, extrahepatic metastasis, vascular invasion, tumor number, and tumor diameter. Local ablative therapy is currently considered the best therapeutic modality for patients with early-stage HCC.1) In local ablative therapy, the larger the tumor diameter, the more difficult it is to obtain good treatment results. Recently, combination therapy with transcatheter arterial chemoembolization (TACE) has been considered to compensate for the shortcomings. It was suggested that radiofrequency ablation (RFA) in combination with TACE is more effective than RFA alone for dilating the excision area.2) Thus, local therapy is one of the important treatments for HCC.
We have reported that organ-selective drug delivery is possible by dropping a drug solution on the surface of the liver.3) Furthermore, we have reported that this method changes the absorptivity and organ selectivity by controlling the organ contact area of the drug solution with a viscous additive.3,4) Therefore, we attempted to apply the hepatic surface administration method having such characteristics to the pharmaceutical formulation.5)
Controlled drug retention around the organ surface has been emphasized to improve drug availability to the diseased region.6,7) Furthermore, it is impossible to apply a drug to the organ surface by employing an administration device of the diffusion cell in the clinical therapy. Different types of drug delivery system formulations, such as gels,8) patch,8) and implant9) have been developed of anticancer drugs, like 5-fluorouracil (5-FU). We considered sheet-type formulation as the appropriate application strategy for the organ surface in the peritoneal cavity. There are several features of sheet-type formulations, from the stand points of controlled release, biocompatibility, and simultaneous insertion of antimetabolite10) and absorption enhancer,11) among others. In addition, it seems possible to reduce drug release into the peritoneal cavity by combining drug-containing sheet and drug non-containing sheet, so as to prevent serious side-effects in vivo.
In the present study, we attempted to produce the new two-layered sheet formulation by utilizing poly(lactic-co-glycolic acid) (PLGA) and other formulation additives. After examining drug release property from the formulation in vitro and in vivo, we determined drug distribution in the liver and plasma after application of the two-layered sheet to rat liver surface.
We selected PLGA as the base additive to form the sheet and hydroxypropyl cellulose (HPC) as the additive; both have such precedents and biodegradable properties.12–14) Several variations of HPC content have been prepared to evaluate the effect of HPC content on sheet properties. The weights of the produced two-layered sheets at different HPC concentrations were 91.72 ± 12.30, 84.73 ± 12.90, and 80.24 ± 26.20 mg, respectively, in the case of HPC 0%, HPC 2%, and 10% HPC (w/w). 5-FU contents in sheet were 4.32 ± 0.46, 9.15 ± 2.12, and 5.37 ± 0.31% (w/w), respectively. In the case of HPC 2% (w/w), the amount of 5-FU incorporated in the two-layer sheet was higher.
In Vitro Release of 5-FU from the Two-Layered SheetAs shown in Fig. 1, the in vitro release of 5-FU from 5-FU-containing sheet side or cover side in the case of 2% HPC (w/w) were examined. The cumulative release ratio of 5-FU from the 5-FU-containing sheet side was approximately 60% for 14 d. On the contrary, the cumulative release ratio of 5-FU from the cover side was less than 10% until day 7, but the cumulative release ratio from day 8 was approximately 20% cumulative release ratio on day 14. These results suggest that the cover side broke down as time progressed and the 5-FU-containing side of the sheets was in contact with phosphate buffered saline (PBS).

Each point represents the mean + standard deviation (S.D.) of at least three experiments. ○: Released percentage of 5-FU from 5-FU-containing sheet side, ●: released percentage of 5-FU from cover side. Significantly different from the result at the sheet side: ** p < 0.01 compared with the cover side.
The effect of HPC content on release profiles of 5-FU was evaluated (Fig. 2). The initial 5-FU release was increased according to HPC concentration. HPC is a water-soluble polymer,13) but PLGA is a less hydrophilic polymer. It suggests that HPC dissolution is involved in the initial drug release. The cumulative release rate of 5-FU in 10% HPC (w/w) reached a plateau in day 1, indicating that the content should be less than 10% for sustained release. The suppressive effects of 5-FU release from the two-layered sheet were probably due to prolonged diffusion in the matrix consisting of PLGA, because the 5-FU release rate from the two-layered sheet obeyed Higuchi equation (data not shown).
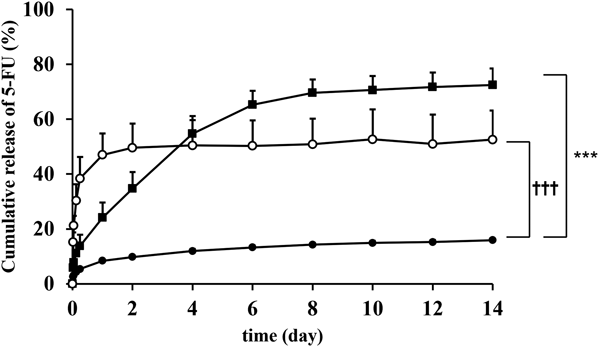
The error bar of HPC at 0% (w/w) is within the symbol. Each point represents the mean + S.D. of three or four experiments. ●: HPC at 0% (w/w), ■: HPC at 2% (w/w), ○: HPC at 10% (w/w). Significantly different from the result at HPC at 2% (w/w): *** p < 0.001 or HPC at 10% (w/w): ††† p < 0.001 compared with HPC at 0% (w/w), respectively.
The cumulative release ratio of 5-FU after applying the two-layered sheet on the liver surface in the rats was evaluated. Figure 3 illustrates the cumulative release ratio (%) of 5-FU for 28 d after application of the two-layered sheets. Under each condition, 70 to 90% of 5-FU was released from the two-layered sheet in vivo. In the 0% HPC sheet, it was observed that the release from the sheet increased sharply after day 14. In addition, 5-FU in vivo release was enhanced by adding HPC similar to the in vitro conditions. However, there was no difference in the release of 5-FU from the two-layered sheet between HPC 2 and 10% (w/w) in vivo. The effect of HPC content was large in vitro but small in vivo. It was presumed that the hydrolysis of PLGA and the dissolution of HPC did not proceed in vivo because there was not enough water on the sticking surface, resulting in different release behavior in vitro and in vivo, with little different depending on the HPC content.

