Abstract
Although aryl hydrocarbon receptors (AhRs) are related to the metabolic pathway of xenobiotics, recent studies have revealed that this receptor is also associated with the life cycle of viruses and inflammatory reactions. For example, flutamide, used to treat prostate cancer, inhibits hepatitis C virus proliferation by acting as an AhR antagonist, and methylated-pelargonidin, an AhR agonist, suppresses pro-inflammatory cytokine production. To discover a novel class of AhR ligands, we screened 1000 compounds derived from fungal metabolites using a reporter assay and identified methylsulochrin as a partial agonist of the aryl hydrocarbon receptor. Methylsulochrin was found to inhibit the production of hepatitis C virus (HCV) in Huh-7.5.1 cells. Methylsulochrin also suppressed the production of interleukin-6 in RAW264.7 cells. Furthermore, a preliminary structure–activity relationship study using sulochrin derivatives was performed. Our findings suggest the use of methylsulochrin derivatives as anti-HCV compounds with anti-inflammatory activity.
Introduction
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that belongs to the basic helix-loop-helix superfamily. AhR recognizes compounds such as polycyclic aromatic hydrocarbons and induces the expression of its target genes by binding to xenobiotic-responsive elements contained in the promoter region.1,2) AhR-regulated genes include members of the CYP family such as CYP1A1, CYP1A2, and CYP1B1. CYP1A1 is strongly induced by AhR and plays an important role in the metabolism of xenobiotics including environmental pollutants and drugs.2–6) In addition to xenobiotic homeostasis, recent studies have revealed that the AhR-CYP1A1 pathway is associated with several biological processes.
Flutamide, used to treat prostate cancer, has been found to exhibit anti-hepatitis C virus (HCV) activity. Our mechanistic study revealed that flutamide antagonized AhR activation and downregulated CYP1A1 expression to inhibit the accumulation of lipid droplets where HCV particles are assembled, which suggested the possibility of AhR antagonists as anti-HCV agents.7) Additionally, previous studies have suggested that AhR is involved in immune regulation.8,9) AhR agonists suppress the production of proinflammatory cytokines via the activation of AhR and ameliorate colitis and skin inflammation.10–12) Thus, AhR can be an attractive target for the development of both anti-HCV and anti-inflammatory agents. The aim of the present study was to discover a novel class of AhR ligands and clarify their biological activities. To identify AhR ligands, we used an in-house natural product library derived from fungi as a screening target.
Secondary metabolites of microbes have diverse structures and physiological activities and have been used in the treatment of various diseases.13,14) In our previous studies, we have focused on fungal metabolites as a source of novel bioactive compounds and found physiologically active compounds such as antiviral, anticancer, and neuroprotective molecules.15–17)
In this study, we screened a natural product library containing secondary metabolites that were partially purified from extracts of fungal culture broths using a reporter gene assay and identified methylsulochrin as an AhR partial agonist. Methylsulochrin decreased HCV production and suppressed the production of inflammatory cytokine interleukin-6 (IL-6) in cells. AhR ligand activities of methylsulochrin derivatives were also evaluated to reveal the structure–activity relationship (SAR). Methylsulochrin and its derivatives might constitute a new class of AhR ligands possessing antiviral and anti-inflammatory activities.
Results and Discussion
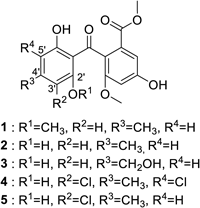
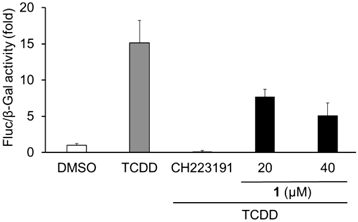
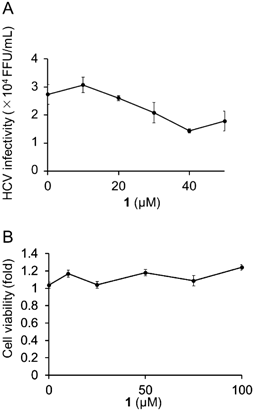
We previously constructed a library containing more than 1000 fractions, which were partially purified from the culture broths of fungi.17) The library was screened for AhR antagonists using a reporter assay with a xenobiotic response element (XRE)-driven luciferase reporter plasmid. The human liver carcinoma cell line Huh-7 was used for screening. We examined whether each library compound antagonizes AhR activation induced by tetrachlorodibenzo-p-dioxin (TCDD), an AhR agonist. An active fraction was purified and analyzed using NMR and MS, and the active compound was identified as methylsulochrin (1) (Fig. 1). Methylsulochrin was previously isolated from the fungi Rhizoctonia sp. and Aspergillus sp. as an anti-Helicobacter pylori or an anti-promastigote compound.18,19) We previously identified its related compound sulochrin (2) as an anti-HCV compound by screening a natural product library for anti-HCV activity.20) Compound 1 inhibited TCDD-induced AhR transactivation in the luciferase assay system (Fig. 2). As compound 1, similar to flutamide, exhibited AhR-antagonistic activity in the presence of TCDD, we evaluated the anti-HCV activity of compound 1. Compound 1 reduced HCV production to 45% at 40 µM without cytotoxicity (Fig. 3). These results are consistent with our previous study finding that the AhR-antagonistic activity of flutamide is associated with the inhibition of HCV particle formation.
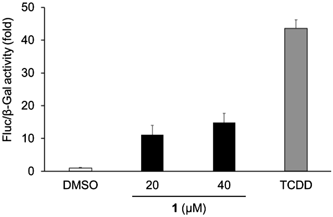
As the anti-HCV activity of compound 1 was likely to be low compared with that of flutamide, we also assessed the AhR-agonistic activity of compound 1. As a result, the treatment with compound 1 in the absence of TCDD induced transactivation of AhR although the activation was less than that of TCDD (Fig. 4). This observation suggests that compound 1 is not a full antagonist but a partial agonist.
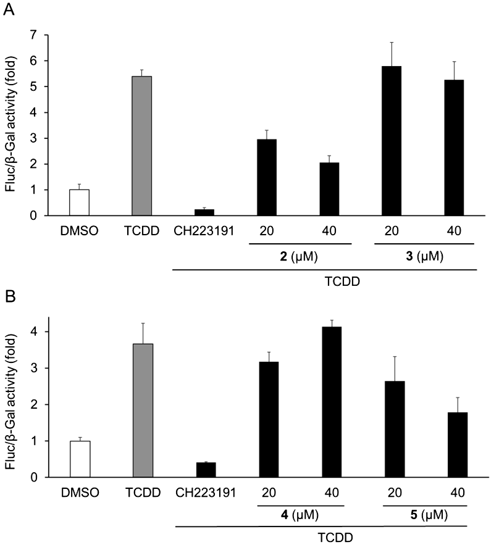
To clarify the relationship between the structure and AhR agonistic or antagonistic activities, AhR activities of methylsulochrin-related compounds 2–5 were also evaluated. Sulochrin (2) showed AhR-antagonistic activity in the presence of TCDD but did not show AhR-agonistic activity in its absence (Figs. 5A, 6A). Considering that methylsulochrin (1), a 2′-O-methyl derivative of sulochrin, displayed agonistic activity (Fig. 4), the methoxy group at C2′ contributes to the agonistic activity of AhR. Hydroxysulochrin (3) did not show AhR ligand activity in the presence and absence of TCDD (Figs. 5A, 6A), which suggests that the C4′ methyl group is related to the antagonistic activity of sulochrin. Dihydrogeodin (4), a 2′,5′-dichloro derivative of sulochrin, did not exhibit AhR-ligand activity, and monochlorosulochrin (5), a 2′-chloro derivative of sulochrin, displayed antagonistic activity but did not exhibit agonistic activity (Figs. 5B, 6B). These findings suggested that the 5′-chloride group decreased AhR-antagonistic activity.
In our previous study, sulochrin (2) significantly suppressed viral production and inhibited viral entry in the HCV life cycle.20) Here, we revealed the AhR-antagonistic activity of compound 2, indicating that the compound impairs the accumulation of lipid droplets and the formation of HCV particles. It can be speculated that compound 2 suppresses both entry and particle assembly of HCV and exerts an antiviral activity. Further studies are required to reveal the detailed mechanism of action.