Each point represents the mean ± standard error (S.E.) of three or four experiments. ●: HPC at 0% (w/w), ■: HPC at 2% (w/w), ○: HPC at 10% (w/w). Significantly different from the result at HPC at 2% (w/w): * p < 0.05 or HPC at 10% (w/w): † p < 0.05 compared with HPC at 0% (w/w), respectively.
The 5-FU concentration in the liver was determined after application of the two-layered sheet to the rat liver surface. As shown in Fig. 4 and described in Experimental, the liver was divided into three sites (the region under the sheet attachment site (site 1); the applied lobe except for site 1 (site 2); and the non-applied lobes (site 3)). The 5-FU concentration in site 1 was maintained above 0.1 µg/g liver for 28 d. However, the plasma concentration of 5-FU was below detection limit under every condition. The limit of quantitation of 5-FU was 4.3 ng/mL. In the 0% HPC sheet, the site 1 concentration increased until day 28, but the sites 2 and 3 peaked on day 14. At 2 and 10% HPC, the release from the sheet was equivalent to approximately 60% on day 4, while the liver concentration was 2% HPC, which was approximately 6 times that of 10% HPC (Figs. 2, 5). HPC has the characteristics of a viscous additive. In our previous research, absorption after liver surface administration was suppressed when a viscous additive was used.12) At 10% HPC, the absorption suppressive effect was significant, and the concentration might have decreased. It was suggested that the organ concentration changed due to the influence of the additive after the release even if the release property was the same. However, the liver concentration of 5-FU continued to decrease in the 2% HPC group for 28 d, whereas there was an increasing trend in the 10% HPC group. The liver concentration of 5-FU was determined by drug release from the sheet, absorption to the liver surface, and drug metabolism in the liver, but how HPC affects each of these processes has not been assessed separately. Therefore, further study is needed using the physiologically based pharmacokinetic model to evaluate the effect of HPC on drug absorption from the liver surface.

Name of the liver lobes: (1) left lateral lobe, (2) left medial lobe, (3) right medial lobe, (4) right lateral lobe, (5) caudate lobe, (6) quadrate lobe, and (7) papillary process lobe. The left lateral lobe was further separated into the region under the two-layer sheet (site 1) and the region not under the two-layer sheet (site 2). Other lobes were pooled together into site 3. The two-layered sheet was attached to site 1 of the rat liver using Aron Alpha biocompatible glue. All dimensions are approximate.

Each bar represents the mean + S.E. of three or four experiments. Closed column: site 1, slashed column: site 2, open column: site 3. Significantly different from the result at site 3: * p < 0.05, ** p < 0.01 compared with site 1.
Figure 6 illustrates the area under the liver concentration–time curve (AUC) of 5-FU concentration up to 28 d at each site in the rat liver after application of two-layered sheet containing 5-FU with HPC at 0, 2 and 10% (w/w). There was no difference in the AUC among sites 1, 2, and 3 at 0% HPC. However, HPC at 2, and 10% tended to have a higher AUC of site 1 than sites 2 and 3 (Fig. 5). The released HPC from the two-layered sheet was supposed to influence on sustaining 5-FU around the attachment site in the liver.

Closed column: site 1, slashed column: site 2, open column: site 3.
The liver concentration of 5-FU was relatively high (Fig. 5), whereas 5-FU could not be detected in the plasma. Most of the 5-FU released from the two-layered sheet could possibly be taken up by the liver. Regional availability of 5-FU in the applied sites in the liver was significantly enhanced by the addition of HPC in the two-layered sheet as an additive (Fig. 6). HPC had similar enhancement effect on regional availability of 5-FU.
In Vivo Toxicity of the Two-Layered Sheets after Application to the Rat Liver SurfaceThe toxicity of the two-layered sheet was examined by body weight change and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities. As illustrated in Fig. 7, body weight was significantly increased in the 10% HPC group compared to the sham group (the abdominal domain was cut open, followed by suture), but none of the two-layered sheet-applied groups lost weight. In addition, no significant increase in ALT and AST activities at day 28 observed even after application of the two-layered sheet containing HPC as an additive (Fig. 8). These results suggest that in vivo toxicity by two-layered sheet was not appreciable.