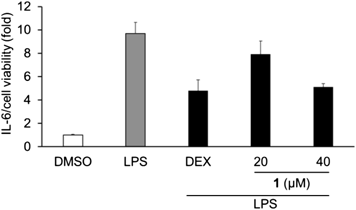
AhR and its ligand are also known to be involved in several physiological processes including immune responses. In the previous study, methylated-pelargonidin functioned as an AhR agonist and attenuated IL-6, a pro-inflammatory cytokine, secretion in an AhR-dependent manner.12) As compound 1 also showed AhR-agonistic activity, we evaluated the effect of 1 on the production of IL-6 in RAW264.7 macrophages. Compound 1 suppressed lipopolysaccharide (LPS)-induced IL-6 production without cytotoxicity (Fig. 7), which supported previous study findings that AhR activation is associated with inflammatory responses.12)
Conclusion
In the present study, compound 1 was identified as a partial agonist of AhR, and found to possess anti-HCV and anti-inflammatory activities. Our preliminary SAR study demonstrated that compounds 2 and 5 are AhR antagonists. We aim to culture the methylsulochrin-producing fungus under various conditions and search methylsulochrin-related compounds from the culture broths. Further structure–activity relationship and molecular mechanistic studies of methylsulochrin-related compounds may yield more potent antiviral and anti-inflammatory compounds. Methylsulochrin derivatives might constitute a new class of AhR ligands possessing antiviral and anti-inflammatory activities.
Experimental
ChemicalsThe natural product library used in this research was constructed as described previously.17) Methylsulochrin and hydroxysulochrin used for the measurement of biological activities were purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A.). Sulochrin was purchased from Santa Cruz Biotechnology (Dallas, TX, U.S.A.). Dihydrogeodin and monochlorosulochrin were isolated from extracts of fungal culture broths. Culture broths were extracted with CH2Cl2, and the crude extracts were separated using silica gel column chromatography to purify compounds. The compounds were identified by comparing their reported NMR and MS data.21–23)
Luciferase AssayOn day 0, Huh-7 cells (3 or 5 × 103 cells/well) were seeded in a 96-well plate containing Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, U.S.A.) supplemented with 10 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) (pH 7.4), 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, U.S.A.), 100 units/mL penicillin, and 100 µg/mL streptomycin sulfate (PS; Thermo Fisher Scientific). On day 1, the cells were co-transfected with 20 ng/well XRE-Fluc (a reporter plasmid carrying the binding element of AhR upstream of the firefly luciferase), 45 ng/well pSV-β-Gal (a reporter plasmid carrying SV40 promoter upstream of the β-galactosidase) along with expression plasmids for 2.5 ng/well AhR and 2.5 ng/well Arnt, using 0.2 µL/well Lipofectamine 2000 reagent (Thermo Fisher Scientific). After incubation for 6 h, the cells were treated with the compounds (6.25 µg/mL) in the presence of 5 nM TCDD (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan.). After 22–24 h of incubation, the cells in each well were lysed, and the firefly luciferase activity and β-galactosidase activity were measured using a Luciferase Assay System (Promega, Madison, WI, U.S.A.) and β-Galactosidase Enzyme Assay System with Reporter Lysis Buffer (Promega). All luciferase assay data were normalized to β-galactosidase level.
Anti-HCV and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) AssaysThe anti-HCV and MTT assays were performed as described previously.7)
Anti-inflammatory Activity Measurement and Cell Viability AssayOn day 0, RAW264.7 cells (1 × 105 cells/well) were seeded in a 96-well plate containing Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 100 units/mL, and 100 µg/mL PS. On day 1, RAW264.7 cells in DMEM supplemented with 2% FBS and 1% PS were treated with indicated concentrations of compound 1 or 100 µM dexamethasone (FUJIFILM Wako Pure Chemical Corporation) for 24 h. On day 2, 1 ng/mL LPS (Sigma-Aldrich) was treated for 4 h. The concentration of IL-6 was measured using the ELISA kit (Biolegend, San Diego, CA, U.S.A.). Cell viability was measured using a WST assay with Methoxy PMS and WST-1 (Dojindo Laboratories, Kumamoto, Japan). The production of IL-6 was normalized to cell viability.
Acknowledgments
We acknowledge support from Dr. Yoshihisa Sei and the Materials Analysis Division, Open Facility Center, Tokyo Institute of Technology, for NMR analysis. We would like to thank Professor Kouji Kuramochi for his encouragement and experimental support. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Private University Research Branding Project (2016–2020), the Japan Society for the Promotion of Science [KAKENHI 18K05343, 20H03499, and 21K05299], the Agency for Medical Research and Development (AMED) [JP22fk0310504 and JP20fk0210036], Takeda Science Foundation, Terumo Life Science Foundation, and Center for Human and Animal Symbiosis Science, Azabu University.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1) Guarnieri T., Abruzzo P. M., Bolotta A., Am. J. Physiol. Cell Physiol., 318, C1078–C1082 (2020).
- 2) Ye W., Chen R., Chen X., Huang B., Lin R., Xie X., Chen J., Jiang J., Deng Y., Wen J., FEBS J., 286, 4215–4231 (2019).
- 3) Nebert D. W., Roe A. L., Dieter M. Z., Solis W. A., Yang Y., Dalton T. P., Biochem. Pharmacol., 59, 65–85 (2000).
- 4) Shimada T., Oda Y., Gillam E. M., Guengerich F. P., Inoue K., Drug Metab. Dispos., 29, 1176–1182 (2001).
- 5) Rendic S., Guengerich F. P., Chem. Res. Toxicol., 25, 1316–1383 (2012).
- 6) Nebert D. W., Dalton T. P., Okey A. B., Gonzalez F. J., J. Biol. Chem., 279, 23847–23850 (2004).
- 7) Ohashi H., Nishioka K., Nakajima S., Kim S., Suzuki R., Aizaki H., Fukasawa M., Kamisuki S., Sugawara F., Ohtani N., Muramatsu M., Wakita T., Watashi K., J. Biol. Chem., 293, 19559–19571 (2018).
- 8) Schiering C., Wincent E., Metidji A., Iseppon A., Li Y., Potocnik A. J., Omenetti S., Henderson C. J., Wolf C. R., Nebert D. W., Stockinger B., Nature (London), 542, 242–245 (2017).
- 9) Trikha P., Lee D. A., Biochim. Biophys. Acta Rev. Cancer., 1873, 188335 (2020).
- 10) Smith S. H., Jayawickreme C., Rickard D. J., et al., J. Invest. Dermatol., 137, 2110–2119 (2017).
- 11) Lv Q., Wang K., Qiao S., Yang L., Xin Y., Dai Y., Wei Z., Cell Death Dis., 9, 258 (2018).
- 12) Biagioli M., Carino A., Fiorucci C., Annunziato G., Marchiano S., Bordoni M., Roselli R., Giorgio C. D., Castiglione F., Ricci P., Bruno A., Faccini A., Distrutti E., Baldoni M., Costantino G., Fiorucci S., Nutrients, 11, 1820 (2019).
- 13) Harvey A. L., Curr. Opin. Chem. Biol., 11, 480–484 (2007).
- 14) Newman D. J., Cragg G. M., J. Nat. Prod., 79, 629–661 (2016).
- 15) Murakami H., Murakami-Kawai M., Kamisuki S., Hisanobu S., Tsurukawa Y., Uchiyama J., Sakaguchi M., Tsukamoto K., Virology, 562, 1–8 (2021).
- 16) Myobatake Y., Kamisuki S., Tsukuda S., Higashi T., Chinen T., Takemoto K., Hachisuka M., Suzuki Y., Takei M., Tsurukawa Y., Maekawa H., Takeuchi T., Matsunaga T. M., Sahara H., Usui T., Matsunaga S., Sugawara F., Bioorg. Med. Chem., 27, 115149 (2019).
- 17) Kanno K., Tsurukawa Y., Kamisuki S., Shibasaki H., Iguchi K., Murakami H., Uchiyama J., Kuramochi K., J. Antibiot., 72, 793–799 (2019).
- 18) Ma Y. M., Li Y., Liu J. Y., Song Y. C., Tan R. X., Fitoterapia, 75, 451–456 (2004).
- 19) Silva-Silva J. V., Moreira R. F., Watanabe L. A., de Souza C. S. F., Hardoim D. J., Taniwaki N. N., Bertho A. L., Teixeira K. F., Cenci A. R., Doring T. H., Júnior J. W. C., de Oliveira A. S., Marinho P. S. B., Calabrese K. S., Marinho A. M. R., Almeida-Souza F., Front. Cell. Infect. Microbiol., 12, 974910 (2022).
- 20) Nakajima S., Watashi K., Kamisuki S., Tsukuda S., Takemoto K., Matsuda M., Suzuki R., Aizaki H., Sugawara F., Wakita T., Biochem. Biophys. Res. Commun., 440, 515–520 (2013).
- 21) Liu R., Zhu W., Zhang Y., Zhu T., Liu H., Fang Y., Gu Q., J. Antibiot., 59, 362–365 (2006).
- 22) Liao W. Y., Shen C., Lin L., Yang Y., Han H., Chen J., Kuo S., Wu S., Liaw C., J. Nat. Prod., 75, 630–635 (2012).
- 23) Inamori Y., Kato Y., Kubo M., Kamiki T., Takemoto T., Nomoto K., Chem. Pharm. Bull., 31, 4543–4548 (1983).