Each bar represents the mean ± S.E. of at least three or four experiments. ●: Control, ■: sham, △: HPC 0% (w/w), 〇: HPC 2% (w/w), □: HPC 10% (w/w) . Significantly different from the result at HPC at 10% (w/w): * p < 0.05 compared with sham.

Each bar represents the mean + S.E. of at least three or four experiments.
A two-layered sheet containing 5-FU for application to liver surface using HPC as an additive was developed. The 5-FU release rate increased due to the properties of the hydrophilic viscous HPC additive. Accumulation of the released 5-FU at the application site was confirmed, and the effect on hematological toxicity was not confirmed. This new two-layered sheet formulation containing additives shows sufficient flexibility to deliver the anticancer drug in the regions covered by the attached sheet and may contribute to the development of topical therapy for treating hepatocellular carcinoma.
The 5-FU was purchased from Nacalai Tesque, Inc. (Kyoto, Japan). PLGA (Lactic acid: Glycolic acid = 65 : 35, Mw 40000–75000) and HPC (Mw 110000–150000) were obtained from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.) and FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). All other reagents were of commercial grade.
Preparation of the Two-Layered Sheets Loaded with 5-FUPLGA 10% (w/v) was dissolved in acetone, followed by mixing 2 mg/mL 5-FU and 1 or 4% (w/v) HPC solutions in acetone. The mixed solution was added into the diffusion cell made by glass (i.d. 9 mm), followed by incubation at room temperature overnight. The HPC concentration in these sheets were at 0, 2, 10% (w/w). The cover sheet consisting of PLGA and 2% (w/w) HPC was obtained by the same procedure. The cover sheet and the 5-FU-containing sheet were combined by Aron Alpha A (Daiichisankyo Co., Ltd., Tokyo, Japan).
In Vitro Drug Release ProfileThe two-layered sheets loaded with 5-FU were floated on 10 mL PBS (pH 7.4) in the test tube (37 °C, 180 rpm) for 14 or 8 d. Drug release from the surface of the 5-FU-containing PLGA sheets with HPC additives or the 5-FU free (cover) sheet side was performed individually. After sampling (1 mL), 1 mL PBS was added back into the residue. The 5-FU concentration in each sample was measured spectrophotometrically using UV spectrometer (SHIMADZU UV-1600) at absorbance of 266 nm. Release ratio of 5-FU (%) was calculated by the following formula.
 |
All animal experiments in this study conformed to the Guidelines for Animal Experimentation at Nagasaki University (approval number: 1006100863-2). Eight-week-old male Wistar rats (250–270 g) were obtained from CLEA Japan, Inc. (Tokyo, Japan) and maintained on a standard laboratory diet (MF; Oriental Yeast, Co., Ltd., Tokyo, Japan) and water ad libitum. Male Wistar rats were anaesthetized with mixed drugs (butorphanol, medetomidine, and midazolam) (intraperitoneally (i.p.)). The 5-FU-containing PLGA sheets with HPC additives of the two-layered sheet loaded with 5-FU (9 mm, 0.64 cm2) was attached to the liver surface (left lateral lobe) using Aron Alpha after laparotomy. The abdominal domain of the rats was cut open, followed by the application of the two-layered sheet on the liver surface. The sheet was removed from the rat liver surface at 2, 7, 14, and 28 d after application, followed by collection of blood and liver samples. The liver was excised following perfusion with saline from the portal vein. The excised liver was divided into three sites; the region under the sheet attachment site (site 1); the applied lobe except for site 1 (site 2); and the non-applied lobes (site 3). In addition, the removed two-layer sheet was assessed to determine the remaining amount of 5-FU. Each liver sample was weighed and homogenized using two-fold volumes of their weight in isotonic phosphate buffer (pH 7.4). 5-FU concentrations in the liver homogenate, the plasma and the solution in which the two layer sheet was dissolved in dichloromethane and extracted were measured using previously reported methods10) with some modifications.14)
Area under the blood concentration time curve was calculated using a linear trapezoidal formula.
In Vivo Drug Release ProfileAfter collecting the sheets from the liver surface, they were dissolved in dichloromethane and 5-FU was extracted in liquid–liquid extraction. The concentration of 5-FU was measured in the same way as in vitro. The release ratio of 5-FU (%) was calculated by the following formula.
 |
The blood sample was collected and centrifuged at 15000 rpm for 10 min. The serum ALT and AST activity were analyzed using the Wako Transaminase CII test (FUJIFILM Wako Pure Chemical Corporation) following the procedure method.
Statistical AnalysisThe statistical analysis was performed by Dunnett’s or Tukey’s test, after examining by one-way ANOVA or two-way repeated measures ANOVA. p-Value <0.05 was recognized to be significantly different.
This work was supported by JSPS KAKENHI Grant No. 21K06513.
The authors declare no conflict of interest.